Abstract
The derived materials obtained from the sol-gel process have been used in various technological applications, such as solar cells, intelligent coatings, catalysis, and, more recently, the fabrication of bioreceptors. The objective of this study was to develop a bioreceptor consisting of a titania-based nanostructure, which was synthesized using the sol-gel method. This nanostructure was immersed in a solution containing laccase and Nafion and integrated into a graphite-based electrode (TiO2/NAF/LAC). This device is called a bioreceptor and is used to detect gallic acid. The nanostructure was characterized by x-ray diffraction, Raman spectroscopy, and scanning electron microscopy (SEM). Particle size was measured using a nanosizer. Cyclic voltammetry (CV) tests were performed on a bioreceptor. In this study, the predominant phase of TiO2 was anatase, and the obtained nanoparticles had an average size of 66 nm. The CV tests of the bioreceptor showed an oxidation response that increased as the concentration of gallic acid in the solution increased, with a detection limit of 0.125 μM, as well as a wide linear range that varied from 0.125 to 175 μM and a factor correlation of 0.9968. As a result, it was possible to develop a bioreceptor capable of immobilizing laccase to detect gallic acid.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Sol-gel technology is used to produce a colloidal suspension of TiO2 by the hydrolysis of titanium alkoxides and is used in various applications [1]. It offers a simple and versatile means of combining the favorable properties of organic–inorganic materials with those of metal nanoparticles. This new combined functionalized sol-gel material is gaining importance in electro-analytical chemistry, particularly in the field of chemical sensors and biosensors [2]. The high compatibility of inorganic supports with immobilized species, such as enzymes, has increased the interest in the use of sol-gel technology for various applications. Sol-gel electrode-based sensors and biosensors have numerous attractive properties, such as simplicity of preparation, low-temperature processing, tunable porosity, chemical inertness, negligible swelling, mechanical stability, and high sensitivity [3, 4]. Additionally, enzyme immobilization in biosensors is an efficient strategy for enhancing catalytic performance in continuous industrial practices [5]. Kim et al [6] reported the use of a nanoporous sol-gel structure derived from a zirconia/Nafion composite film as the encapsulation matrix of the enzyme glucose oxidase (GOx) on a platinized glassy carbon electrode for the development of an amperometric glucose biosensor. Wang et al [7] immobilized horseradish peroxidase in a sol-gel film of silica on a glassy carbon electrode modified with carbon nanotubes. Singh et al [8] developed a combined method by applying sol-gel and gold nanoparticles stabilized with polyvinyl pyrrolidone (PVP) to immobilize the matrix in the tyrosinase enzyme. Furthermore, Viticoli et al [9] used TiO2 to immobilize several enzymes and biological molecules in biosensors based on the direct electrochemistry of enzymes. Generally, enzymes are immobilized on the sensor surface either by cross-linking with, for example, glutaraldehyde, or by protecting with a thin gel or polymer layer of, for instance, Nafion, to avoid enzyme loss. New materials and methods have been explored to find more active and stable biosensors based on immobilizing enzymes [10, 11].
Sol-gel TiO2 nanoparticles have some advantages over other processes used to obtain nanoparticles, as they possess unique properties, such as high mechanical strength, low price, physical and chemical stability, low toxicity, coordination ability with amine and carboxyl groups, good biocompatibility, and environmental safety. Consequently, positively charged nano-TiO2 particle can adsorb negatively charged materials via electrostatic interactions, which is beneficial for the design of electrochemical sensors [11–15]. Hence, nanoparticles produced by the sol-gel TiO2 process are ideal for enzyme immobilization in biosensor applications. Kochana et al [16] developed a biosensor-immobilizing tyrosinase/laccase bi-enzyme system biosensor in a titania gel matrix to detect phenolic compounds. Li et al [17] reported a mesoporous titanium dioxide biosensor that immobilizes glucose-oxidase on a glassy carbon electrode. Kochana [18] immobilized tyrosinase enzyme in a sol-gel composite of titania/Nafion in an amperometric biosensor. In this study, we developed titania nanoparticles synthesized by the sol-gel process and used them as a bioreceptor nanostructured matrix to immobilize laccase. Additionally, the bioreceptor nanostructure was employed to detect gallic acid and prove its effectiveness, as measured by volti-amperometry tests.
