Abstract
The vasculature is an integral unit of the tissue microenvironment due to providing nutrients and oxygen to surrounding cells. Therefore, pro-angiogenic biomaterials have the potential to improve the success of a wide range of medical therapies, including tissue engineering, wound healing, and drug delivery. Herein, we decellularized bovine spinal cord meninges with Triton X-100 and digested them with pepsin to obtain a hydrogel (MeninGEL). The cryogel form of the MeninGEL was also prepared by lyophilization process (named as MeninRIX). DNA content analysis showed that the nuclear content was significantly reduced by 98.6% after decellularization process. Furthermore, the effect of decellularization on extracellular matrix components was investigated with glycosaminoglycan (GAG) and hydroxyproline (HYP) content analyses. Tensile, compression, and suture retention tests were performed to elucidate the mechanical properties. The physiological degradation behavior of the bioscaffolds was investigated by hydrolytically. Both MeninGEL and MeninRIX have good biocompatibility and pro-angiogenic properties, as proved by the Chick Chorioallantoic Membrane (CAM) assay. Moreover, SEM and histological analyses indicated cellular migration, attachment, and dynamism on the bioscaffolds' surfaces. On the basis of these data, MeninGEL and MeninRIX are pro-angiogenic structures and have adequate mechanical properties, which makes them promising candidates for soft regenerative medicine applications.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
To date, no synthetic substitute has yet been developed as effective as the original tissues due to the prodigious complexity of biological systems. Hence, the concept of 'decellularization' emerged as an ingenious idea to utilize existing and well-functioning tissue ultrastructures instead of constructing new ones from scratch. This tissue ultrastructure, called extracellular matrix (ECM), besides serving as a support material consisting of biomolecules such as collagen, laminin, fibronectin, glycoprotein, and proteoglycan, is also a dynamic 4-dimensional (4D) structure tailored by host cells [1]. Principally, the unique architecture of ECM contains crucial specific mechanical and biochemical cues (growth factors, cytokines, and adhesion molecules, etc) that regulate cell proliferation, migration, differentiation, and behavior [2]. That is, the cells and the ECM are in a 'dynamic mutuality' correlation for tissue homeostasis [3]. Furthermore, the cells are able to sense the stiffness of the environment; different stiffnesses affect cell behavior by triggering signaling pathways [4]. Therefore, the mechanical properties of the developed biomaterials are as pivotal as their biochemical ones. During the process of forming new tissues, oxygen, and nutrients must reach every area of the applied biomaterial. Unfortunately, structures without a vascular system, which is thicker than 200 μm (the diffusion limit), lead to cell death as a result of hypoxia. Thus, biomaterials that spark vascularization are a vital degree substantial in tissue engineering and regenerative medicine [5]. In addition, pro-angiogenic growth factors are usually immobilized and embedded in biomaterials to achieve that goal [4]. Indeed, decellularized ECM may automatically involve these bioactive signals and mechanical features by inheriting the properties of native tissues [6]. In light of this information, decellularized materials can be considered as an optimum platform that provides the necessary mechanical stiffness and biochemical signals to cells to repair or remodel tissues.
Almost all tissues and organs, such as the brain [7], heart [8], spinal cord [9, 10], trachea [11], and cartilage [12], have been successfully decellularized in the last few decades. The spinal meninges is a three-layered membrane (dura mater, arachnoid mater, and pia mater) ensheathing the spinal cord and defends it from extraneous factors. Identical to the brain and spinal cord, its ECM contains GAGs, collagen (I, III, IV, V, and VI), perlecan, fibronectin, laminins, Galectin, and Neurofascin. However, unlike the meninges, the decellularization process of the brain and spinal cord is problematic owing to their high fat content. The presence of residual fat is undesirable since it prevents cell attachment to biomaterials [6].
