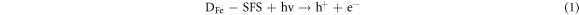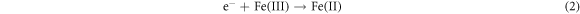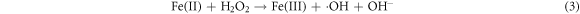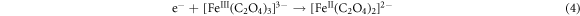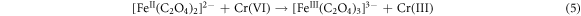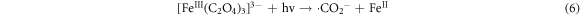Abstract
Heavy metal ions and organic pollutants cause irreversible damage to water environment, thereby posing significant threats to the well-being of organisms. The techniques of adsorption and photocatalytic degradation offer versatile solutions for addressing water pollution challenges, attributed to their inherent sustainability and adaptability. Silicates exhibit exceptional practicality in the realm of environmental protection owing to their structural integrity and robust chemical/thermal stability during hybridization and application process. Furthermore, the abundance of silicate reserves, coupled with their proven effectiveness, has garnered significant attention in recent years. This detailed review compiles and analyzes the extensive body of literature spanning the past six years (2018–2023), emphasizing the pivotal discoveries associated with employing silicates as water purification materials. This review article provides a comprehensive overview of the structure, classification, and chemical composition of diverse silicates and offers a thorough descriptive analysis of their performance in eliminating pollutants. Additionally, the utilization of diatomite as either precursors or substrates for silicates, along with the exploration of their corresponding purification mechanisms is discussed. The review unequivocally verifies the efficiency of silicates and their composites in the effective elimination of various toxic pollutants. However, the development of novel silicates capable of adapting to diverse environmental conditions to enhance pollution control, remains an urgent necessity.

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Amid the challenges of a burgeoning global population and the rapidly evolving global climate, there is an imminent surge in the demand for fresh water [1]. However, the presence of hazardous substances in polluted water further exacerbates the depletion of freshwater [2, 3]. Although wastewater recovery and centralized treatment are acknowledged as fundamental strategies for sustainable water management, the effective elimination of stubborn water pollutants (heavy metal ions, antibiotics, dyes, etc) poses significant challenges to realizing these approaches [4]. According to Global Environmental Protection research, approximately 2 billion people lack access to safe drinking water, with a pronounced effect in underdeveloped regions (primarily in sub-Saharan Africa and southern Asia). The accumulation of heavy metal ions and organic pollutants in rivers stimulates the growth of microorganisms, thereby disrupting the balance of entire ecosystem [5–8]. Over the past few decades, a variety of methods have been used to eliminate wastewater pollution, including coagulation, ultrafiltration, coprecipitation, etc [9–11]. Nevertheless, these methods often require a large amount of costs and complex operational requirements [12, 13]. Among the employed remediation techniques, photocatalysis and adsorption stand out for their simplicity, environmental friendliness, and cost-effectiveness, rendering them particularly suitable for practical production [14–16]. The photocatalysis and adsorption purification processes are shown in figure 1. In photocatalysis, when photocatalysts receive effective stimulation from light, the electrons located at the valence band (VB) absorb energy, and transfer to the conduction band (CB), generating positively charged holes in the VB. This transition facilitates the formation of various reactive oxygen species (ROS) for oxidation–reduction purposes [17–20]. Adsorption is a typical mass transfer process that can be divided into physical and chemical adsorption according to the nature of surface interaction forces [21, 22]. The exploration of adsorbents and photocatalysts for wastewater purification has recently expanded to include metallic oxides [23, 24], biochars [25], emerging 3D printed materials [26], and organic/inorganic hybrid materials [27, 28], etc. Silicates offer a relatively clean and inexpensive option for removing contaminants from aqueous medium, attributed to their high ion exchange capacity, reactive silanol groups, low or null toxicity, and high swelling/expansion capacity.
Figure 1. The schematic illustration of water purification process by (a) photocatalysis and (b) adsorption.
Download figure:
Standard image High-resolution imageSilicates, as the most prevalent minerals in the Earth's crust, are characterized by large, thick ore bodies that are easily accessible through exploration and mining [29]. This accessibility and advantages of environmental friendliness have spurred significant research interests and widespread applications in diverse fields [30, 31]. Notably, silicates have found utility in drug delivery platforms [32], energy conversion [33], lithium-ion batteries [34], and wastewater treatment [35, 36]. In the specific context of water purification, silicates, whether synthesized or natural silicate materials have emerged as promising catalysts or adsorbents for removing contaminants from water [37, 38]. Their superior adsorption performance towards heavy metal is primarily due to the presence of numerous replaceable metal ions within the chains of the silica-oxygen tetrahedral framework [39, 40]. In addition, in comparison to metal oxides, most silicates exhibit more disordered crystal structures and a greater occurrence of defects such as oxygen vacancies [41, 42], which may enhance their catalytic potential. However, the application of unmodified silicates in wastewater remediation faces several challenges, including a limited adsorption capacity, poor photocatalytic activity due to their photo inertness, and difficulties in separation and reuse [43–45]. To address these issues, a variety of modifications have been proposed to enhance the functionality of silicates. These include the adjustment of their regulating structure [46–48], surface organic modification [49, 50], and the creation of composites through the integration of silicates with other functional materials [51, 52]. Such advancements provide inspiration for further exploration and applications of silicates in water treatment by the scientific community.
In recent decades, numerous reviews have been conducted on the utilization of silicates in water treatment. By 2022, the cumulative number of studies examining the efficacy of silicates in mitigating water pollution totaled 6543, among which 1,142 focused on photocatalysis, while 5,401 addressed adsorption techniques (figure 2). However, the majority of these reviews have tended to concentrate on narrow domains, such as the elimination of particular pollutants or the exploration of certain silicate varieties. This trend has resulted in a notable scarcity of comprehensive reviews that cover the wide array of silicates and their multifaceted roles in water treatment. This review aims to furnish a systematic and comprehensive overview of the recent research progress in employing silicates for adsorption and photocatalysis in water purification. It meticulously elucidates the distinct characteristics of diverse functional silicates and investigates their surface and layered structure properties. Additionally, diatomite is one of the important precursors and substrates of silicates [53–55]. This review also encapsulates the use of diatomite-based adsorbents and catalysts in wastewater remediation, summarizing the mechanisms underpinning their effectiveness. Through an analysis of the current state of research, our objective is to facilitate and inspire further exploration and development in this field. It is our aspiration that this review will serve as a catalyst for innovative approaches and solutions, thereby catalyzing significant progress and breakthroughs in the application of silicates for water treatment.
Figure 2. Research trends of silicates for water purification. The number of annual journal papers retrieved on the topics of 'photocatalysis', 'adsorption', 'water treatment' and silicate. (Data were collected from web of science, date of search: November 30, 2023).