2. Materials and methods
2.1. Materials and support preparation
Titanium isopropoxide and Nafion were purchased from Sigma-Aldrich. Graphite electrodes (GE) (ø = 0.6 cm) were of material grade: ST-21, Bay Carbon, Inc. (Bay City, MI, USA). Phosphate buffer 0.1 mol l−1 (PBS) was prepared using Na2HPO4 and KH2PO4 at the original pH of the solution and was used as the electrolyte support. Gallic acid was purchased from Sigma-Aldrich, and HNO3 was purchased from Baker.
l−1 (PBS) was prepared using Na2HPO4 and KH2PO4 at the original pH of the solution and was used as the electrolyte support. Gallic acid was purchased from Sigma-Aldrich, and HNO3 was purchased from Baker.
For the preparation of titania sol, the process known as sol-gel was used, as reported by Kochana et al [16] and Romero-Arcos [19]. The first stage consisted of preparing the precursor solution, which was made by adding two milliliters of titanium isopropoxide to 10 milliliters of 2-propanol at room temperature. Next, the solution was added with 80 μl of acetic acid and 160 μl of nitric acid. In the second stage, 24 ml of cold distilled water was added to the precursor solution, and it was homogenized by stirring. The titania sol was aged under storage at room temperature for a period of fifteen days.
On the other hand, the graphite electrodes (GE) were treated manually with sandpaper (1200 and 1500), followed by a stage of applying slurries of metallic alumina powder (0.3 and 0.05 μm), until a shiny surface was obtained. After rinsing twice with distilled water, the shiny GE were sonicated first with 0.1 mol  l–1 HCl, followed by absolute ethanol for 20 min, deionized water for 5 min, and finally, they were dried at room temperature, as described by Romero-Arcos et al [19].
l–1 HCl, followed by absolute ethanol for 20 min, deionized water for 5 min, and finally, they were dried at room temperature, as described by Romero-Arcos et al [19].
A laccase solution with a concentration of 20 mg ml−1 was prepared in 1 M PBS (pH 6.68). The bioreceptor TiO2/NAF/LAC was attained by mixing appropriate amounts of sol-titania with Nafion, resulting in composite titania-Nafion in identical proportions (1:1 v/v). An aliquot of 23 μl was taken from the end solution and deposited onto the surface of the graphite electrode. Finally, the prepared electrodes were immersed in a 1 M solution at pH = 6.68 SPB for washing.
2.2. Characterization techniques
The structural determination of the titania sol-gel samples was characterized by x-ray diffraction in the range of 20°–80° of 2θ with an x-ray diffractometer (RIGAKU DMAX/1200), using Cu Kα radiation, operating at 30 KV and 16 mA. The surface morphologies of the bioelectrodes were observed using a JOEL JSM-7600F field emission scanning electron microscope (SEM). The particle size of TiO2 was measured using a Horiba particle analyzer (Scientific Nanosizer SZ-100). The vibrational modes in the multi-layer coatings were obtained through Raman dispersion spectroscopy using DilorLampram II equipment at a temperature of 21 °C with a relative humidity of 37%, 20 mW of power, the integration time of 120 s, 1009 objective, spot of 1 μ, and a helium-neon laser.
2.3. Electrochemical measurements
Electrochemical tests were performed with Femtostat FAS2TM Gamry Instruments equipment. The experiments were carried out with a conventional three-electrode system, where the first electrode working was the GE coated with the composite, the second was the platinum counter electrode, and the third was the saturated Calomel reference electrode (SCE). According to Romero-Arcos et al (2019), the test was carried out using 0.1 M phosphate buffer (pH 6.68), 0.1 M KCl via open circuit potentials. A mixture (1:1) of 0.5 mM [Fe(CN)6:K4Fe(CN)6] was used as a redox probe. The tests were carried out at 25 °C.
3. Results and discussion
The XDR patterns of the TiO2 samples observed in figure 1(a) show five typical diffraction peaks located at 2θ: 25.3°, 37.9°, 48.15°, 54.05°, and 55.28°. Moreover, the sample had preferential orientation planes of (101), (004), (200), (105), and (211), corresponding to the body-centered tetragonal phase of TiO2 (anatase phase) [20, 21]. As previously observed, the anatase phase was the dominant phase observed in the samples [22–24]. Finally, figure 1(b) shows that in the bioreceptor that contains the immobilized laccase enzyme (TiO2/NAF/LAC), the characteristic peaks of the anatase phase are still present, which indicates that the immobilization process of the laccase enzyme did not change the phase transformation of TiO2.