Among other biomaterials, decellularized hydrogels have some extra advantages such as 3D structure, injectability, ease of application using minimally invasive techniques to fill irregularly shaped spaces, similar bioactivity to the natural matrix, high fluid content, and tunable physical properties [13, 14]. MeninGEL is a hydrogel form produced from decellularized spinal meninges by pepsinization, while MeninRIX is a cryogel form obtained with the lyophilization of MeninGEL. Our prior study showed that MeninGEL stimulates neurogenic differentiation of hMSCs and angiogenic sprouting of HUVECs in vitro. In addition our previous study, this paper addresses the two critical biomaterials properties: pro-angiogenic and mechanical properties. For this purpose, the chorioallantoic membrane (CAM) assay, which is an effective in vivo alternative, was performed, and unlike our previous study, compression, tensile strength, and suture retention tests were conducted. CAM assay also allows monitoring biodegradability, graft-tissue compatibility, and monitoring of the angiogenic response of the material. Also, in CAM assay, animal facilities are unnecessary, and this method ensures that testing is conducted safely and ethically [15]. Moreover, biodegradation, scanning electron microscopy (SEM), histology, and biochemical analyses such as double-stranded DNA (dsDNA), glycosaminoglycan (GAG), and hydroxyproline (HYP) content were performed. Based upon the aforementioned dominances, the spinal meninges tissue may be a highly sought-after source for regenerative medicine applications.
2. Materials and methods
2.1. Materials
Bovine spinal meninges tissue was received from a local slaughterhouse in Lapseki, Çanakkale; also, chick embryos were obtained from a local farm in Çanakkale, Türkiye. Unless otherwise indicated, all chemicals and reagents were acquired from Merck (Sigma-Aldrich).
2.2. Decellularization and hydrogelation of spinal meninges
The spinal meninges was decellularized [16] and digested with pepsin [6] as previously described with some alterations. Briefly, meninges were treated with 1% Triton X-100. Subsequently, they were incubated with DNase/RNase solution to minimize nuclear residues. Then, they were decontaminated with ethanol/peracetic acid solution. After being rinsed, the decellularized spinal meninges (dSM) were homogenized and digested with 1 mg ml−1 pepsin (Sigma-77160) in 0.01 M HCI for 72 h at RT under rotation. Ultimately, MeninGEL was obtained by neutralizing the dSM pre-gel with 7.5% sodium bicarbonate. A cryogel form of MeninGEL was named as 'MeninRIX'. The whole decellularization process requires five days to be completed. MeninGEL can also be acquired within eight days. Finally, MeninRIX requires an extra day due to the lyophilization process. The overall experimental design is given in figure 1.
Figure 1. An overview of the methodology.
Download figure:
Standard image High-resolution image2.3. Biochemical analyses
dsDNA, HYP, and GAG content assays are the universal methods used to prove the success of the decellularization process. dsDNA of MeninGEL and native spinal meninges (nSM) was isolated with a DNA purification kit (Thermo Scientific, GeneJET, USA) following the kit instructions. Isolated dsDNAs were detected with the Qubit 4 Fluorometer using the Qubit 1X dsDNA HS Assay Kit (Invitrogen, ThermoFisher Scientific, USA) according to the manufacturer's specifications. GAG content was verified via the dimethylmethylene blue assay (DMMB), as formerly explained by our group [12]. Finally, HYP content was identified with the HYP calorimetric analysis kit (Sigma-MAK008) by applying the kit directives.
2.4. Mechanical tests
Tensile strength analysis was conducted to decellularized spinal meninges (wet form), and compression analysis was applied to MeninGEL and MeninRIX. Furthermore, a 5-0 surgical silk suture (Surgilactin, DW11860U, U.K.) was passed through the spinal meninges membranes for the suture retention test. Then, specimens on the counter side of the suture were clamped, and the suture was pulled until failure. A 50 N load cell was used for tensile strength and suture retention analysis, while a 10 N load cell was utilized for compression analysis. All tests were carried out on a CellScale Biomaterials Testing (Univert, Canada) at 0.05 mm s−1.