Download figure:
Standard image High-resolution image2. Structural features and framework types of layered silicates
The majority of silicates, including kaolin, vermiculite, glauconite, talc, and those composing rocks for other minerals exhibit a layered structure [56]. The crystal structure of these layered silicates is built upon two types of structural elements: silicon-oxygen tetrahedron and aluminum-oxygen octahedral (both with oxygen and hydroxyl groups), which combine in various configurations [57, 58]. These layers are arranged in different proportions (1:1, 2:1, 2:1:1), resulting in similar structures but various physical (especially the distribution of negative and positive charges on the surface, as well as the types of bonds between atoms and molecules) and chemical properties (interlayer exchangeable ions) [59–61].
Some examples of typical representative layered minerals are presented in table 1. The performance of silicates demonstrates certain superiority in water treatment applications, owing to their unique layered structures. In comparison to other materials, the incorporation of natural silicates leads to a reduction in synthetic material content, lower costs, and minimized pollution arising from metal leaching.
Table 1. Performance comparison of layered silicates with different structures.
| Type | Layered silicate | Composite based-silicate | Pollutant | Removal method | Removal efficiency | Conditions | References |
|---|---|---|---|---|---|---|---|
| 1:1 | Kaolinite | Kaolinite-GO-PVA | MB | adsorption | 98% | 25 °C, 200 mg L−1 | [62] |
| Kaolinite | exfoliated kaolinite nanolayers | Organic pollutants | photocatalysis | RhB:92.3% PNP:99.7% | 25 °C, 30 mg L−1 | [63] | |
| Kaolinite | kaolinite-humic acid | glyphosate | adsorption | — | 28 °C, 30 mg L−1 | [64] | |
| 2:1 | vermiculite | VE/COFTP-TTA | Cr | Adsorption + photocatalysis | 100% | 25 °C, 30 mg L−1 | [65] |
| Montmorillonite | hitosan-Montmorillonite | MO | adsoption | 99% | 25 °C, 30 mg L−1 | [66] | |
| illite | Sodalite-bearing modified illite | Fe2+ Mg2+ | adsorption | Fe2+:99% Mg2+:97% | 25 °C, 1 mg L−1 | [67] | |
| muscovite | muscovite/phillipsite | Phosphate and ammonium | adsorption | Phosphate:83% | 25 °C, 300 mg L−1 | [68] | |
| 2:1:1 | chlorite | — | As(V) | adsorption | 200 mg g−1 | — | [69] |
2.1. Type 1:1 silicates
For type 1:1 (type 'TO') silicates, the crystal layer unit comprises a single sheet of Si-O tetrahedra connected to a sheet of Al-O or Mg-O octahedra (figure 3(a)). Within the crystal structure, the Si-O tetrahedron exhibit a higher negative charge, whereas the Al-O or Mg-O octahedra exhibit a higher positive charge, resulting in an internal charge imbalance within the crystal structure [72]. The kaolinite group, a typical representative of type 1:1 silicates [73, 74], is distinguished by its large specific surface area, chemical and mechanical stability, and abundant surface hydroxyl radicals. This type of silicates are commonly employed as a carrier for loading other photocatalysts through chemical bonds. Furthermore, their chemical attributes, including resistance to acid and alkali resistance, high cation exchange ability, and excellent electron capture capability, render them highly suitable for co-doping with semiconductors [75, 76]. However, the application of 1:1 type silicates in adsorption and photocatalysis is significantly limited due to their extremely fixed crystal structure and restricted cation exchange capacity. Therefore, it becomes crucial to overcome these limitations by enhancing the properties of 1:1 type silicates through surface modification.
Figure 3. (a) Schematic representation of Type 1:1 silicate structure. (b) Schematic diagram of ZnO immobilization over the acid-activated kaolinite and the mechanism of photocatalytic activity of AAK/ZnO nanocomposites. Reprinted from [70], Copyright (2023), with permission from Elsevier. (c) Flowchart of H(OH) KAc-Kaolin preparation. Reprinted from [71], Copyright (2021), with permission from Elsevier. (d) Snapshot of kaolinite-PMG and kaolinite-HA-PMG after equilibrium of the system. Reprinted from [64], Copyright (2021), with permission from Elsevier.
Download figure:
Standard image High-resolution imageSeveral methods have been proposed in the as-published literature. For example, Md. Fardin Ehsan et al (2023) prepared nanocomposites of acid-activated kaolinite and ZnO (AAK/ZnO) using a co-precipitation method (figure 3(b)) [70]. The inclusion of kaolin greatly avoids the drawbacks of aggregation and enhances the otherwise low specific surface area of ZnO. Acid treatment not only enhances the surface negative charge of kaolinite but also introduces pores within its structure, thereby facilitating the adsorption of cationic dye methylene blue (MB). Moreover, the nanocomposite material demonstrates superior optical properties including a reduced bandgap in comparison to pure ZnO. Such a reduction in bandgap effectively minimizes electron–hole recombination, thus extending the lifetimes of electrons and holes. As a result, the composite shows excellent degradation performance in the degradation of MB dye under ultraviolet light, suggesting that kaolinite has greater potential to be a support matrix of photocatalysts. Besides serving as a photocatalyst carrier, modified kaolinite also shows commendable efficacy in the adsorption of pollutants. Zhong et al (2021) combined kaolin intercalation-exfoliation with acid/alkali modification to enhance the adsorption capabilities and porosity of kaolinite, thereby improving its adsorption efficiency [71]. The XRD pattern shows that the (001) peak of kaolin modified with potassium acetate (KAc) weakens, indicating the expansion of the interlayer spacing in kaolin due to the insertion of KAc. In addition, acid/alkali modification introduces Al3+ defects on the surface of kaolin, generating more adsorption active sites and enhancing porosity. It thus leads to more effective removal of Pb(II), Cd(II), Zn(II), and Cr(III) (figure 3(c)). Guo et al (2021) combined kaolinite with humic acid (HA) to improve the removal efficiency of glyphosate (PMG) from aqueous solutions [64], and elucidated the adsorption mechanism through thermodynamic calculations. These simulations indicate that PMG molecules exhibit parallel adsorption to both studied surfaces with the adsorption process occurring through hydrogen-bonding interaction involving the carboxyl, amino, and phosphonyl groups on PMG molecules, which form bonds with the surface groups of kaolinite modified by HA. This construction of hydrogen bonding networks significantly increases the adsorption capacity for PMG (figure 3(d)). beyond the aforementioned methods, several other modification methods are available, including calcination [77], chemical impregnation [78, 79], and combination with other active species [80], etc, further diversifying the functional aspects of 1:1 type silicate materials.
