Abstract
Poly(ethylene furanoate) (PEF) biocomposite films incorporating zinc oxide nanoparticle (ZnO NPs) were prepared using a solvent casting method. The ZnO NPs were homogeneously dispersed within the PEF films with the aid of γ−aminopropyltriethoxylsilane (APTES). The water vapor barrier, optical transmittance and antimicrobial properties of the PEF/ZnO films were tested. Water vapor permeability (WVP) and transmittance in the visible (400–800 nm) region of control PEF film were 6.92 × 10–12 g·m m−2 · s·Pa and 87.3%, respectively. WVP value of PEF films decreased 43.2% through ZnO NPs compounding. On the contrary, transmittance of PEF films decreased 6.8% due to the absorption and scattering of ZnO NPs. In addition, the PEF film with modified ZnO NPs exhibited a bacteriostatic rate up to 97.0% after 3 h. Thus, the PEF/ZnO films show great potential in the field of food packaging.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Over the past few decades, many petroleum based polymers, such as polyethylene (PE), polypropylene (PP) and polyethylene terephthalate (PET) have been extensively studied as packaging materials due to the cost-effective, outstanding processing performance and acceptable water vapor barrier properties [1–3]. Nevertheless, the extensive use of petroleum-based polymers might result in accelerated depletion of oil resources and increased carbon dioxide emissions. Thus, bio-based polymers have been proposed as the alternative of fossil-based products [4–6]. Among the novel bio-based polyesters, polyethylene furanoate (PEF) shows a potential to replace the conventional PET on account of its better mechanical and barrier properties [7–9].
At present, studies related to PEF mainly focus on the following aspects, including the synthesis of copolymers and the industrial production of PEF films [10, 11]. Despite the mechanical and barrier properties of PEF film has been extensively reported, their antibacterial property of the film was never investigated before and is of great interest as a packaging alternative material to PET [12, 13].
Zinc oxide nanoparticles (ZnO NPs) have aroused great research interest as an important inorganic antimicrobial agent due to their non-toxic, eco-friendly, biocompatibility and availability of surface modification [14–16]. They have significant antimicrobial effects against both Gram-positive and Gram-negative bacteria, even at a low concentration [17–19]. Moreover, several researches have indicated that ZnO NPs can greatly improve the mechanical properties of the polymer matrices when they are used as fillers [20–22]. Ana M. D´ıez-Pascual et al [23] investigated the mechanical and antibacterial properties of poly(ether ether ketone)/ZnO (PEEK/ZnO) nanocomposites with different contents of modified ZnO additive. The tensile strength and antibacterial activity were enhanced with increasing ZnO content until a concentration of 5.0 wt%. Roberto Pantani et al [24] reported poly(lactic acid) (PLA)/ZnO nanocomposite films with 3% ZnO rod-like nanoparticles. The well dispersed ZnO nanofillers were acquired by silanization with triethoxy caprylylsilane, which were effective in increasing the water vapor barrier and antibacterial activity of PLA nanocomposite films.
In this work, ZnO NPs have been incorporated in the bio-based PEF matrix via a solution casting technique with the aid of γ−aminopropyltriethoxylsilane (APTES). Moreover, we investigated the influence of ZnO NPs on the transmittance, water vapor barrier and antibacterial properties of bio-based PEF nanocomposite films, which show great potential for food packaging applications.
2. Experimental
2.1. Materials
PEF amorphous granules with a shape of long strip were made in Ningbo Institute of Materials Technology and Engineering of China. ZnO NPs with ∼50 nm particle size was supplied by Ningbo Sunlit Electronic Material Co., Ltd. (China). APTES and Ethanol (EtOH) were supplied by Hefei Kinu Biotechnology Co., LTD. (China). Phenol (C6H5OH) and tetrachloroethane (C2H2Cl4) were purchased from Anhui Kuer Bioengineering Co., LTD. (China).