2.5. In vitro biodegradation
To simulate the biodegradation of MeninGEL and MeninRIX, two sets were designed. In the first set, the samples (n = 3) were incubated in PBS containing 0.05% sodium azide at 37 °C and 100 rpm for 15 days, and in the second set for 30 days under the same conditions. All PBS (pH 7.2–7.4) solutions were refreshed weekly. The masses of the samples were recorded before (m0) and after (mt) the experiment, and the biodegradation percentages were calculated with the following formula:

2.6. The chick embryo chorioallantoic membrane (CAM) assay
The CAM assay was accomplished following our group's previous research methodology with slight modifications [17]. Initially, fertilized chicken eggs in embryonic development day 0 (EDD0) were first incubated vertically for 72 h at 37.5 °C and 60% relative humidity. The incubator (Brinsea Ovation 28 Advance Ex, U.K.) is programmed to rotate the eggs 45 degrees every 120 min. After that, each egg's shell was gently cut out close to the top of the chalaza using a mini drill (Snaiker, Germany), and eggs were incubated for a further 7 days under the same conditions without rotation. Then, MeninGEL and MeninRIX were grafted on the upper of CAM and between the central allantoic veins. Images of samples were captured after grafting (EDD10) and before sacrificing (EDD14) via a Stemi 305 stereo microscope integrated with Axiocam 105 color camera (Zeiss, Germany). Ultimately, specimens were harvested for SEM and histology analyses, and embryos were sacrificed. To quantitate the vascular density, captured images were processed using ImageJ software, version 1.53t (National Institutes of Health, Bethesda, Maryland, USA). The measure of vascular density refers to the proportion of space taken up by blood vessels. Vascular density was computed over two distinct areas: the foveal and parafoveal regions. The foveal zone was specified as a central ring in diameter of the specimen implanted in EDD10, and the parafoveal zone as a 1 mm broad circle coating the foveal zone. To ensure correct and precise comparison, the diameter of the foveal region in EDD14 was determined as the diameter of the specimen implanted in EDD10. The vascular index was determined according to the equation presented below:

2.7. Scanning electron microscopy
The surface morphologies of MeninGEL and MeninRIX, as well as the attachment, migration, and proliferation of CAM cells on the MeninGEL and MeninRIX, were observed by SEM (FE-SEM JFM 7100 F EDS, JEOL, Japan). As preparation for SEM, the samples were treated with a 2.5% glutaraldehyde prepared in PBS solution for at least 24 h. Subsequent to this process, the samples were dried at room temperature after passing through a sequence of ethanol solutions with varying concentrations (50%, 70%, 80%, 90%, 95%, and 100%, respectively) for 15 min each. Before microscopy, samples were coated with Au (80%)—Pd (20%) for 90 s using a Mini Sputter Coater (SC7620, U.K.). Once the process was completed, SEM micrographs were captured under a high vacuum at 10 kV with different magnifications.
2.8. Hematoxylin and eosin staining
The specimens were first immersed in a 10% neutral buffered formalin solution for 48 h. Afterward, they were embedded in paraffin and then sliced to 3–5 μm thickness. The customary hematoxylin and eosin (H&E) staining procedure was carried out as described in our previous study [17]. The images were taken through a light microscope equipped with an Axiocam 105 color camera (Zeiss, Germany) for histological assessment.
2.9. Statistical analyses
Experimental outputs were processed using Microsoft Office 365 Pro Plus Excel, and the obtained data were represented as mean values ± the standard deviation. Statistical significance was achieved between the two related groups via the Tukey test one-way analysis of variance (ANOVA) utilizing Origin 2023 (Origin Lab Corporation, MA, USA). A p-value less than 0.05 was attributed statistical significance.