2.2. Type 2:1 silicates
The 2:1 type of silicates (type 'TOT') consist of two sheets of Si-O tetrahedron flanking a central sheet of Al-O or Mg-O octahedra, forming a three-layered SiO4-AlO4(OH)2-SiO4 structure (figure 4(a)). Different from the 1:1 silicates, the particle-silicate interactions in 2:1 type silicates are predominantly influenced by a permanent charge present in the structure [83]. Isomorphic substitution exists in both the tetrahedral and octahedra layers, resulting in negative surface charges that are beneficial for the adsorption of cations. Moreover, the structural configuration of 2:1 silicates allows each tri-layer unit to be relatively independent as there is no hydrogen bond linking the layers. This characteristic structure provides ample space for interlayer cations (such as Ca2+, Mg2+, Na+, etc). Therefore, 2:1 type silicates possess exceptional ion-exchange capability, rendering them highly promising as potential adsorbents in water treatment.
Figure 4. (a) Schematic representation of type 2:1 silicate structure. (b) Schematic diagram of the synergetic removal of Cr(VI)/Cr(III) and the recycling process of photocatalyst. Reprinted from [65], Copyright (2023), with permission from Elsevier. (c) Adsorption mechanisms of BDIEP-Vt for contaminants. Reprinted from [81], Copyright (2021), with permission from Elsevier. (d) Schematic illustration of the selective adsorption and migration process of Cs+ onto illite. Reprinted from [82], Copyright (2019), with permission from Elsevier. (e) Atomic structure of chlorite, e.g. of 2:1:1 mineral.
Download figure:
Standard image High-resolution image2:1 type mineral silicates include the expanding minerals (montmorillonite and vermiculite) and non-swelling minerals (illite and muscovite). The characteristic expansion of layered silicates results from the intercalation of hydrated cations in the interlayer spaces. In contrast, non-swelling minerals lack the embedding of water molecules due to the strong bond of potassium ions that prevents the incorporation of water molecules [82, 84, 85]. The applications of 2:1 type silicates in the field of sewage treatment are extensively documented in the literature. For example, Zhang et al (2023) conducted a study to evaluate the efficacy of hexadecyltrimethylammonium cations (HDTMA+) modified montmorillonite (Mt) and unmodified Mt in removing MB [86]. The presence of interlayer water molecules in Mt influences the orientation of MB molecules, which tend to align horizontally within the Na-Mt interlayers. In comparison, MB can be adsorbed on the surface of montmorillonite in a horizontal or inclined manner in the presence of HDTMA+. However, the influence of surfactants is further modulated by the water content. The N(CH3)3 groups in HDTMA occupy the active sites on Mt, leading to a decrease in its adsorption efficiency. As a result, the unmodified clay adsorbent demonstrates higher efficiency in adsorbing dye molecules at this stage. As the water content increases, the modified Mt demonstrates an enhanced capacity for dye molecules adsorbing. This enhancement is attributed to the hydrophobic nature of the HDTMA molecules, which tend to aggregate and separate from water. Therefore, a larger quantity of MB molecule is physically encapsulated by the HDTMA alkyl chains and retained within the interlayer space of Mt, exhibiting reduced mobility. From these findings, it is clear that the modification of Mt with cationic organic compounds does not significantly improve the adsorption capacity under specific circumstances. Nevertheless, other organic compounds have been found to effectively boost the adsorption effect of expansive silicates. Recently, an innovative total Cr (Cr(T)) removal material system was developed by modifying expanded vermiculite with a donor–acceptor type photoactive covalent organic framework (COF) [65]. The application mechanism is shown in figure 4(b). Initially, the photoactive COF polymer reduces Cr(VI) to Cr(III) under light irradiation. Subsequently, Cr(III) is adsorbed by vermiculite through ion exchange. Furthermore, the newly formed Cr (III) species interact with unreacted Cr (VI) to generate a novel photocatalyst, Cr5O12. This photocatalyst exhibits potential for the oxidation degradation of ciprofloxacin and the conversion of NO gas. This strategy also provides innovative perspectives for the recycling of waste photocatalysts. This is not an isolated instance. Mao et al (2021) designed an imidazole-ether-containing surfactant as a modifier for Vermiculite (BDIEP-Vt) [81]. The resulting composite serves as adsorbent for the removal of triclosan (TCS), p-chlorophenol (p-CP), and Cr(VI), leveraging the interaction between the adsorbent and contaminant. Meanwhile, the detailed adsorption mechanism is elaborated through DFT calculations, supplemented by a series of microstructural characterizations. As depicted in figure 4(c), for organic pollutants, the insertion of the imidazole modifier enhances the π–π interaction between BDIEP-Vt and TCS/4-CP; In the case of Cr(VI), it can be removed by electrostatic interaction according to the positively charged nature of BDIEP-Vt. Another type of non-expansive silicate has been studied for wastewater treatment. Park et al (2019) summarized the adsorption and fixation mechanisms of Cs+ by non-swelling minerals (illite) [82]. As illustrated in figure 4(d), two primary mechanisms are identified: (1) the hydrated Cs+ ions undergo dehydration, resulting in the collapse of the illite layer, which allows the Cs+ ions to migrate into deeper interlayers; (2) another fixed mechanism can be explained by the differential hydration energies of Cs+ and K+. Given that K+ has a higher hydration energy than Cs+, it preferentially combines with the hydrated molecules of Cs+. Thus, the positions of Cs+ and K+ are switched, leading to the irreversible fixation of Cs+ within the interlayer. Muscovite is also a typical non-expanded 2:1 mineral silicate. Current research on muscovite modification is primarily focused on chemical activating its surface. Rashed et al (2023) prepared low-cost and eco-friendly muscovite adsorbents through a straightforward activation process using H2O2/HCl for Rhodamine B (RhB) dye removal [87]. The simulation results pertaining to reaction kinetics and thermodynamics, suggest that the adsorption system is likely operates through physical adsorption, with RhB dye spontaneous migration towards the surface and interlayer of muscovite. Overall, 2:1 type silicates have emerged as the most commonly employed mineral silicates due to their distinctive layered structures, negative surface charge, and sufficient interlamellar space.