2.2. Surface modification of ZnO NPs
The preparation principle of surface-modified ZnO (m−ZnO) NPs is the reaction between various groups of APTES and hydroxyl groups on the surface of ZnO. Typically, a 5 ml APTES solution was mixed with a 5 ml EtOH solution and stirred for 1 h at room temperature. Then, 0.5 g ZnO NPs were injected into the above mixture. Afterwards, the mixed solution was sonicated for 0.5 h and refluxed at 80 °C for 2h. Finally, the obtained m−ZnO NPs were washed with EtOH solution to remove excess impurities and dried in a draught drying cabinet at 100 °C.
2.3. Fabrication of PEF/ZnO composite thin films
The pure PEF solution was prepared using C6H5OH and C2H2Cl4 as solvents. Typically, 1g PEF granules, 5 ml C6H5OH and 5 ml C2H2Cl4 were injected into a 25 ml flask and stirred at 90 °C for 4 h. Then, a certain quantity of ZnO NPs and m−ZnO NPs were dissolved in the as-prepared PEF solution. The mass ratio of ZnO NPs to PEF solution is about 5%. Subsequently, the PEF/ZnO solutions were stirred at 45 °C for 2 h. Finally, an 8 ml PEF/ZnO solution was dripped onto a clean slide surface and transferred to an oven at 90 °C for 3 h to evaporate the solvent. The PEF/ZnO film can be removed from the surface of the slide by immersing it in deionized water for 1 h. The thickness of PEF/ZnO films was measured using a polymer film thickness meter (FPTHE-FR-Thermal, China). The thicknesses of the PEF/ZnO films were 86 μm ± 8 μm.
2.4. Characterization and measurement
Surface modification of ZnO NPs was analyzed and characterized by a Fourier Transform Infrared Spectrophotometer (FTIR) (Nicolet 6700, Thermo, USA). The scanning range and resolution were 4000–450 cm−1and 1 cm−1, respectively. The morphologies of PEF/ZnO films were measured using a scanning electron microscope (SEM) (Regulus 8230, Hitachi, Japan) at an accelerating voltage of 15 kV. The surface wettability of PEF/ZnO films was obtained by using an optical contact Angle measuring instrument (SDC-200S, SINDIN, China). The transmittance of those films was characterized with an ultraviolet and visible spectrophotometer (Gold S54T, Lengguang, China). The test band ranges from 300 nm to 1100 nm.
Water vapor permeability (WVP) of PEF/ZnO films was measured by a water vapor transmittance test system (W3/060, Labthink, China), according to the ISO 2528 international standard [25]. The temperature and humidity inside the test instrument were set to 38 °C and 89% RH, respectively. The test area of the sample was set as 33 cm2. The weight of the remaining water in the cup was tested every three hours for up to 18 h. Finally, WVP of those films was calculated by instrument built-in software based on weight loss versus time curves.
The antimicrobial activity of PEF/ZnO films was tested against Escherichia Coli (E. coli) ATCC 29522 by the film attachment method. The standard number of the test is ISO 22196:2007 [26]. The pure PEF film was tested as control sample. Specifically, place the test sample (50 mm × 50 mm) in a Petri dish and inoculate the upper surface with 200 μl of bacterial suspension (105 ∼ 106 CFU·ml−1). Polyethylene film (40 mm × 40 mm) was used as a covering film. Subsequently, samples were held at 37 °C for 3 h. Afterwards, test samples were washed with 20 ml saline containing 0.2 vol% tween 80 and then viable bacterial were enumerated by performing 10-fold serial dilutions of saline. Place 1 ml of each dilution into separate sterile Petri dishes and pour 15–20 ml nutrient agar (46 ∼ 48 °C) into each dish. Once the agar has solidified, the Petri dishes were inverted and incubated at 37 °C for 18 h. Finally, count the number of colonies in the dish containing 30 to 300 colonies. Bacteriostatic rates (%) were obtained by the following formula:
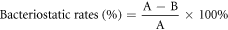
Where A and B are colony counts (CFU·ml−1) of the control and sample groups, respectively.
