Abstract
The inherent brittleness of poly(lactic acid) (PLA) usually limits its application in the high ductile requirements sector. To address the above issue, n-ethyl-p-toluenesulfonamide (N-PTSA) as the toughening agent, was utilized to optimize the rheological and mechanical performance of the PLA matrix. The microstructure, thermal stability, rheological behavior, and mechanical properties of PLA/N-PTSA were systematically investigated. A rheological test showed that N-PTSA can reduce the melt processing viscosity of PLA matrix and the decreasing of processing viscosity strongly depended on the N-PTSA content rather than shear rate. Mechanical properties results confirmed that the 14.7% N-PTSA can improve the toughness of PLA/N-PTSA without deteriorating the tensile strength and Yong's modulus. This reason was ascribed to the reduction of hydrogen bonds of PLA matrix caused by N-PTSA to accelerate the movement of PLA molecular chain meanwhile forming new hydrogen bond between PLA and N-PTSA. By adding N-PTSA, the thermal stability of PLA was decreased. With the synergistic effect of plasticizing and toughening N-PTSA, the rheology and mechanical properties of PLA had been effectively improved.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
The difficult natural degradation of traditional petroleum-based polymers is leading to a large amount of plastic pollution which causes damage to the environment [1, 2]. Recently, the research on environmentally friendly and sustainable green materials has attracted more and more attention. Polylactic acid (PLA), one of the bio-based green materials, has been widely developed and used in daily life [3, 4]. Due to the merits of being biodegradable and renewable, it is a potential alternative polymer material to replace the traditional petroleum-based materials [4, 5]. However, some unfavorable factors, such as inherent brittle texture, low toughness, and flexibility (elongational break is below 8% and toughness below 13 J m−1) of PLA lead to the failure to meet the industrial production requirements, which hinder its application and development in packing material, electronic device and in the high ductile required sector [6–9]. As a consequence, many chemical modifications and physical doping (fillers or additives) have been utilized to improve the mechanical properties of PLA-based composites through a relatively simple approach meantime maintaining the biocompatible and biodegradable PLA composites [10–12]. Especially, the introduction of natural nanofillers and green additives into PLA matrix endow many advantages to regulating the physical properties of PLA-based composites [13].
Currently, there are two universal approaches to improving the toughness of the PLA matrix. One is the grafting of flexible molecular chains into PLA to improve the ductility of the PLA matrix. Such as block or grafted copolymers like the PLA-g-PCL, and PLA-b-PCL [6, 10]. These resulting copolymers exhibited excellent mechanical properties and further improved their processability and degradation properties. Another one is to introduce some additives, such as PU elastomer [14], fillers (cellulose nanocrystals) [15], or plasticizers [16]. In the case of PLA/PU blend [14] or PLA/cellulose nanocrystals [15]. Interfacial compatibility of PLA matrix with PU elastomer was enhanced through in situ blending reaction, further favoring their mechanical performance. Additionally, the dispersion and mechanical performance of PLA/CNCs were synchronously improved via in situ surface modification of cellulose nanocrystals (CNCs) to achieve good interfacial compatibility. However, the synthesis process of the copolymers or chemical modifiers of fillers surface is complex and high cost in general. Meanwhile, it should be noted that interfacial modifiers used in PLA blend is nonbiological additives, hindering its application in biomedical fields. No one can ignore the fact that whatever the introduction of fillers or elastomers will obviously increase the melt processing viscosity. Therefore, plasticizer is still deemed as a promising additive to optimize the rheology and mechanical performance of PLA. These plasticizers are usually chosen by their inherent nature to endow the PLA with specific desirable properties depending on the final use purpose. For instance, to form highly flexible materials with ideal properties for food packaging, PLA and PLA blends have been combined with numerous plasticizers such as poly-(ethylene oxide) (PEO), polyethylene glycol (PEG), triethylcitrate (TEC), tributyl citrate (TBC), acetyl-tributyl citrate (TBAC), or epoxidized vegetable oils [17–19]. Among them, citrate ester-based plasticizers are widely used because of their good miscibility with the PLA and their biocompatibility, idea for food contact applications.