2.3. Type 2:1:1 silicates
There is also an additional group of silicates, the 2:1:1 layer type, which comprises 2:1 layers. For instance, vermiculite clay, in combination with a magnesium dominated tri-octahedral sheet constitutes a 2:1:1 ratio (figure 4(e)). The chlorite group, exemplifying the 2:1:1 type of silicate possesses a principal chemical formula of (Mg, Fe, Li)6AlSi3O10(OH)8 [88]. The chlorite group is differentiated into dioctahedral and trioctahedral structures, with the former being comparatively less common [89]. Lin et al (2020) investigated three types of silicates (1:1 layer, 2:1 layer, and 2:1:1 layer) for As(V) adsorption [69]. This study indicates that chlorite (2:1:1) exhibits superior adsorption capabilities for As(V) compared to other silicate varieties. This superiority can be explained by the basic properties of chlorite, which is rich in iron and contains a large amount of FeO and Fe2O3. Previous research has shown the potent adsorption capacity of iron oxides for As(VI). Furthermore, the 2:1:1 layered structure consists of 2:1 layers plus one interlayer hydroxide sheet, which is negatively charged because of ion substitution. Notably, the interlayer hydroxide sheet is instrumental in the effective removal of As(V). Overall, the 2:1:1 type of clay silicates has exhibited remarkable adsorption performance for heavy metals. Nevertheless, the limited diversity of this type of silicate necessitates further investigation to explore its potential for wastewater treatment applications.
3. Water treatment applications of silicate materials
3.1. Photocatalysis
As a fundamental class of substances, silicates are widely used in the photocatalytic water purification, attributed to their substantial specific surface area and stability. Moreover, the inclusion of transition metals within silicates enables the SiO4 tetrahedron to construct an internal polar electric field through deformation and polarization [90]. Therefore, transition metal silicates have potential to promote electron transfer and augment light absorption. However, the photocatalytic properties of silicate materials have been limited due to rapid recombination of photogenerated electron–hole pairs [43, 91]. To address this challenge, various strategies have been developed to tackle these problems, including defect engineering, constructing heterojunctions, and structure modulation.
3.1.1. Defect engineering
Recently, defect engineering of semiconductors such as element doping and the introduction of vacancies, has been recognized as a promising strategy for enhancing the photocatalytic activity of silicate photocatalysts [92–94]. The key lies in how to introduce defects into silicates by convenient, precise, and manageable ways. He et al (2022) developed a facile ball-milling method to obtain sodium iron silicate with iron defects (DFe-SFS), wherein the concentration is adjustable through the modification of milling conditions [95]. The primary cause of chemical bond disruption in the NaFeSi2O6 crystal is the Fe-O bond. In SFS, three types of oxygen bonding are identified: Si-O-Fe, Si–O–Si, and Fe-O-Fe. It is important to note that the existence of transition metal Fe significantly polarizes the silicate tetrahedra, thereby resulting in a much lower bond energy for Fe-O bonds in comparison to Si-O bonds (figure 5(a)). During the ball-milling process, the Fe-O bonds are broken, releasing Fe3+ from the crystal lattice. The introduction of Fe-deficiency promotes the separation of photogenerated electron–hole pairs (figures 5(b), (c)) and modulates the band structure of SFS (figure 5(d)). Exposed Fe-deficiency is pivotal in facilitating the activation of hydrogen peroxide and oxalate (OA), thereby generating active radicals essential for the degradation of ciprofloxacin and reduction of Cr (VI). The mechanism of photo-fenton catalytic reactions is illustrated in figure 5(e). Firstly, the semiconductor catalyst is excited by incident light, leading to the generation of electrons in the CB. These electrons are subsequently attracted to the sites of Fe-deficiency in SFS, which promotes the reduction of Fe(III) to Fe(II). Secondly, Fe(II) reacts with H2O2 to produce •OH and Fe(III), thus establishing a cyclic reaction involving iron. Throughout this cycle, ciprofloxacin is decomposed due to the strong oxidative capability of •OH. The specific reaction equation is presented below:
In addition, oxalic acid complexes with Fe(III) to form [Fe(III)(C2O4)3]3− system, which is subsequently reduced to [Fe(II)(C2O4)3]2− upon receiving photogenerated electrons. The complex plays a significant role in facilitating the reduction of Cr(VI). Moreover, a portion of [Fe(III)(C2O4)3]3- can be photoexcited, leading to the generation of •CO2, which further contributes to the conversion of Cr(VI) to Cr(III). The reaction equations are as follows:
The findings suggest that the Fe-deficient SFS exhibits a superior removal efficiency compared to the original SFS sample. This substantiates the claim that incorporating defects can markedly enhance the photocatalytic performance. Similarly, existing literature suggests that the creation of oxygen vacancies [96] and transition metal doping [97] are commonly employed to promote the photocatalytic capabilities of silicates. Sarkar et al (2021) synthesized Bi2SiO5 nanoparticles endowed with rich oxygen vacancies (BSO NPs) [98]. The structural and morphological features of the BSO NPs are adjusted by the incorporation of halide ions (Cl-, Br-, and I-), which initiated the development of monoclinic phases within the BSO NPs. The introduction of oxygen vacancy defect sites serves to counterbalance the surface charge discrepancy generated by the halide ion doping. The formation of oxygen vacancies changes the valence and conduction bands, leading to a decrease in resistance to charge transfer and an increase in charge transfer efficiency. BSO nanoparticles, enriched with oxygen vacancies, exhibit an enhanced ability to eliminate harmful anionic dyes and antibiotics from wastewater compared to the unmodified BSO. Long et al (2022) prepared Cu-doped cobalt silicate using a universal alcohol-thermal method [99]. The incorporation of Cu not only promotes the radical pathway but also enhances the non-radical pathway through the generation of more oxygen vacancies. The effective degradation of the target pollutant tetracycline suggests a synergistic effect of bimetallic compounds in silicates.
Figure 5. (a) Schematically formation of DFe-SFS. (b) Photo-luminescence (PL) and (c) transient photocurrent responses (TPR) of pristine SFS and DFe-SFS-10 (DFe-SFS after ball-milling ten minutes). (d) Band position diagrams of pristine SFS and DFe-SFS-10. (e) A plausible charge transfer mechanism of DFe-SFS materials for photoFenton-like redox reactions under visible-light irradiation. Reprinted from [95], Copyright (2022), with permission from Elsevier.
Download figure:
Standard image High-resolution imageHence, the introduction of defects provides a new avenue for the design and optimization of silicate catalysts. However, it is essential to optimize the concentration and distribution of defects to achieve enhanced performance. More efforts should focus on elucidating the complex interplay between the characteristics of defects and photocatalytic activities, enabling the construction of photocatalysts with superior efficiency.