3. Results and discussion
Figure 1 shows the preparation process of PEF/ZnO composite membrane, including the preparation of PEF solution, modification of ZnO NPs, mixing of PEF solution and film formation of PEF solution. In general, the performance improvement of nanocomposites depends on the interaction between the filler and the polymer interface, and the uniform dispersion of the filler in the matrix. However, the large surface energy of ZnO NPs usually leads to aggregation, which affects their uniform distribution in the polymer medium. Moreover, the interaction between the unmodified ZnO NPs and the PEF matrix is weak due to the large surface energy difference. Therefore, it is necessary to surface modify ZnO NPs before they are blended with PEF solution.
Figure 1. The preparation flow chart of Poly(ethylene furanoate) (PEF) film, Poly(ethylene furanoate)/ZnO (PEF/ZnO) composite film and Poly(ethylene furanoate)/ surface-modified ZnO (PEF/m−ZnO) composite film.
Download figure:
Standard image High-resolution imageIn this study, ZnO NPs were treated by APTES solution, which is a common inorganic nanoparticle surface modifier. The principle of modification is usually substitution reaction between hydrogen bonds on the surface of ZnO NPs and functional bonds on the surface of APTES. Figure 2 shows the FTIR spectra of ZnO NPs, APTES solution and m−ZnO NPs to prove the successful modification of ZnO NPs. A broad peak at 3438 cm−1 was observed in the spectrum of ZnO NPs, which assigned to the stretching of hydrogen-bonded –OH groups on ZnO NPs surface [27]. A sharp peak at 956 cm−1 was observed in the spectrum of APTES solution, which assigned to the Si-O groups. Furthermore, the other two peaks at 2974 cm−1 and 2885 cm−1 represent symmetrical and symmetrical stretching vibrations of C–H in CH3 and CH2 groups, respectively [28]. In the spectrum of m−ZnO NPs, some new peaks were observed as compared to that of ZnO NPs. The peak at 872 cm−1 indicates the existence of Zn–O–Si [23]. The peak at 1456 cm−1 was assigned to CH3 asymmetric bending. Moreover, the peak at 3342 cm−1 was assigned to the asymmetric stretching mode of NH2. Another new peak at 3504 cm−1 was related to the presence of Si-OH. According to the above peaks, we can basically infer that the functional groups of APTES are associated with the surface groups of ZnO NPs, which confirms the success of the modification.
Figure 2. FT-IR spectra of ZnO nanoparticle (ZnO NPs), surface-modified ZnO nanoparticle (m−ZnO NPs) and APTES.
Download figure:
Standard image High-resolution imageIn order to observe the distribution of ZnO NPs in the PEF matrix, SEM surface images of pure PEF film and PEF composite films are displayed in figure 3. In the figure 3(a), the surface of pure PEF film shows the texture structure of different sizes. Similar phenomena can be referred to the literature [29]. In the figures 3(b) and 3c, some quasi-spherical shape white dots are randomly placed on the surface of the PEF film, which correspond to the ZnO NPs and m−ZnO NPs. There is no obvious agglomeration of randomly distributed ZnO NPs in the PEF films due to a low ZnO content. In addition, m−ZnO NPs in the PEF film revealed better homogeneity as compared to that of ZnO NPs. It is mainly attributed to the organic groups of APTES that change the surface characteristics of ZnO NPs, which improved the compatibility between nanoparticles and polymer substrates [27].
Figure 3. Typical SEM images of Poly(ethylene furanoate) film (a), Poly(ethylene furanoate)/ZnO composite film (b) and Poly(ethylene furanoate)/ surface-modified ZnO composite film (c).
Download figure:
Standard image High-resolution imageFigure 4 shows the wetting angles of the PEF films and the glass slide were measured at room temperature. In general, microbial adhesion strongly depends on the hydrophilic and hydrophobic properties of the interacting surfaces. In contrast to hydrophobic surfaces, hydrophilic surfaces have been shown to improve cell adhesion, growth, and differentiation [30]. In this study, all PEF films showed better hydrophobic effect compared to slides. The wetting angle of the pure PEF film abruptly increased due to the surface chemical characteristics, as compared with that of the glass slide. In contrast, the PEF/ZnO film exhibits a reduced water contact angle, which was ascribed to the increased surface roughness and hydrophilic hydroxyl group. The further decrease was observed for the PEF/m−ZnO film, which may be due to the residual -NH2 in m−ZnO.