Figure 1. X-ray diffraction: (a) TiO2 nanostructure and (b) bioreceptor TiO2/NAF/LAC.
Download figure:
Standard image High-resolution imageFigures 2(a) and (b) show the scanning electron microscope (SEM) micrographs of titanium oxide synthesized by the sol-gel method. We observed particles of different sizes, ranging from 1 μm to 100 nm, and formed irregularly sized clusters. In figure 2(c), the immobilized enzyme in the titania nanoparticles is appreciated because of the inherent luminescence of the laccase enzyme.
Figure 2. SEM micrographs: titanium oxide synthesized by the sol-gel method (a) 10000X, (b) 50000X; (c) the immobilized enzyme in the nanoparticles of titania.
Download figure:
Standard image High-resolution imageThe particle size distribution of titanium oxide, presenting a narrow range, is concentrated between 0.012 and 0.096 μm (approximately 12–96 nm) with an average size of 0.066 μm (66 nm) (figure 3). On the other hand, the surface area value obtained by BET method was 441.07m2 g−1, which was significantly higher compared to that reported by Watson et al [25], Kontos et al [26], and Choi et al [27]. The results of this research are promising, as it is assumed that the particles obtained have significant potential for use in the manufacture of TiO2 composite films, as they present low resistance and high retention of organic compounds.
Figure 3. Average size distribution of TiO2.
Download figure:
Standard image High-resolution imageRaman spectroscopy is one of several techniques used to characterize TiO2 and has been widely used because it allows rapid application of the crystal morphology on the surface of TiO2 nanoparticles [28]. Figure 4(a) shows the Raman spectrum of the laccase enzyme where no band was present because the enzyme was luminescent. Figure 4(b) shows the Raman spectra of the TiO2 samples synthesized by the sol-gel method, in which the bands around 148, 396, 515, and 630 cm−1 are; attributed to the active Raman modes of the anatase phase with symmetries Eg(1), B1g(1), A1g or B1g , and Eg(3) [29, 30], which is consistent with the results reported in the literature for a typical nanocrystal anatase TiO2 [31, 32]. Furthermore, the absence of 446 and 609 cm−1 bands—characteristic of the rutile crystal phase—suggests that the powders are composed of only the anatase phase [33]. Finally, figure 4(c) shows the nanostructure of the support that contains the immobilized laccase enzyme (TiO2/NAF/LAC) in which, in the Raman spectrum of the support, it is shown that the bands belong to the anatase phase, which has a lower intensity attributed to the presence of the immobilized laccase enzyme within the matrix of TiO2 nanoparticles.
Figure 4. Raman spectra of the support: (a) Pure TiO2; (b) Inserted graphic top right of the graph is Raman spectra Laccase and (c) support nanostructure TiO2/NAF/LAC.
Download figure:
Standard image High-resolution imageThe electrochemical impedance behavior (IES) of the graphite electrode (GE) and nanostructured support of the TiO2/NAF/LAC electrode are shown in figure 5. The Nysquit graphs of the graphite electrode (a) show a linear behavior that is characteristic of a limiting step in the electrochemical process. The TiO2/NAF/LAC nanostructured bioreceptor (b) presents a resistance value of 9.44 ohms, which indicates a lower resistance to electron transfer and therefore a better conductivity of the composite. This is due to the moderate hydrophobicity of TiO2 and Nafion, which is necessary to maintain the activity of the enzyme, as reported by Romero-Arcos et al and other authors [18, 19, 34, 35].
Figure 5. The impedance spectra (a) graphite (GE); and (b) bioreceptor nanostructure TiO2/NAF/LAC.
Download figure:
Standard image High-resolution imageCyclic voltametric behavior: The assembly-modified behavior of the TiO2/NAF/LAC electrode was verified through cyclic voltammetry (CV) tests, and its response was compared with that of a graphite bar. To conduct these experiments, an electrochemical solution consisting of 0.1 MKCl + 0.5 mM [Fe(CN)6/K4Fe(CN)6] was used. According to Singh et al (2013), when an electrode surface is modified, the electron transfer kinetics of Fe(CN)6 3−/4− are also altered. Therefore, the Fe(CN)6 system was selected as an indicator to investigate the changes in the behavior of different electrodes following modification [8, 19].