3.1.2. Heterostructure construction
The heterostructure engineering of two semiconductors provides novel and efficient photocatalytic materials that promote charge separation and enhance photocatalytic efficiency [100]. Through an analysis comparing the CB and VB positions of TiO2 (TO) with Ag10Si4O13 (ASO), Li et al (2022) built a staggered type II heterojunction between these two components by a sol–gel assisted thermal synthesis technique [101]. The effective crystal cell of Ag10Si4O13 is more efficient due to its smaller size, leading to enhanced ion mobility and charge carrier separation. Under light irradiation, electrons excited in TiO2 migrate to the CB of Ag10Si4O13, while photo-generated holes transfer spontaneously from the VB of Ag10Si4O13 to the VB of TiO2. This process facilitates the degradation of MB by the active species (•OH and h+) (figure 6(a)). The construction of heterojunctions is an effective means of enhancing photocatalytic efficiency. However, the preparation method is also crucial for identifying a simple and cost-effective approach. Recently, our group has designed a novel S-scheme NaFeSi2O6/Fe3O4-Fe2O3 (SFS/M-FO) composite through a one-step hydrothermal in situgrowth method [102] (figure 6(b)). Given the challenges associated with low carrier migration efficiency and unsuitable separation in either α-Fe2O3 or NaFeSi2O6, the in situ synthesized heterojunction of NaFeSi2O6/Fe2O3, featuring tightly bound interfaces, exhibits a robust redox capability and high electron–hole separation efficiency. The introduction of magnetic Fe3O4 enables rapid separation and recycling of photocatalysts from the aqueous solution. Meanwhile, Fe3O4 serves as an excellent conductor material, enhancing the facilitation of electronic transmission. The S-scheme charge transfer mechanism is depicted in figure 6(c). Compared to the type-II heterojunctions, the S-scheme NaFeSi2O6/Fe3O4-Fe2O3 hetero-junction effectively separates photogenerated electron–hole pairs while preserving the maximum redox potential. The holes generated in the VB of SFS directly contribute to the decomposition of TC. Simultaneously, electrons generated in the CB of FO reduce Fe(III) to Fe(II). Furthermore, the recycling of Fe(III)/Fe(II) pairs leads to the decomposition of H2O2, creating reactive oxygen species that contribute to the degradation of tetracycline.
Figure 6. (a) Photocatalytic mechanism of ASO/TO (2:1). Reprinted from [101], Copyright (2022), with permission from Elsevier. (b, c) Schematic illustration of the preparation procedure of samples by one-step in situ growth method (b), and of degradation mechanism of tetracycline over NaFeSi2O6/Fe3O4–Fe2O3 in photo-Fenton-like reaction (c). Reprinted from [102], Copyright (2023), with permission from Elsevier. (d, e) TEM images of copper silicate nanotube-assembled hollow sphere and (f) corresponding chemical mechanism. Reprinted with permission from [103]. Copyright (2018) American Chemical Society.
Download figure:
Standard image High-resolution image3.1.3. Morphological modulation
The catalytic performance of photocatalysts frequently depends on the morphological characteristics of the materials [104]. For instance, 2D silicates developed recently have shown promising high adsorption and catalytic activities in water treatment, attributed to their considerably larger specific surface areas compared to traditional silicates [105, 106]. Therefore, specific structural features of nanostructured catalysts (such as hollow-nanostructured, layered structure, etc) often demonstrate superior catalytic performance. A reasonable design of the catalyst's structure provides a facile way to explore the influence of structural variations on catalytic processes.
Figures 6(d)–(f) display the catalytic activity of copper silicate hollow sphere assembled by nanotubes, towards hydrogenation of dimethyl oxalate [103]. This structure facilitates the enrichment of hydrogen on the concave surfaces of the nanotubes and hollow spheres, resulting in remarkable activity and stability. Alamgholiloo et al (2022) synthesized a bimetallic-ZIF@silicate derived Co/NC@mSiO2 hollow sphere [107]. The results obtained indicate that the Co site within the hollow sphere serves as efficient active sites for activating persulfate, thereby generating HO•, SO4 •- , and 1O2 radicals for the degradation of ciprofloxacin. The specific silicate architectures have gained considerable interest owing to their high catalytic efficiency and widespread applications. It is evident that optimized surface morphology, alongside a significant specific surface area, holds significant potential for enhancing photocatalytic activities.
To facilitate a clearer understanding, table 2 presents a summary of the various modification methods previously discussed, highlighting their respective advantages and limitations. In fact, the modification methods for photocatalysts, including morphology modification, heterojunction construction and defect introduction, are multifaceted and should be tailored based on the intrinsic properties of the materials to enhance their strengths and avoid their weaknesses. Continued investigation into these areas holds promise for improving photocatalytic efficiency and expanding the application of photocatalysis in water pollution treatment.
Table 2. Different methods of modification of silicates and their advantages and limitations.
| Method | Silicate | Type | Pollutant | Advantage | Disadvantage | References |
|---|---|---|---|---|---|---|
| defect | cobalt silicate | Mn-doping | MO | Suppressing hole electron pair recombination | The reaction process is difficult to regulate | [108] |
| sodium ferric silicate | Fe-deficiency | Cr(VI);CIP | [95] | |||
| Heterojunction | silver silicate | Type II | TC;MO;MB | Rapidly separated electron–hole pairs | Poor redox | [44] |
| sodium ferric silicate | S-scheme | TC | Electrons with high reducibility and Holes with high oxidation capacity | High-requirement of band structure | [102] | |
| bismuth silicate | Z-scheme | RhB | Electrons with high reducibility and Holes with high oxidation capacity | PH sensitive | [109] | |
| Morphological modulation | copper silicate | hollow sphere | dimethyl oxalate | Increase light absorption, effectively separate electron–hole pairs | The synthesis is relatively complex | [103] |
3.2. Adsorption
Silicates have undergone extensive research over recent years for their potential as adsorbents in the removal of different poisonous contamination, owing to their stability, low cost, and non-toxic nature [110]. Table 3 summarizes and reorganizes studies on their capabilities in the elimination of pollutants and adsorption mechanisms, demonstrating that these adsorbents exhibit strong adsorption capacities and versatility in pollutant removal. This section of the review provides insights into the use of diverse silicates and their modified forms as adsorbents, with the goal of advancing the development of silicate-based materials for wastewater purification.