In recent years, n-ethyl-p-toluenesulfonamide (N-PTSA) as plasticizers have been selected to regulate the rheology and mechanical performance of PLA matrix. N-PTSA imparts flexibility, reduces water vapor permeability, and imparts resistance to oils, greases, and solvents [1, 20]. The fact that both of these additives were detected in cellulose ester fabrics, a knitting needle, is consistent with these being desirable properties for a domestic product that is handled frequently [21]. Herein, the addition of N-PTSA into the PLA matrix (PLA/N-PTSA) by directly melting blending can not only effectively reduce the melting processing viscosity, but also lower the cost. In the meantime, the rheological behavior, thermal properties, micromorphology, and mechanical performance of PLA/N-PTSA with different content of N-PTSA were systematically discussed to explore the toughen mechanism. Through the above-mentioned studies, N-PTSA is extended to the possibility of producing high-performance PLA-based nanocomposites at a large scale.
2. Materials and methods
2.1. Materials
PLA (Ingeo™, 6201D, 1.2 × 105 g mol−1, melt flow index of 7 g/10 min at 210 °C) was purchased from NatureWorks LLC (Minnetonka, USA). N-ethyl-p-toluenesulfonamide (>98 wt%) was supplied by Aladdin Biochemical Technology Co., Ltd, China. Antioxidant 1098 (98 wt%) was supplied by Aladdin Biochemical Technology Co., Ltd, China. Other reagents were provided by Shanghai Titan Technology Co., Ltd.
2.2. Preparation of PLA/N-PTSA
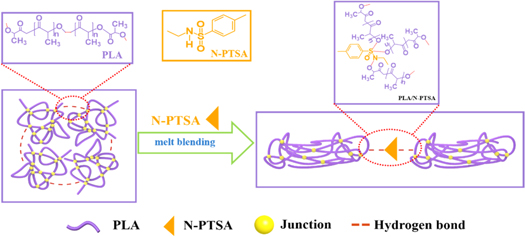
PLA and N-PTSA were dried in a vacuum drying oven at 80 °C for 8 h to remove moisture before use. The PLA/N-PTSA composites were prepared by melt compounding on a Haake rheometer at 170 °C and 60 rpm for 10 min The contents of N-PTSA in the resulting blends were 3.69%, 7.34%, 10.93%, 14.70%, and 17.95%, respectively. After the melt compounding, the composite product was compressed by a hot press to prepare standard impact (ASTM D256) and tensile (ASTM D638) specimens. The mold temperature and pressure and time is 185 °C, 10 MPa, 10 min, respectively. The preparation process was shown in scheme
Scheme 1. The preparation of PLA/N-PTSA via melt blending.
Download figure:
Standard image High-resolution image2.3. Characterization
Rheological measurements were performed by using a HAAKE MARS III rheometer (Thermo Fisher Scientific, Germany) with parallel plate of 25 mm in diameter at 180 °C. The average gap distance was kept at about 1.2 mm for all tests. The G' (defined as storage modulus) and G'' (defined as loss modulus) were measured at a fixed angle frequency (ω) of 6.28 rad s−1 in the strain range of 10−1–103 at 180 °C. Dynamic frequency sweep was measured at ω range of 0.1 to 600 rad s−1 at 180 °C. A steady rheological test was carried out in the frequency range of 0.01–40 s−1 180 °C. The average measurement thickness of all samples was 1.2 mm. Here, Payne effect was defined that straining the compounds that cause G' and G'' to decay and in some cases, a G'' peak appears before its sustained decay. Critical strain (γc ) means the critical transition strain from the linear region to the nonlinear region. The frequency corresponding to the intersection of the storage G' and the G'' is defined as the crossover frequency. This is calculated at which G' = G'' corresponds to the frequency.