3. Results and discussion
3.1. Biochemical analyses
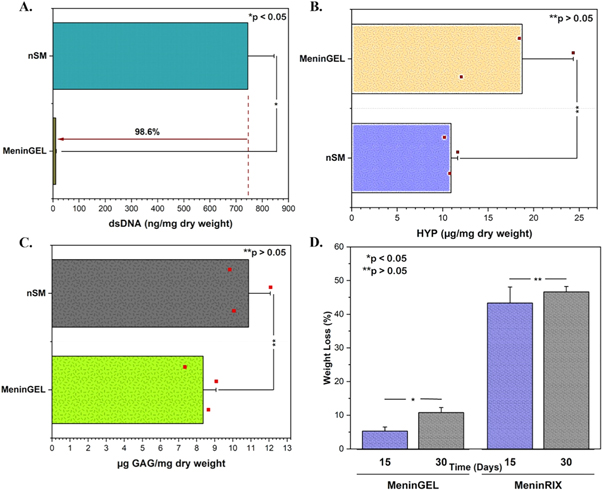
Assessing the quality of decellularized tissue requires dsDNA, HYP, and GAGs content assays to ensure the tissue is free of cells and the ECM is preserved for safe and effective use in regenerative medicine. While reducing the dsDNA content to below 50 ng mg−1 dry weight, maintaining the integrity of the ECM is essential to acquire material with the primary tissue characteristics and avoid any potential immune responses [18]. nSM and dSM contained 744.87 ± 99.77 ng mg−1 and 10.39 ± 2.05 ng mg−1 of dsDNA, respectively. According to the findings, the quantity of dsDNA was reduced by 98.6% following the decellularization procedure (n = 3; p < 0.05; ANOVA; figure 2(A)). The ECM is sophisticatedly woven by cells and comprises many tissue-specific structural and functional proteins, glycoproteins, GAGs, and growth factors [19]. It plays a crucial role in maintaining the physical integrity of multicellular organisms and supplies essential biochemical and biophysical signals for cell survival, organization, and differentiation [20]. Therefore, keeping the dynamic structure and activity of the ECM post-decellularization is imperative to fabricate a bioactive biomaterial. The amounts of hydroxyproline and collagen directly correlate, as hydroxyproline constitutes approximately 14% of collagen, the most copious ECM protein [21, 22]. During decellularization, the ECM may damage due to detergents, enzymes, and physical forces. Hence, GAG and HYP content analyses were handled to nSM and MeninGEL for detecting ECM destruction. HYP amounts of native tissue and MeninGEL was measured as 10.91 ± 0.72 μg mg−1 and 18.74 ± 5.62 μg mg−1 (n = 3; p > 0.05; ANOVA; figure 2(B)), and GAG amounts were 10.87 ± 1.20 μg mg−1 and 8.36 ± 0.71 μg mg−1 (n = 3; p > 0.05; ANOVA; figure 2(C)), respectively. As shown in figures 2(B)–(C), there was no significant difference in GAG and HYP contents between nSM and MeninGEL. Thus, it has been proven that we reduce the DNA content to the safe zone (<50 ng mg−1 dry weight) for a controlled immune response without significantly damaging the matrix. Finally, we revised the tissue digestion step in the gelation process, increasing the yield to approximately 99% (data not shown).
Figure 2. Biochemical characterizations. (A) dsDNA content. (B) Hydroxyproline content. (C) Glycosaminoglycan content. (D) Biodegradation test.
Download figure:
Standard image High-resolution image3.2. In vitro hydrolytic degradation
The degradation rate could affect cell integration and neo-tissue genesis in the lesion cavity. Therefore, comprehending and regulating biomaterials' degradation rate is critical to ensuring their biocompatibility, functionality, and long-term performance in biomedical applications [23]. Biodegradation also avoids surgical removal owing to degradation and resorption within the host body [24]. The weight losses of MeninGEL and MeninRIX were calculated as 5.31 ± 1.18% and 43.34 ± 4.75% on day 15, respectively. Besides, MeninGEL and MeninRIX lost 10.81 ± 1.48% and 46.63 ± 1.61% of their weights at the end of 30 days, respectively. A significant difference in weight loss of MeninGELs was observed between on days 15 and 30 (n = 3; p < 0.05; ANOVA; figure 2(D)). In contrast, there was an insignificant difference in MeninRIXs' between the two sets (n = 3; p > 0.05; ANOVA; figure 2(D)). The lyophilization notably amplified the degradation. As a matter of fact, lyophilized collagen scaffolds can hydrolytically degrade (without enzyme) by about 75% within 28 days in PBS [25].