Table 3. Application of different silicate materials and their composites in pollutant adsorption.
| Silicate | Silicate composite material | Pollutant | Removal efficiency | Adsorption mechanism | References |
|---|---|---|---|---|---|
| Magnesium silicate | sepiolite-based alkali-activated material | MB | 99.92 mg g−1 | Electrostatic attraction | [111] |
| sodium silicate | Silicate-modified oiltea camellia shell-derived biochar | Cd(II) | 211 mg g−1 | Ion exchange with Na+, surface precipitation; coordination with π electrons | [112] |
| palygorskite | bismuth silicate | chlortetracycline | 329.84 mg g−1 | Electrostatic attraction | [113] |
| zeolites | copper silicate | Pb(II) | 252.7 mg g−1 | Chemical adsorption | [114] |
| calcium silicate | Fe(III) modified calcium silicate hydrates | Cu(II), Cd(II), and Zn(II) | 99.94%; 99.98%; 99.99% | surface precipitation | [115] |
3.2.1. Heavy metals
Numerous studies have been conducted on the adsorption properties of silicates towards heavy metal ions, With an emphasis on quantifying adsorption performance through the analysis of various influencing factors such as time, initial concentration, pH, etc to explain the adsorption processes. Recently, Fe(III) modified calcium silicate hydrates have investigated their properties and application in removing As(V), Cu(II), Zn(II), and Cd(II) from acidic mine drainage [115]. With a pH range of 2–4, an equilibration time of 120 min, Fe(III)-calcium silicate hydrates (CSHs) achieved removal efficiencies of 99.94% for Cu(II), 99.98% for Cd(II), 99.99% for Zn(II), with the maximum arsenic loading capacity approaching 55 mg g−1. Kinetic experiments confirm that the chemical adsorption process is controlled by a pseudo-second-order kinetic model, and the synthetic adsorbent exhibits a high adsorption rate for all tested ionic species. The modification process entails substituting a portion of Ca in nanosized CSH with Fe(III), leading to the development of insoluble and stable double calcium and iron arsenate. This approach significantly enhances the removal efficiency of As(VI) from acidic mine drainage, while also preserving the adsorption capacity of nano CSH to remove other metal ions. Similarly, Sun et al (2022) proposed a strategy of adsorption-decalcification utilizing steel slag to construct CSH adsorbents [116]. The negative surface charge of CSH gives it a strong affinity for metal cations, facilitating further adsorption through chemical precipitation and ion exchange. Subsequently, surface functionalization of the spent adsorbent is realized via CO2 weathering decalcification followed by H2 chemical reduction, resulting in the production of SiO2 materials loaded by metal/metal oxide nanocrystals. Thus, this idea provides a promising solution for reusing adsorbents burdened with heavy metals. Deng et al (2020) prepared a highly efficient silicate-hydrochar (MgSi-HC) composite and studied its adsorption behavior (figure 7(a)) [117]. The adsorption isotherm shows that the maximum adsorption capacity of MgSi-HC for Cu(II), Zn(II), and tetracycline are 214.7, 227.3, and 361.7 mg g−1, respectively. The mechanism experiments reveal that Cu(II) and Zn(II) adsorption onto MgSi-HC occurs through pore filling, co-precipitation, and surface complexation. Meanwhile, Cu(II) and Zn(II) as cations, can undergo ion exchange with the cations in MgSi-HC. Tetracycline can then chelate with these metals to create a complex, thereby improving the adsorption capacity of tetracycline. It is evident that the adsorption performance of adsorbents primarily depends on their pore structure and chemical composition. To increase the exposure of active sites and provide rapid transport channels, Bao et al (2023) developed a series of magnesium silicates with layered pore structures and high specific surface area by further modification with sodium dodecyl sulfonate [118]. The morphological analysis in figure 7(b) indicates that the obtained samples contain a large number of mesopores and macropores, expediting the rapid transport of adsorbates within the pores, thereby accelerating the adsorption rate of Cd(II). The maximum adsorption capacity reaches 244.5 mg g−1, with the adsorption mechanism for Cd(II) primarily credited to electrostatic interaction, exchange of metal ions and surface group interaction with solution. The ion exchange behavior is demonstrated in figure 7(c). However, in response to the strategic requirements of the global situation and economic development, numerous silicate adsorbents synthesized from industrial solid waste have been developed recently. Fly ash, a by-product of coal-fired power plants predominantly consisting of silica and alumina, serves as one of raw materials for these novel developed adsorbents. Li et al (2022) have reported a zeolite-calcium silicate hydrate composite was obtained through one-step hydrothermal synthesis using activated fly ash as the source of silicon [120]. A series of experiments have confirmed the remarkable capacity for adsorbing different heavy metals (Pb(II), Ni(II), Cd(II), Zn(II), Cu(II), and Cr(III)), leveraging the ion exchange properties of zeolite and hydrated calcium silicate. Additionally, silicate waste sourced from the Syah-Kamar Polymetal Porphyry mine subjected to acid and alkaline modification has been used in MB and Pb(II) removal (figure 7(d)) [119]. This adsorbent not only exhibits a high adsorption capacity but also shows renewable performance, indicating that using natural or abundant mining waste for the treatment of wastewater containing toxic elements and dyes represents a cost-effective approach. In most of the studies, adsorption has been shown to be significantly influenced by pH, implying a likely contribution from the surface complexation mechanisms. Moreover, the excellent ion exchange performance of silicates further enhances their adsorption effectiveness.
Figure 7. (a) The synthesis flowchart of MgSi-HC and its adsorption mechanisms. Reprinted from [117], Copyright (2020), with permission from Elsevier. (b) SEM images of magnesium silicate samples and (c) the concentration relationship between the released Mg(II) and adsorbed Cd(II) in the solution. Reproduced from [118], with permission from Springer Nature. (d) Schematic diagram of renewable utilization of silicate waste. Reprinted from [119], Copyright (2022), with permission from Elsevier.
Download figure:
Standard image High-resolution image3.2.2. Organic and biological pollutants
In comparison to inorganic cationic pollutants, organic or biological pollutants often exhibit higher levels of toxicity and persistence. Therefore, the adsorption mechanisms for these two types of substances differ significantly. The removal of inorganic pollutants is eliminated through electron exchange to establish chemical bonds, while organic and biological pollutants are eliminated through van der Waals forces, hydrogen bonding, polarity and π–π interactions [121, 122]. Although silicates serve as affordable and safe adsorbents, the adsorption capacities of unmodified silicates are unsatisfactory. Numerous studies have attempted to improve the removal efficiency of organic and biological pollutants through surface activation with acids, surfactants, and polymers. For example, acid leaching treatment disrupts the Si-O-M bonds, resulting in an increased SiO2 content in sepiolite. As a result, the fiber morphology of acid leached silicate can serve as a template to synthesize talc-type magnesium silicate (MMSCs) [123]. Based on the Langmuir model, the maximum adsorption capacity for aflatoxin has been calculated as 21.26 mg g−1. The desirable behavior is attributed to the synergistic effect of the flower morphology and mesoporous structure, which increases the surface area and active sites. The primary adsorption mechanisms include electron donor acceptor interactions and hydrogen bonding (figures 8(a) and (b)). Moreover, a tremella-like mesoporous CSH with a tremella-like structure (AP-CSH) was prepared to investigate its effectiveness in adsorbing formaldehyde [125]. The results indicate that AP-CSH with multi-pleated surface structure possesses a vast specific surface area, facilitating the rapid removal of formaldehyde from water and exhibiting a removal efficiency of 98.94%. The high adsorption efficiency can be attributed to the connection between the electronegative oxygen atoms in formaldehyde molecules and the silicon groups present on AP-CSH, in contrast to other materials reported. Recent studies have also investigated the structures of rod-like [126], cage-like [127], and vesicle-like silicates, underscoring the significance of structure in their application for wastewater treatment.