Thermal analysis of PLA/N-PTSA composites was conducted on a differential scanning calorimetry (3500 Sirius). The samples were heated from 20 °C to 200 °C, holding for 5 min to eliminate thermal history, then cooled to 20 °C, sequent heating to 200 °C. The heating and cooling rates were 10 °C min−1. The heat of fusion of 100% crystalline PLA is 93.6 J g−1 [22]. The degree of crystallinity (Xc) was determined as follows,
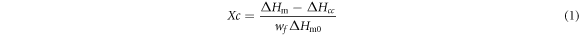
where wf is the weight fraction of PLA. and are the enthalpy of fusion of samples and the equilibrium melting enthalpy, respectively. is the cold crystallization enthalpy.
FTIR (Tensor-27, Bruker) spectroscopy was used to characterize the hydrogen bond interaction between PLA and N-PTSA in the range of 4000 cm−1 to 400 cm−1 at 64 scans with an average scanning rate of 4 cm−1.
The mechanical properties of all samples were performed by using Instron-5976 (Instron Limited, USA) at room temperature at a crosshead speed of 50 mm min−1 on dumbbell specimens with the average measurement values of 115 mm (length) × 6 mm (width) × 2 mm (thickness). Its mechanical property parameters are calculated from stress-strain curves according to established equations [23]. In each case, five specimens were tested and the results were reported as average values. Here, the maximum stress that a material can withstand at tensile fracture is defined as the critical stress (or ultimate tensile strength). The stress-strain curves were calculated from the load-displacement data from tensile tests. The critical stress was the maximum load divided by cross-section area of tensile specimens. The strain was the extension divided by the gage length of tensile specimens.
The morphology was studied by scanning electron microscopy (SEM) using X-650 (Hitachi, Japan SEM was performed by X-650 (HITACHI Co., Japan) to observe the surface morphology of samples at an accelerating voltage of 30 kV after gold-sputtering treatment.
3. Results and discussion
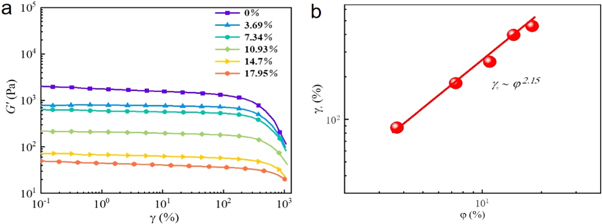
Generally, the addition of plasticizer into polymers matrix will influence their viscoelastic behavior during melting processing, which can be monitored by their rheological behavior. A dynamic strain sweep at a fixed frequency of 6.28 rad s−1 for each sample was conducted to determine the linear region of the polymer, as shown in figure 1(a). For pure PLA, there existed a wider linear region from 0.1% to 50% while the PLA/N-PTSA exhibited typical 'Payne effect' when the deformation was over than critical strain [24, 25]. Moreover, the critical strain (γc ) and G' strongly depended on the N-PTSA. the γc increased with increasing the N-PTSA content while G' sharply decreased, which was consistent with the plasticizing effect of N-PTSA. To clarify the relationship between γc and φ of all samples, the corresponding values of γc as a function of φ of N-PTSA were shown in figure 1(b). Interestingly, the γc value obeyed power-law dependences γc ∞ φi x . Here, the subscript i represents N-PTSA. The x was estimated at 2.15. A positive x value of about 2.0 is usually revealed in filled polymer melts whose linear viscoelasticity is governed by the particle-particle interaction [26]. In this system, it was believed that N-PTSA not only reduced the hydrogen bonding interaction between PLA macromolecules but also enhanced the compatibility between N-PTSA and PLA macromolecules. The γc value was dependent on N-PTSA content further disclosed that the addition of N-PTSA improved the molecular flexibility and interface compatibility between N-PTSA and PLA.
Figure 1. (a) Storage modulus (G') as a function of strain amplitude (γ) and (b) corresponding critical strain (γc ) as a function of volume fraction (φ) of PLA/N-PTSA.