3.3. Mechanical properties
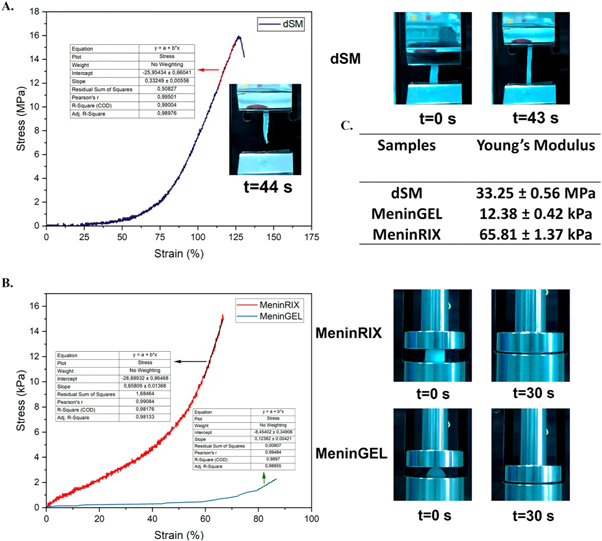
Human tissues are subjected to different forces, such as tension, compression, fluid shear, and stretching. Each tissue must overcome distinct forces and mechanical limitations to sustain its own 'mechanical homeostasis' [26]. Severe soft tissue defects often require the use of prosthetic materials. For such applications, the mechanical properties of an ideal substitute were obliged similar to the original tissue [27]. Thus, the tissue function might continue properly without any hindrances. Extensive mechanical tests such as tensile strength, compression, and suture retention were conducted to figure out mechanical properties. The maximum tensile strength of the dSM membranes was 19.71 ± 3.24 MPa (n = 3, figure 3(A)), and the elastic modulus was found to be 33.25 ± 0.56 MPa (n = 3, figure 3(C)). Consistent with our data, a study published by Kızmazoğlu et al in 2019 showed that the dura mater, one of the three layers of the spinal meninges, had a tensile strength of 7.01 ± 0.77 MPa and an elastic modulus of 60.18 ± 10.77 MPa. In addition, the tensile strength of the commercial Durepair® (Medtronic Inc.) biomaterial, which is a collagen matrix used for the repair of the meninges during neurosurgical procedures, was determined as 19.59 ± 0.65 MPa, and the elastic modulus as 54.16 ± 4.82 MPa [28]. The more elastic spinal meninges membranes may be a result of the decellularization process's treatments (mechanical, detergent, enzyme, etc) weakening a bit their compact structure.
Figure 3. Mechanical properties of decellularized spinal meninges membranes, MeninGEL and MeninRIX. (A) Tensile strength of decellularized spinal meninges membranes. (B) Compression strength of MeninGEL and MeninRIX. (C) Young's moduli of all.
Download figure:
Standard image High-resolution imageAnother critical factor to consider is the suture retention capacity of biomaterials employed in anastomosis. Suture retention refers to the capability of a suture to uphold its strength until the tissue is capable of supporting itself [29] The maximum suture retention strength of dSM membranes (9.66 ± 0.76 N, n = 3, lyophilized form) complies with Dura-Guard (10.02 N) and Durepair (12.38 N) [30].
Understanding the compressive strength of biomaterials is absolutely critical in assessing their functionality within the body. Meninges is a central nervous system tissue like the brain and spinal cord, and we mentioned above that its biochemical content is similar to that of the brain and spinal cord. That is, the meninges can be used in brain and spinal cord injuries so that their properties can be compared with those of the brain and spinal cord. According to this, the compressive strength of an adult spinal cord is 8 kPa, and Young's modulus changes between 0.3–1.4 MPa. Conversely, brain tissue has a compressive strength between 0.47 and 1.6 kPa and Young's modulus in the range of 40 Pa-20 kPa and displays nonlinear viscoelastic behavior [31]. MeninGEL and MeninRIX exhibited maximum compressive strengths of 2.57 ± 0.01 kPa and 15.65 ± 0.3 kPa, respectively (n = 3, figure 3(B)). Their Young's moduli were also determined to be 12.38 ± 0.42 kPa and 65.81 ± 1.37 kPa, respectively (n = 3, figure 3(C)). The mechanical properties of meninRIX and the spinal cord are closely matched, whereas those of MeninGEL overlap with the brain. MeninGEL and MeninRIX exhibit satisfactory mechanical properties and adequate biodegradation rates for advanced research. Furthermore, by utilizing crosslinking techniques, it is possible to enhance the mechanical properties and adjust the biodegradation rate.