Figure 8. (a) Percentage content of Si-O, Si-O-Mg and Si-OH in MMSC-12 before and after adsorption and (b) adsorption mechanisms of MMSC-12 for AFB1. Reprinted from [123], Copyright (2022), with permission from Elsevier. (c) Silicate/carbon composite adsorbent for organic pollutants removal from water. Reprinted from [124], Copyright (2022), with permission from Elsevier.
Download figure:
Standard image High-resolution imageSome novel methods have been developed to address the limitations of conventional methods in the fabrication of silicate-based composite adsorbents. For instance, Wang et al (2019) successfully converted palygorskite into novel silicate/carbon composite adsorbents, demonstrating remarkable efficiency in the removal of dyes and antibiotics (figure 8(c)) [124]. The adsorption isotherm and kinetic fitting results show that organic molecules are adsorbed through monolayer coverage and chemical adsorption plays a pivotal role. These non-toxic silicate-based adsorbents are considered ideal for the removal of organic pollutants.
4. Diatomite-based composites
4.1. The characters of diatomite
Diatomite is a silicate rock with high surface area formed from the fossilized skeletons of unicellular algae [128, 129]. The main component of diatomite is silicon dioxide (SiO2), along with considerable amount of quartz, carbonate, clay minerals, and organic matter. It has been mined in nearly 30 countries around the world for a period of time. At present, the main producers of diatomite are the United States and China, jointly contributing to nearly 50% of the world's total production [130]. Diatomite is characterized by unique structural properties, such as graded pore organization and unique three-dimensional structure (figure 9), alongside its lightweight and high surface area, which have garnered attention. Therefore, diatomite has been found widely used in various fields, including drug delivery, catalysts, supporting material, supercapacitors, etc [132–134] The application of diatomite in water purification can be traced back to the early stages of World War I, when it was employed to remove particulate matter from drinking water [135]. In recent years, there has been an endless stream of research on diatomite-based composites, and a review of a selection of this research combined with the work of our research group is now presented.
Figure 9. Skeleton and structure of disk-shaped diatomite. Reprinted from [131], Copyright (2012), with permission from Elsevier.
Download figure:
Standard image High-resolution image4.2. Diatomite as a carrier for composited materials
The attachment of dispersed nanoparticles onto diatomite featuring a graded pore structure can effectively avoid the loss of active sites caused by agglomeration. Specifically, the plentiful silanol groups that exist on the surface of diatomite have demonstrated the ability to support a variety of nanosized metal oxides or silicates [136, 137]. Based on the characteristics of diatomite, our research group has developed a series of diatomite-based composites, broadening their applications in sewage purification. Employing a universal synthesis approach, the solution-phase route was developed by Wu et al (2019) to synthesize nanostructured niobates on natural mineral diatomite (figures 10(a)–(c)) [141]. Interestingly, the system of niobates@diatomite can be adjusted using (NH4)2C2O4. The generated H2C2O4 dissolves Nb2O5 to form a complex, while another hydrolysis product NH3•H2O initiates diatomite's surface etching, making the heterogeneous crystallization process controllable. As illustrated in figures 10(d) and (e), the as-obtained MnNb2O6-, SnNb2O6-, and ZnNb2O6-diatomites are used for the photoreduction of Cr(VI) to Cr(III), the physical eliminating Fe(III), and the direct chemisorption of Pb(II). The integration of diatomite into active niobate materials yields a highly effective and promising composite for environmental purification. Additionally, Sun et al (2019) utilized a facile hydrothermal process to construct Zn2SiO4 or Mn7O8(SiO4) with diverse structures and morphologies on diatomite, showing a high adsorption capacity for typical heavy metal Pb(II) and Cd(II) [138]. The hierarchical silicate nanostructures provide a broad space for Zn2SiO4 or Mn7O8(SiO4) growth, resulting in a sizeable specific surface area and generous active sites. In the case of Zn2SiO4-diatomite (ZnDt), silicate anions easily hydrolyze, releasing OH− ions that subsequently interact with metal ions to form insoluble hydroxides on the surface of ZnDt. This research inspires the development of novel diatomite-based adsorbents. Moreover, a composite material (Fe2O3-MnO2/Diatomite) with excellent As(VI) adsorption performance and reusability has been developed (figure 10(f)) [139]. Nanostructured Fe-Mn oxides on diatomite contain rich surface functional groups that collaboratively facilitate the removal of As(VI) by complexing with surface hydroxyl groups. After five cycles of testing, the As(VI) removal efficiency of Fe2O3-MnO2/Diatomite remains above 90%. These results prove significant advancements in the development of diatomite-based adsorbents. However, there have been limited systematic studies on the potential recycling of spent adsorbent loaded with toxic micro pollutants such as heavy metal ions. There is a growing concern about the secondary pollution caused by the used conventional adsorbents. Addressing this issue, Liu et al (2021) obtained an environmentally friendly diatomite-based adsorbent (Dt@ZnS-TA) by introducing ZnS or Zn(II)-tartaric acid complex. The composite efficiently removes heavy metal ions such as Pb(II), Cu(II), and Cd(II) [140]. The surface of the synthesized Zn-based composite displays wire- or sheet-like morphology, enhancing its dispersion and separation from the solution. The experimental results indicate that the maximum adsorption capacities for Pb(II), Cd(II) and Cu(II) are 132.6, 12.6, and 56.7 mg g−1, respectively. Subsequently, Dt@ZnS loaded with Cd(II)/Cu(II) is converted directly into diatomite@Cu-/Cd-doping-ZnO photocatalysts through calcination, exhibiting good Cr(VI) photoreduction performance under sunlight irradiation (figure 10(g)). Furthermore, the adsorption capacity for Cd(II)/Cu(II) can be adjusted to fine-tune light absorption, enabling the complete removal of Cr(VI) from wastewater. This work shows the potential of diatomite-based composites as effective materials for decontamination and explores the possibility of reusing toxic adsorbent waste. Diatomite emerges as an excellent catalyst carrier, attributed to its large specific surface areas and high surface activity. The synthesized diatomite-based composites have shown significant improvement in adsorption/catalytic efficiency, reusability, controllable cost, and other relevant aspects, thereby making a considerable contribution to eliminating targeted pollutants from wastewater.