Download figure:
Standard image High-resolution imageDue to the overlapping of some rheological curves, the G' and G'' curves of all samples are vertically shifted by a factor of 10n. A dynamic frequency sweep of all samples was studied, as shown in figure 2. In the low and moderate frequency region, G'' > G' indicated liquid-like behavior. When the φ ≥ 14.7%, the viscous behavior played a predominant role even in the high frequency region. This behavior implied that the polymer network is not truly 'solid' and may not be fully percolated, since it indicates the flow at long timescales. Rheological results show that the addition of N-PTSA can enhance the molecular chain flexibility of PLA, which is beneficial to reduce the melt processing viscosity of PLA. The influence of N-PTSA on the relaxation time of the PLA matrix was further examined by investigating the G' and G'' crossover frequency (G' = G'' corresponding to the frequency) as a function of N-PTSA content [27], as shown in figure 2(b). The reciprocal of crossover frequency (ωc) is usually defined as a characteristic relaxation time [28]. The ωc values also strongly depended on N-PTSA content, which obeyed the power-law relationship ωc ∞ φi 2.29. The index value of 2.29 was nearly consistent with 2.15 from γc ∞ φi x . The relaxation time of PLA/N-PTSA decreased with the increasing of the N-PTSA content. The results demonstrate that N-PTSA can regulate the relaxation time of PLA molecule chains and weaken the direct binding forces among the macromolecules, resulting in an increase of the strain at break and a decrease of the stiffnes, which are highly desirable properties for molding and shaping processes 29].
Figure 2. (a) Storage modulus (G') and loss modulus (G'') as a function of ω at 180 °C and (b) cross-frequency (ωc) as a function of φ for PLA/N-PTSA.
Download figure:
Standard image High-resolution imageThe influence of N-PTSA on the melting processing of PLA matrix was investigated, as shown in figure 3. Obviously, the addition of N-PTSA decreased the processing viscosity. When  < 10 s−1, all samples exhibited newton fluids. When
< 10 s−1, all samples exhibited newton fluids. When  > 10 s−1, the shear thinning behavior appeared and became weaker with the increasing N-PTSA content. The η strongly depended on the N-PTSA content rather than
> 10 s−1, the shear thinning behavior appeared and became weaker with the increasing N-PTSA content. The η strongly depended on the N-PTSA content rather than  showing the predominant of N-PTSA to accelerate the disentanglement of PLA molecular chains.
showing the predominant of N-PTSA to accelerate the disentanglement of PLA molecular chains.
Figure 3. Steady viscosity (η) versus shear rate ( ) curves for PLA/N-PTSA.
) curves for PLA/N-PTSA.
Download figure:
Standard image High-resolution image3.1. DSC and FTIR analysis of PLA/N-PTSA
Figure 4(a) showed DSC curves of PLA with different contents of N-PTSA. The thermal parameters of all samples were summarized in table 1. Compared with pure PLA, the glass transition temperature (Tg), cold crystallization temperature (Tcc), and melting temperature (Tm1, Tm2) of the composites showed a similar decreasing trend with the increase of N-PTSA content, showing that the thermal stability of PLA was decreased. It can be speculated that the hydrogen bond interaction between PLA molecular chains was reduced with the addition of N-PTSA, leading to movement enhancement of the PLA molecular chain. The results were consistent with previously reported small molecule plasticizers such as PEG200 [30], ATBC [31], and TBC [32]. It was worth noting that N-PTSA hardly influenced the degree of crystallinity. When φ ≤ 10.93%, it maintained about 4%. Even when φ > 10.93%, it was only reduced to 3.7%, indicating the weak inhibitory effect on the PLA crystallization. Figure 4(b) showed the FTIR spectra in the N-H stretching region of all samples. With the addition of N-PTSA, two weak peaks appeared at 3379 cm−1 and 3283 cm−1 and gradually become stronger. There was only one N-H bond in the FTIR spectrum of N-PTSA, it reasonably be expected that the N-H bond in N-PTSA formed a hydrogen bond with PLA. When a part of N-H bonds formed a hydrogen bond with PLA, its absorption peak decreased by about 100 cm−1. Therefore, the hydrogen bond interaction between PLA and N-PTSA played a key role to regulate the physical properties rather than degree of crystallinity.