3.4. Angiogenic assessments
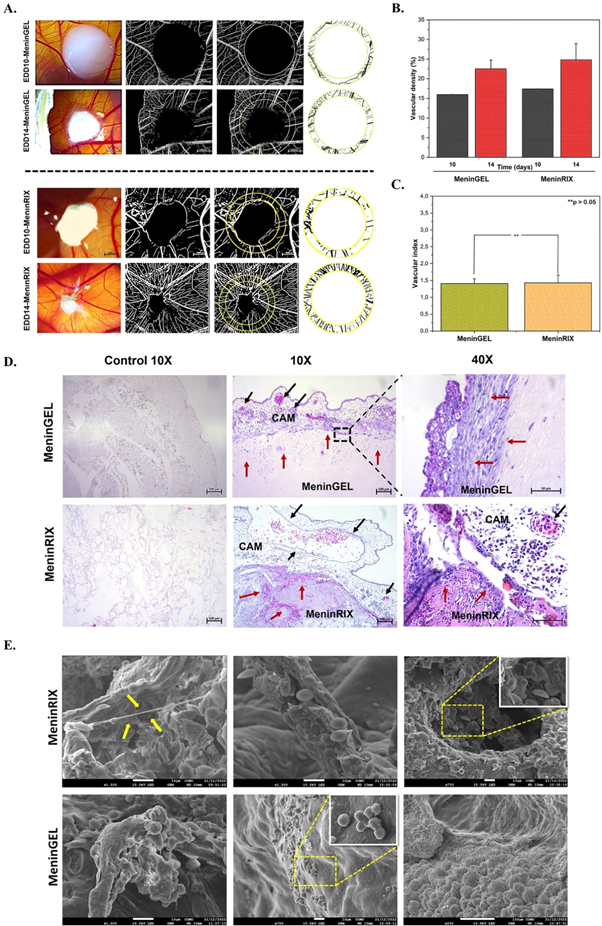
The role of vasculature goes beyond just supplying nutrients and oxygen to neighboring cells. It also delivers key signals that can greatly affect biological outcomes, such as regeneration or disease. The signals are derived from the cells that make up the blood vessels, along with the surrounding ECM's changing physical and chemical properties during vascular growth and modification [32]. In this regard, the pro-angiogenic property of biomaterials is of prime importance as it induces the formation of new blood vessels. The angiogenic potential of MeninGEL and MeninRIX was evaluated by CAM assay (figures 4(A)–(C)). The macroscopic assessment demonstrated the growth of vessels towards the scaffolds. Furthermore, at the end of the experiment, it was found that the scaffolds were entirely surrounded by the chorioallantoic membrane. Vascular densities for MeninRIX on EDD10 and EDD14 were 17.38 ± 0.04% and 24.82 ± 4.12% (n = 3, figure 4(B)), respectively; For MeninGEL, they were found to be 15.97 ± 0.03% and 22.52 ± 2.24% (n = 3, figure 4(B)) on EDD10 and EDD14, respectively. With these data, the vascular indexes of MeninRIX and MeninGEL were calculated as 1.43 ± 0.23 and 1.41 ± 0.14 (figure 4(C)), respectively. According to statistical analysis, there were no significant differences between the vascular index values of MeninRIX and MeninGEL. This in vivo analysis showed the scaffold-host tissue integration and genesis of new blood vessels during recellularization. Thereby, the biocompatibility and pro-angiogenic properties of MeninGEL and MeninRIX were approved.