Figure 4. Contact Angle bar graph of Glass slide, Poly(ethylene furanoate) (PEF) film, Poly(ethylene furanoate)/ZnO (PEF/ZnO) composite film and Poly(ethylene furanoate)/ surface-modified ZnO (PEF/m−ZnO) composite film.
Download figure:
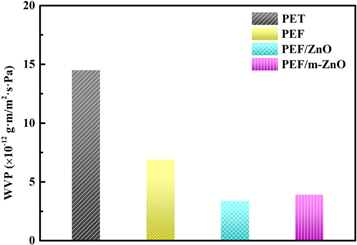
Standard image High-resolution imageAs a potential packaging film, it is necessary to evaluate its water vapor barrier and visible light transmittance. The corresponding results of the WVP tests with films of PET and PEF/ZnO are presented in figure 5. Commercially purchased PET film (∼90 μm) was used as a control in this test. Compared with PET films (WVP : 1.45 × 10–11 g·m m−2 · s·Pa), all PEF films have lower WVP values, which indicates that PEF films have better water vapor barrier properties. Moreover, the WVP values of PEF/ZnO composite films are much lower than that of the pure PEF film (6.92 × 10–12 g·m m−2 · s·Pa). The main reason is that the impermeable ZnO NPs forms a tortuous diffusion path in the PEF matrix, thus increasing the length of the effective diffusion path [31]. Unexpectedly, the WVP value of the PEF/m−ZnO composite film (3.93 × 10–12 g·m m−2 · s·Pa) is slightly higher than that of the PEF/ZnO film (3.41 × 10–12 g·m m−2 · s·Pa). This phenomenon may be related to the increased hydrophilicity on the surface of PEF/m−ZnO composite film (figure 4).
Figure 5. Water vapour permeability of Polyethylene terephthalate (PET), Poly(ethylene furanoate) (PEF) film, Poly(ethylene furanoate)/ZnO (PEF/ZnO) composite film and Poly(ethylene furanoate)/surface-modified ZnO (PEF/m−ZnO) composite film.
Download figure:
Standard image High-resolution imageFigure 6 shows the average transmittance in the visible spectral region (400–800 nm) of prepared PEF composite films with a size of 2 × 5 cm2. In the inset, all PEF films exhibit acceptable visible light transmittance. Among these films, pure PEF films exhibit the highest visible light transmittance of up to 87.3%.With the addition of ZnO NPs, the transmittance of PEF films decreased significantly, especially the unmodified ZnO NPs resulted in a decrease of 11.5%. This drastic reduction is mainly attributed to the absorption and scattering of visible light by ZnO NPs [32]. Interestingly, the transmittance of PEF/m−ZnO film was 80.5%, which was only 6.8% lower than that of the pure PEF film. This may be related to the better dispersion of the m−ZnO NPs in the PEF film with the aid of APTES.
Figure 6. Photograph and transmittance of Poly(ethylene furanoate) (PEF) film, Poly(ethylene furanoate)/ZnO (PEF/ZnO) composite film and Poly(ethylene furanoate)/surface-modified ZnO (PEF/m−ZnO) composite film.
Download figure:
Standard image High-resolution imageCompared with conventional packaging materials, antibacterial packaging materials can effectively improve food shelf life and safety. To determine the antimicrobial property of PEF/ZnO composite films against E. coli, the pure PEF film was chosen as a references group. Figure 7(a) shows the antimicrobial results of PEF/ZnO composite films. The bacteriostatic rates of PEF/ZnO film and PEF/m−ZnO film reached 93.4% and 97%, respectively, which demonstrated excellent antimicrobial properties. In addition, PEF/m-ZnO composite films exhibit a lower survival ratio than that of PEF/ZnO films, which can be attributed to the improved ZnO NPs dispersion and organic groups on the surface of m−ZnO NPs [33]. Figure 7(b) shows the photographs of the Petri dishes with bacterial solution of different dilutions. When the dilution of bacterial solution reached 105, there were almost no macroscopic colonies in the Petri dishes of PEF/ZnO and PEF/m−ZnO samples. On the contrary, there were hundreds of colonies in the Petri dishes of PEF samples. Therefore, ZnO NPs give PEF film an obvious antibacterial effect.