Figure 4. (a) DSC curves of PLA with different contents of N-PTSA and (b) FTIR spectra in the N-H stretching region of PLA with different contents of N-PTSA.
Download figure:
Standard image High-resolution imageTable 1. DSC data of PLA different contents of N-PTSA.
| Sample | Tg (
| Tcc (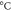 ) ) | Tm1 (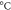 ) ) | Tm2 (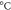 ) ) | Xc (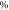 ) ) |
|---|---|---|---|---|---|
| 0% | 62.5 | 108.7 | 162.1 | 169.4 | 4.2 |
| 3.69% | 58.3 | 106.5 | 158.6 | 167.3 | 4.4 |
| 7.34% | 54.1 | 104.1 | 156.0 | 165.3 | 3.9 |
| 10.93% | 49.5 | 101.7 | 151.2 | 163.4 | 4.0 |
| 14.70% | 46.2 | 99.1 | 148.1 | 160.8 | 3.8 |
| 17.95% | 40.5 | 95.4 | 143.5 | 157.9 | 3.7 |
Figure 5 showed the stress-strain curves of all samples. All samples yielded similar strains, that can be attributed to local plastic deformation due to stress concentration at the entanglement of PLA/N-PTSA molecular chains under external stress [23, 33]. When φ ≤ 7.34%, the samples exhibited typical brittle fracture without toughness effect. When φ > 7.34%, the samples started to transition from brittle fracture to ductile fracture. In particular, when φ ≥ 14.70%, the plasticizing effect was very obvious. The relative mechanical properties were listed in table 2. Here, the elongation at break and toughness of PLA/N-PTSA (14.7%) were 480.4% and 20.96 J m−1, respectively, which was 63.2 times and 1.51 times than that of pure PLA. Meanwhile, its ultimate tensile strength and Young' s modulus were 36.4 MPa and 718.6 MPa, respectively, which still maintained 70% and 80% of pure PLA without severely sacrificing its high plasticity. When φ = 19.95%, its high toughness and elongation are at the expense of its high plasticity in a significant way, which is not beneficial to its mechanical behavior. Therefore, the mechanical property can be rationally optimized when the N-PTSA concentration is 14.70%.
Figure 5. Stress-strain curves of PLA with different contents of N-PTSA.
Download figure:
Standard image High-resolution imageTable 2. Mechanical properties of PLA with different contents of N-PTSA.
| Sample | Ultimate tensile strength (MPa) | Yield strength (MPa) | Young's modulus (MPa) | Elongation at break (%) | Toughness (J m)−1 |
|---|---|---|---|---|---|
| 0% | 50.8 ± 3.3 | 49.47 ± 0.8 | 888.2 ± 18.8 | 7.6 ± 1.5 | 13.86 ± 0.84 |
| 3.96% | 46.5 ± 4.2 | 43.91 ± 2.4 | 843.1 ± 15.6 | 7.9 ± 2.2 | 12.19 ± 0.69 |
| 7.34% | 47.4 ± 2.5 | 41.96 ± 2.8 | 863.7 ± 32.5 | 7.8 ± 2.6 | 15.87 ± 1.17 |
| 10.93% | 42.5 ± 2.8 | 41.46 ± 2.3 | 880.7 ± 24.1 | 12.5 ± 4.1 | 20.79 ± 2.27 |
| 14.70% | 36.4 ± 1.8 | 35.9 ± 1.0 | 718.6 ± 35.6 | 480.4 ± 33.3 | 20.96 ± 1.79 |
| 17.95% | 17.78 ± 1.6 | 17.31 ± 1.2 | 423.8 ± 55.3 | 602.4 ± 46.6 | 28.14 ± 1.56 |
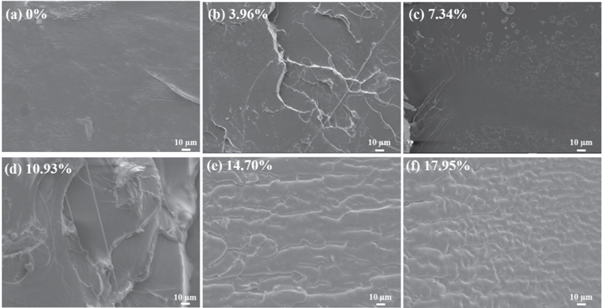
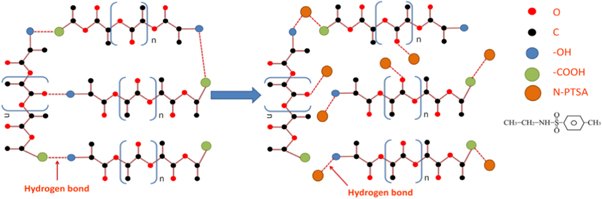