Figure 10. (a)–(c) TEM images of (a) MnNb2O6, (b) ZnNb2O6, (c) SnNb2O6 nanostructures on diatomite, and (d) their Cr(VI) removal performance and (e) Fe(OH)3 adsorption efficiency. Reproduced from [138], with permission from Springer Nature. (f) TEM images of the Fe2O3-MnO2/diatomite sample. Reprinted from [139], Copyright (2022), with permission from Elsevier. (g) Dt@ZnS sample for Cu(II) and Cd(II) removal and the recycled diatomite@Cu-/Cd-doping-ZnO photocatalysts for Cr(VI) reduction. Reprinted from [140], Copyright (2021), with permission from Elsevier.
Download figure:
Standard image High-resolution image4.3. Diatomite as a silicon source for silicates
Various sources of silicon are used in the production of silicates, such as organic silicon, kaolin, and molecular sieves. Among these, diatomite stands out for its safety, cost-effectiveness, and environmentally friendly attributes, effectively addressing the limitations arising from the synthesis of composites [142]. Up to now, the use of diatomite as a raw material has led to the creation of various types of silicates [143, 144].
Zeolites are a group of aluminosilicates composed of silica and alumina tetrahedra, offering advantages such as uniform porosity, high cation-exchange capacity, and shape adjustability. In order to overcome the challenge of controlled synthesis of zeolite, our research group has successfully synthesized sodalite zeolite using diatomite via an alternative solution-mediated crystallization route. This obtained material proves effective for cations (Pb(II), Cd(II), Zn(II), and Cu(II)) removal [145]. The material's high adsorption capacity is largely attributed to the ion radii's similarity, which favors the ion exchange process (figures 11(a) and (b)). In a related study, Yao et al (2021) synthesized X zeolite from diatomite and explored its ability to adsorb Cu (II) and Zn (II) in obtained samples [146]. The meso-microporous structure of X zeolite, with its expansive cavity volumes and channels, improves the diffusion and transport of Cu(II) and Zn(II). These findings suggest that the design of advanced zeolite materials using diatomite for heavy metal removal is a feasible strategy. However, the aluminum tetrahedron's permanent negative charge significantly limits its ability to remove anionic species (such as Cr2O7 2−, MnO4 −, AsO4 3−, etc).
Figure 11. (a) SEM images of Na8(AlSiO4)6(OH)2 zeolite on diatomite and (b) corresponding mechanism of ion exchange. Reprinted from [145], Copyright (2020), with permission from Elsevier. (c) SEM images of Mg4Si6O15(OH)2-diatomite adsorbent and (d) corresponding mechanism for Cr removal. Reprinted from [54], Copyright (2021), with permission from Elsevier.
Download figure:
Standard image High-resolution imageBoth diatomite and silicates are composed of silicon oxygen tetrahedra, which allows silicates to connect with the diatomite substrate through Si-O chemical bonds. The synthesis of new silicate/diatomite composite materials using diatomite as both the substrate and silicon source is considered an effective strategy [147, 148]. As shown in figure 11(c), Wang et al (2021) presented a sepiolite phase Mg4Si6O15(OH)2-diatomite adsorbent for Cr(VI) wastewater purification. The composite adsorbent achieves a maximum adsorption capacity of 1292 mg g−1 [54]. The removal mechanism is outlined in figure11(d), involves the corrosion by ammonia to form silicate anions SiO3 2−, which then reacts with Mg2+ to form Mg4Si6O15(OH)2. Meanwhile, the diatomite serves as a template for the growth of magnesium silicates and prevents the aggregation of nanomaterials, thus making more active sites available. The specific adsorption mechanism can be categorized into three parts: Firstly, the as-obtained magnesium silicate/diatomite composite possesses abundant adsorptive sites, including Si-O-Mg bonds, Si-O-dangling bonds, etc, which can combine with Cr(VI). Secondly, Cr(VI) reacts with magnesium silicate/diatomite composite to produce MgCrO4 precipitate. Lastly, a portion of Cr (VI) is reduced to Cr(III), which then undergoes ion exchange with interlayer cations to form MgCr2O4. Similarly, a silicate/diatomite adsorbent was fabricated for the purification of uranium-containing wastewater [149]. A series of experiments demonstrated that the composite has a substantially higher maximum adsorption capacity for U(VI). Therefore, utilizing diatomite as a silicon source for the synthesis of silicates presents an efficient and cost-effective approach for the removal of heavy metal ions and organic pollutants.
5. Conclusion and outlook
The implementation of the overall trend towards green and sustainable development relies on the utilization of non-toxic and renewable materials. Silicates are the preferred candidates for adsorbents because of their large specific surface area and high adsorption capacity for targeted substances. Furthermore, enhancing the photocatalytic performance is achievable by dispersing photocatalysts across the surface of silicates, thereby increasing the availability of active sites. Many studies have demonstrated that silicates play a vital role in the transformation and purification of polluted water. In this review, we have come up with some observations and recommendations.
- (1)At present, there are numerous studies focused on the effectiveness of silicates in adsorptive removal of pollutants. However, experiments with simulated water do not accurately reflect the performance of new silicate materials in real water environments. There is room for further improvement in the anti-interfering and selective removal performance of silicate or silicate-based materials. The design and production of charged porous materials with unique pore structures and abundant surface functional sites remain future directions in the development of silicate composites.
- (2)The structure of photocatalytic materials exerts a significant impact on their catalytic performance. Type 2:1 silicate is the most frequently and extensively employed silicate structure. Its surface holds negatively charged electrical properties and sufficient spatial structure, which results in its high adsorption capacity. But its potential as a catalyst carrier is frequently disregarded.
- (3)There remain certain unclear aspects pertaining to the comprehensive catalytic mechanisms of silicate catalysts (particularly transition metal silicates), including the electron transfer mechanisms, metal valence transition pathways, and the production of active species, that require immediate attention.
- (4)The production scalability of a product heavily relies on the synthesis method used. From what has been published so far, hydrothermal synthesis and sol–gel methods are the major focus of silicate preparation techniques. However, this may hinder large-scale production. Consequently, there is an urgent need to explore and enhance synthesis techniques, as well as find more effective and practical silicate composites.
Acknowledgments
This work was financially supported by the Natural Science Foundation of China, China (Grant Nos. 52172290 and 51974011).
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).