Abstract
Palygorskite (Pal) is a kind of magnesium aluminum silicate clay mineral with a one-dimensional rod and layer chain structure. Herein, Pal hybrid iron oxide red pigment (Pal/α-Fe2O3) was prepared by the grinding method. Pal acted as a matrix to fix α-Fe2O3 particles and influenced the performances of composite pigments by changing the particle size and distribution of α-Fe2O3. The color difference analysis showed that the color performances of composite pigments were closely related to the ratio of Pal to α-Fe2O3. Compared with the original red iron oxide pigment, the tinting strength of the composite pigment was increased by about 15%. When Pal/α-Fe2O3 was used as the red pigment for coating, it had good storage stability for waterborne coating and improved its adhesion and corrosion resistance. Pal/α-Fe2O3 composite pigments had excellent high-temperature resistance, which could increase the calcination temperature of ceramic pigments and would be beneficial for the ceramic industry. The synthetic method of Pal/α-Fe2O3 composite pigment was simple, low-cost, and environmentally friendly, and could be applied to large-scale industrial production.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Instruction
Red iron oxide, especially α-Fe2O3, is the most popular inorganic coloring agent and is widely used in ink, paint, ceramics, architecture, and other fields because of its advantages of weather resistance, corrosion resistance, strong shading ability, tinting strength, and so on (Hradil et al 2003, Legodi and de Waal 2007, Tian et al 2017a, 2017b, Fiuza et al 2018). Moreover, red iron oxide pigments benefit from the low-cost and easy-to-obtain raw materials, as well as a well-established production process, resulting in a high market share. However, compared with red chrome pigment, red lead pigment, and other heavy metal inorganic pigments, the color of red iron oxide pigment was not so bright, and its application scope was limited (Sotiropoulou et al 2010, Rukhlyadeva et al. 2015, Cao et al 2017). To improve the color performances of red iron oxide pigment, some new synthesis routes such as alkali precipitation and gas oxidation methods, and bio-oxidation methods instead of the traditional wet method were developed (Leskelä and Leskelä 1984, Murray 2000, Chen et al 2014, Li et al 2016). Most of these methods required high temperature, and pressure, and were high-cost, thus limiting their practical industrial application. Therefore, it is very important to develop a low-cost synthesis method to improve the color and light properties of red iron oxide pigments.
Pal is a magnesium-rich aluminosilicate clay mineral with a one-dimensional rod and layer chain structure (Liu 2007, Giustetto and Wahyudi 2011). The unique nanorod and nanoporous structure of Pal make it have a large specific surface area and provide a large number of active adsorption sites. The surface of Pal was rich in hydroxyl and other functional groups, which can be used as an excellent matrix for binding with other inorganic/organic materials (Dejoie et al 2010, Chen et al 2019a) or adsorption of heavy metal ions (Lu et al 2020, Zhang et al 2021), various dyes (Mu and Wang 2016), and others. For example, palygorskite was modified by hydrothermal method to adsorb methylene blue and Cu2+ (Wang et al 2015), and magnetic palygorskite nanocomposites were prepared to adsorption of Ag+ by 'grafting' technology (Mu et al 2013), Palygorskite was used to synthesize of hierarchical porous and hydrophobic composites for the adsorption of benzene by one-pot method (Chen et al. 2019b). Besides Pal, there are many kinds of clays that have been used for the synthesis of clay-based red iron hybrid pigment. Sepiolite (Sep) or halloysite (HNTs) has been used for the clay/Fe2O3 red hybrid pigments by facile one-step reaction with Fe (III) solution. The hybrid pigments demonstrated good color and excellent stability (Tian et al 2017a, 2017b). Therefore, in this work, Pal was used as the matrix to hybrid with α-Fe2O3 to improve the color performances of iron oxide red pigment. It was verified that the composite pigments had excellent improvements in color, tinting strength, and stability. The performance mechanism of composite pigments was analyzed by the characterization of composite structure morphology.