Figure 4. Biological characterizations of MeninGEL and MeninRIX. (A) In ovo CAM assay. Stereo microscop and image j illustrations. Scale bars represent 2000 μm. (B) Vascular density (%) graph. (C) Vascular index graph. (D) H&E staining. Control images shows the elmination of cellular and/or nuclear residue. Red arrows indicate cell migration, and black arrows indicate blood vessels. Scale bars represent 50 μm (40X) and 100 μm (10X). (E) Scanning electron micrographs at varios magnifications (X250, X750 and X1500). Yellow arrows indicate cell connections. Inset micrographs (X1500) indicate cell clusters.
Download figure:
Standard image High-resolution image3.5. Visual evaluations: SEM and histology
SEM and H&E are valuable tools for characterizing the surface morphology of decellularized biomaterials. The histological analysis clearly indicated that any nucleic components and ECM damage were not detected in MeninGEL and MeninRIX after decellularization. H&E analysis also evidently displayed the porous structure of MeninGEL and MeninRIX. Cellular migration into scaffolds and the existence of blood vessels near the implanted scaffolds were shown in figure 4(D). Further, there were no indications of any adverse reactions whatsoever. H&E staining also revealed the vessel formation in MeninRIX and MeninGEL. As made evident by H&E and SEM analyses, cellular migration, attachment, and activation were observed on the microfibrillar surface of MeninGEL and MeninRIX (figure 4(E)). Thus, , these analyses validate our CAM assay, dsDNA, HYP, and GAGs content findings.
4. Conclusion
The meninges is a protective barrier that shields the spinal cord against any possible dangers from external sources. Furthermore, meninges support neural homeostasis in adults by releasing several trophic factors. As envisaged, decellularized biomaterials inherit key biochemical signatures of the native tissue. Undeniable, the vasculature is indispensable as it provides the nutrient, oxygen, and waste cycles of the cells. Hence, decellularized pro-angiogenic biomaterials are promising biomaterials with the potential to improve the efficacy of tissue engineering and regenerative medicine. In this study, we illuminated the mechanical properties of decellularized bovine spinal meninges, MeninGEL, and MeninRIX, which are compatible with native spinal meninges, brain, and spinal cord, respectively. Additionally, we verified their in vivo biocompatibility and pro-angiogenic properties by CAM analysis. Detailed visual analyses demonstrated the cellular migration, attachment, and activation on the microfibrillar surface of MeninGEL and MeninRIX. In conclusion, construing the promising features of MeninGEL and MeninRIX, we envision that they could be advisable resources for regenerative medicine applications.
Acknowledgments
We would like to thank the Çanakkale Onsekiz Mart University Science and Technology Application and Research Center (ÇOBILTUM) staff for their technical assistance. This work was supported by Çanakkale Onsekiz Mart University Scientific Research Projects Coordination Unit (Project ID: FLÖAP-2022-3957). We would also like to thank Mr.Yücel Okatali (MER-TER Medikal) for his assistance in histology studies.
Data availability statement
The data cannot be made publicly available upon publication because no suitable repository exists for hosting data in this field of study. The data that support the findings of this study are available upon reasonable request from the authors.
Author contributions
Aybuke Samancioglu: methodology, formal analysis, data process. Beyza Aydın: methodology, formal analysis, data process. Eren Ozudogru: methodology, material design, formal analysis, data process, writing-original draft preparation. Yavuz Emre Arslan: conceptualization, project administration, methodology, material design, supervision, writing-review, and editing.
Conflict of interest
The sections on the decellularization of spinal cord meninges and the fabrication of MeninGEL and MeninRIX are related to a patent application (app. no: 2021/013582, international app. no: PCT/TR2022/050899, Çanakkale Onsekiz Mart University), which is corresponded by Assoc. Prof. Yavuz Emre Arslan, and Eren Özüdoğru (PhD. candidate). Samancıoğlu A. and Aydın B. contributed equally to this work.
Ethics statement
Ethics committee approval is not required for this study.