Figure 6 showed the cross-section SEM images of all samples. Pure PLA had typical brittle fracture characteristics. When φ ≤ 10.93%, the fracture surface became rough, showing the ductile characteristic. Especially, when φ > 10.93%, the samples exhibited a bicontinuous phase structure, indicating that N-PTSA had good compatibility with PLA. The results further confirmed that high loading on N-PTSA was beneficial to the toughness of PLA. The toughing mechanism was explained as follows (figure 7). On the one hand, the PLA molecular chain contained a rigid carbonyl group, which formed a hydrogen bond with the terminal hydroxyl group, leading to the movement restriction of the PLA molecular chain. With the addition of the toughening agent N-PTSA, N-PTSA can destroy the hydrogen bonding among PLA molecular chains meanwhile the new hydrogen bonds can be rebuilt between the –OH (or C=O) groups of PLA molecular chains and O = S = O (or N-H) of the N-PTSA molecules, resulting in the reduction of hydrogen bond density among PLA molecular chains and enhance the interaction between PLA and N-PTSA. On the other hand, based on the rheological results, the addition of N-PTSA can accelerate the disentanglement of PLA molecular chains and weaken the hydrogen bonding interaction among PLA molecular chains. When the φ = 14.70%, there is a trade-off between viscoelastic behavior and the mechanical properties of PLA. Therefore, the rheology and mechanical properties of PLA had been effectively improved with the synergistic effect of plasticizing and toughening N-PTSA.
Figure 6. SEM images of fracture surface of PLA with different contents of N-PTSA.
Download figure:
Standard image High-resolution imageFigure 7. The toughing mechanism schematic of PLA/ N-PTSA.
Download figure:
Standard image High-resolution image4. Conclusion
In this paper, N-PTSA as a plasticizer and toughening agent was utilized to improve the melting processing and mechanical properties of the PLA matrix. The addition of N-PTSA increased the γc for the transition from linear to nonlinear region meanwhile reducing the molecular chain relaxation time (1/ωc) of the PLA matrix. Interestingly, both γc and 1/ωc obeyed a similar power-law relationship. Steady rheology revealed that the mechanism of viscosity reduction was related to the decrease of hydrogen bond of PLA matrix induced by N-PTSA to inhibit the formation of hydrogen bond of PLA. Thermal analysis and FTIR showed that hydrogen bond interaction between PLA and N-PTSA played a dominant role rather than the degree of crystallinity. Finally, 14.7% N-PTSA as optimum content could significantly improve the toughness and plasticity of PLA without deteriorating the strength and modulus.
Data availability statement
The data that support the findings of this study are available upon reasonable request from the authors.
Funding information
This work was financially supported by the National Natural Science Foundation of China (51973167, 52103062), the Opening Project of Guangxi Key Laboratory of Calcium Carbonate Resources Comprehensive Utilization (HZXYKFKT201808), the State Key Laboratory of Bio-Fibers and Eco-Textiles (Qingdao University), No. K2019-10), and the Innovation and Entrepreneurship Program of Hubei province (201810495060, S201910495047).
Compliance with ethical standards
The authors declare that they have no conflict of interest.