2. Experimental
2.1. Materials
Palygorskite (Pal) with the main composition of CaO (1%), Al2O3 (8%), Na2O (2%), MgO (13%), SiO2 (59%), K2O (1%), and Fe2O3 (5%) was a gift from Jiuchuan Clay Technology Co. (Jiangsu, China). Red iron oxide (α-Fe2O3, 190) was provided by Jiangsu Yuxing Industry and Trade Co. Ltd (Jiangsu, China). The color light of 190 was L* = 37.56, a* = 25.60, and b* = 13.73. Meanwhile, the tinting strength of 190 was L* = 54.78, a* = 24.06, and b* = 3.80. Sodium hexametaphosphate ([NaPO3]6) was purchased from China National Medicines Co. Ltd (Shanghai, China). The dispersant of EDAPLAN®494 for red iron oxide was obtained from MÜNZING (Germany). The deionized water was used throughout the experiments.
2.2. Preparation of the Pal/α-Fe2O3 hybrid pigments
Before the synthesis of the Pal/α-Fe2O3 hybrid pigments, 1 g Pal was mixed with 0.01 g sodium hexametaphosphate. Then, 1, 2, 4, 8, 12, and 15 g red iron oxide dispersed in 0.01, 0.02, 0.04, 0.08, 0.12, and 0.15 g EDAPLAN®494, respectively, were added to the above Pal mixture (The compositions of mixed pigments were referred to table S1 (available online at stacks.iop.org/MRX/9/065202/mmedia)). The resulting hybrid was grounded with ZrO2 microsphere at 1350–1650 r min−1 for 90 min, washed with deionized water three times, filtered with 200 mesh screens, and dried in an oven at 80 °C. After that, the as-prepared pigments were ground with agate mortar and screened with 140 mesh screens to obtain Pal/α-Fe2O3 hybrid pigments named cp1, cp2, cp4, cp8, cp12, and cp15, respectively.
2.3. Characterization
According to the Commission Internationale de L'Eclairage 1976 color space (L*a*b*), the color of each Pal/α-Fe2O3 hybrid pigment was analyzed three times by a Datacolor 500 spectrophotometer (Datacolor, USA). In L*a*b* color space, each color is represented by three numbers L*, a*, and b*, while L* represents brightness, a* represents the green to the red component, and b* represents the blue to the yellow component. The larger L*, the higher the brightness. When L* is 0, it represents black, and when L* is 100, it represents white. When a* and b* are 0, both represent gray. a* and b* change from a negative number to a positive number, and the corresponding color changes from green to red and blue to yellow, respectively. The range of color channels a* and b* are −100 ∼ 100 and −128 ∼ 127. The − presented as ΔE = (ΔL2 + Δa2 + Δb2)1/2, where ΔL = Lcp − L190 is the lightness difference, Δa = acp−a190 is the red/green difference, Δb = bcp−b190 is the yellow/blue difference. C = [(a)2 + (b)2]1/2 is the saturation of the color.
The tinting strength test was performed by diluting the pigment with a white base material (such as ZnO and TiO2) and then measuring the color performance with a Datacolor 500 spectrophotometer. In this case, the sample was prepared by mixing the pigment and TiO2 in a ratio of 1:20. The color performance was compared with the diluted 190 red iron oxide standard sample.
The XRD patterns were collected by the X-ray powder diffractometer (Ultima IV, Rigaku) at a measuring 2θ range of 5 ∼ 80°, with Cu-Kα ration (40 mA, 40KV), 0.025° (2θ) step size, and 10° (2θ) scan rate per minute. The powder sample was ground for 15 min and then laid flat on a glass sample plate with a thickness of about 0.5 mm. The Fourier Infrared Spectrum was conducted by Nicolet IS20 (Thermo Scientific, USA), in a range of 4000–400 cm−1. UV–vis reflectance spectra were collected by UV-2600 (Shimadzu, Japan), in a range of 350–750 nm. The Zeta potential and particle size distribution were determined by NanoBrook Omni (Brookhaven, USA). The morphology of the pigments was observed by a field emission scanning electron microscope (Nova Nano SEM450, FEI, USA). The sample was ultrasonically dispersed in absolute ethanol for about 30 min and then dropped onto the silicon wafer. The thermal gravity (TG) curves were recorded by TG209 F3 thermogravimetric analyzer (NETZSCH, GER) in the heating range from room temperature to 800 °C, and the heating rate of 10 °C min−1. For the high-temperature calcination test, the composite pigments were respectively treated with a muffle furnace (carbonite cwf1100, GER) at 700, 950, and 1100 °C for 30 min under a heating rate of 10 °C min−1. After naturally cooling to room temperature, XRD and ultraviolet diffuse reflection were performed to evaluate the high-temperature resistance. The chemical composition of hybrid pigments was measured by ARL X4200 X-ray fluorescence spectrometer (Thermo Fisher Scientific, USA).