Figure 7. (a) Antimicrobial activity of Poly(ethylene furanoate) (PEF) film, Poly(ethylene furanoate)/ZnO (PEF/ZnO) composite film and Poly(ethylene furanoate)/surface-modified ZnO (PEF/m−ZnO) composite film, and (b) actual images of the incubated E. coli on agar plates with PEF and PEF composite films.
Download figure:
Standard image High-resolution imageTable 1 lists the antibacterial activities of several typical polymer/ZnO composite coatings. From the table, it can be found that all polymer/ZnO composite films have achieved high antibacterial effect even when the concentration of ZnO is lower than 10 wt%. In addition, by comparing the relevant data in the table, it is not difficult to find that the factors affecting the antibacterial effect of polymer/ZnO composite film include the following aspects: the concentration of ZnO NPs, the diameter of ZnO NPs and the type of polymer. For example, the polymer/ZnO composite coatings in references [27, 36] and in this work have an antibacterial rate of ∼97% against Escherichia coli. Among them, the concentration of ZnO NPs in the polymer film in reference [36] is only 2 wt%. However, the antibacterial rate against Escherichia coli was as high as 97.7%, which was mainly related to the interaction between the polymer substrate and ZnO NPs.
Table 1. The antibacterial activities of ZnO nanoparticles (ZnO NPs) with synthetic polymers.
| Materials | Organisms | ZnO NPs content (wt%) | Diameter of ZnO NPs (nm) | Bacteriostatic rates (%) | References |
|---|---|---|---|---|---|
| Poly(3-hydroxybutyrate)/ZnO | E. coli | 10 | <100 | 97.0% | [27] |
| Poly(phenylene sulfide)/ZnO | E. coli | 8 | ∼75 | 86.0% | [34] |
| Poly( N –isopropylacrylamide/ZnO | E. coli | 10 | ∼20 | 100% | [35] |
| High-Density Polyethylene/ZnO | E. coli | 2 | ∼50 | 97.7% | [36] |
| Poly(ethylene furanoate)/ZnO | E. coli | 5 | ∼50 | 97.0% | This work |
At present, there are three main explanations for the antibacterial mechanism of ZnO NPs including Zn ion (Zn2+) release, mechanical damage and reactive oxygen ions (ROS) [37, 38]. In this study, we inferred that the release of Zn2+ and mechanical damage may not be the main factors leading to bacteriostasis. First, the presence of APTES may restrain Zn2+ release and thus reduce the antibacterial effect. In addition, the PFE/ZnO composite films contain a close concentration of ZnO NPs, and the modified ZnO NPs possess larger diameters. Thus, the production of ROS can be considered as a major factor in the antimicrobial activity of the PEF/ZnO composite films.
4. Conclusions
In summary, PEF/ZnO transparent thin films were successfully prepared using a solution casting method with the addition of APTES. FT-IR spectra confirmed the surface modification of ZnO NPs by amino groups and Si–O bonds. SEM images revealed that m−ZnO NPs were uniformly dispersed in the PEF films. The PEF/m−ZnO films exhibited a WVP value of 3.93 × 10–12 g·m m−2 · s·Pa and a visible light transmittance of 80.5%. Furthermore, the PEF/m−ZnO films possess a bacteriostatic rate up to 97.0% after 3 h. Therefore, PEF/m−ZnO films have great potential in the application of food and beverage packaging.
Acknowledgments
This work was supported by the Zhejiang Basic Public Welfare Research Project (Nos. LGF19E020001 and LGF20E050002) and Science and Technology Planning Project of Zhejiang Market Supervision Administration (No. ZC2021A048).
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.