Figure 6(a) shows the separation of the peak (ΔEp) of the 270 mV graphite electrodes (GE), which in turn shows a quasi-reversible reaction [36]. However, when the surface of the GE electrode was modified with the TiO2/NAF/LAC composite (figure 6(b)), the peak current increased compared to the bar graph; however, there was also a decrease ∆Ep (216 mV). The behavior of the TiO2/NAF/LAC-coated electrode, which corresponds to the high peak current and decreased ΔEp, can be attributed to the TiO2 nanoparticle film, which increases the surface area for the immobilization of Laccase enzyme, following an improvement in electron transfer [37]. These results indicate that Laccase was successfully immobilized in the TiO2 matrix.
Figure 6. Cyclic Voltammetry of GE (a); and bioreceptor nanostructure TiO2/NAF/LAC (b).
Download figure:
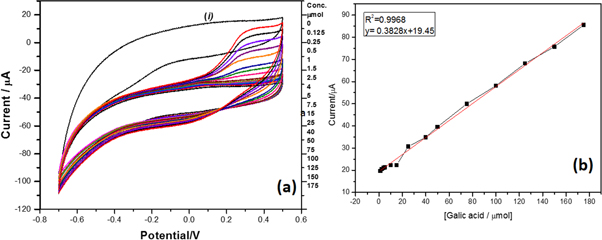
Standard image High-resolution imageVoltamperograms obtained by measuring TiO2/NAF/LAC with different concentrations of gallic acid in the electrochemical cell showed that when gallic acid was added a slight increase in the peak oxidation current was observed, which gradually increased at higher concentrations. It is also evident that this compound exhibits irreversible behavior because once oxidized on the surface of the electrode, it cannot electrochemically return to its previous state, as shown in figure 7(a). The possible reasons for this pattern are as follows: (i) changes in the stability of the phenoxy radical produced by the enzymatic reaction, (ii) solubility of the phenolic compound, (iii) the steric impedance between the phenolic compounds, and (iv) the active site of the enzyme; this behavior agrees with that reported by Singh et al [8], Yang et al [38], Wang et al [39], and Almeida et al [40].
Figure 7. The voltamperograms were obtained using the TiO2/NAF/LAC measurement with gallic acid at different concentrations, insert of linear regression.
Download figure:
Standard image High-resolution imageGraph (i) in figure 7(a) shows the nanostructured support immersed in the buffer solution in PBS 1 mM where it is observed that the current pass faster because there are no ions to oxidize or reduce. Therefore, it remains at higher currents once gallic acid is added the anodic current increases in proportion to the increase in gallic acid concentration.
The electrochemical response of the support nanostructure TiO2/NAF/LAC was studied as a function of the gallic acid concentration (0.125–175 μmol l−1), using cyclic voltammetry (CV) at a scanning rate of 100 mV s−1 (figure 7(a)) in PBS 1 mM. The anodic current increased in proportion to the increase in gallic acid concentration. This can also be perceived from the linear range of the curve (0.125–175 μmol) with a linear regression coefficient of 0.9968. The nanostructured support exhibited a detection limit of 0.125 μmol (figure 7(b)).
4. Conclusions
It has been demonstrated that the sol-gel process can be used to obtain titanium oxide nanostructures in the anatase phase, as identified by x-ray diffraction. The particle size obtained was in the order of 12–96 nm with a surface area of 441.07 m2 g−1. Because the anatase phase was present, it exhibited bioreceptor behavior, which allowed immobilization of the laccase enzyme. This was observed in the SEM micrographs and Raman spectroscopy in which it was verified that the enzyme had a luminescent effect. The support nanostructure TiO2 was sensitive and, able to detect gallic acid at concentrations of 0.125–175 μmol l−1. The nanostructured support exhibited a detection limit of 0.125 μmol.
Acknowledgments
Coordinación de la Investigación Científica—Universidad Michoacana de San Nicolás de Hidalgo for financing the project in 2022.
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).
Declarations of conflict of interest
The authors declare that there are no conflicts of interest.