2.4. Applications of the Pal/α-Fe2O3 hybrid pigments
The waterborne polyurethane composite coating was synthesized by adding 7.5 g waterborne polyurethane, 0.5 g defoamer, and 0.2 g dispersant into a 25 ml beaker, and magnetically stirring for 30 min at the rotation speed of 900–1200 r min−1. Then 1 g of Pal/α-Fe2O3 hybrid pigment and an appropriate amount of deionized water was added to adjust the viscosity. The mixture was kept under magnetic stirring at 1500–1800 r min−1 for 4 h, followed by standing for 12 h for later use.
The ceramics containing 190 red iron oxide or composite pigment cp8 was synthesized by adding 8 g ZnO, 4 g MgO, 21 g B2O3, 12 g SiO2, 10 g Al2O3, and 4 g cp8 into a ball mill tank and mixing them initially. After adjusting the viscosity with a certain amount of water, the mixture was grounded with an F-P2000 (FOCUCY, CHN) high-performance planetary ball mill for 2 h. Then, the mixture was dried in an oven at 100 °C followed by sieving with a 40-mesh screen to obtain the ceramic powder. The resultant powder was pressed into a 1.5 cm diameter disc with a tablet press machine and burned in a muffle furnace at 800 °C for 3 h to ceramic discs. After that, the color performances of the obtained ceramics were analyzed according to CIE 1976 l*a*b*standard colorimetric method.
3. Results and discussion
3.1. Color performances of the Pal/α-Fe2O3 hybrid pigments
The color light and tinting strength played key roles in the property and application of a red pigment (Jose et al 2019). The color light and tinting strength properties of each composite pigment were shown in figure 1 and table 1. After adding Pal to red iron oxide, the color light property of the composite pigment decreased slightly with the increase of Pal content. However, when the Pal/α-Fe2O3 mass ratio exceeded 1:12, the color light performance of the pigment decreased significantly and was close to that of pure red iron oxide. On the other hand, with the increase of Pal content, the tinting strength of the composite pigments increased largely and reached its largest value at the Pal/α-Fe2O3 mass ratio between 1:4 and 1:8. Thus, the optimum ratio of Pal/α-Fe2O3 for the preparation of composite pigments was between 1:4–1:8, which displayed the best color light and tinting strength properties. The composite pigments cp1 and cp2 had the highest color light redness values, but their tinting strength redness values were low (table 1). Meanwhile, the tinting strength of cp12 and cp15 was relatively improved, but their color light performance was poor. On the other hand, cp4 and cp8 had the most balanced color light and coloring intensity, the coloring intensity was higher than other composite pigments, and their color light redness value and tinting strength redness value were about 10% higher than those of 190 iron oxide red, showing the best color performances of Pal/α-Fe2O3 at the optimum ratio of 1:4–1:8. From the result of XRF (table S2), with the increase of Fe content in the hybrid pigment, the shading performance of the hybrid pigment will continue to improve, but when the Fe content reaches more than 92%, the increase in shading will tend to 0. At the same time, the tinting strength of the hybrid pigment will increase with the content of Fe first, reaching a peak when the Fe content is 70%–85%, and then the tinting strength will continue to decrease with the increase of the Fe content.
Figure 1. (a) Pictures of six composite pigments (cp1-cp15, from left to right); (b) Colored light and tinting strength of six composite pigments.
Download figure:
Standard image High-resolution imageTable 1. Color light, tinting strength, absorption edge, and Eg of hybrid pigments.
| Color light | Tinting strength | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample name | ΔL | Δa | Δb | ΔC | ΔE | ΔL | Δa | Δb | ΔC | ΔE | absorption edge (nm) | Eg (eV) |
| cp1 | 2.09 | 3.16 | 3.43 | 4.48 | 5.11 | 2.09 | 0.23 | 3.43 | 4.48 | 5.11 | 618 | 2.00 |
| cp2 | 2.25 | 3.11 | 3.71 | 4.59 | 5.34 | 2.25 | 1.74 | 3.71 | 4.59 | 5.34 | 623 | 1.99 |
| cp4 | 2.21 | 2.74 | 3.49 | 4.22 | 4.96 | 2.15 | 2.54 | 4.61 | 4.23 | 5.69 | 629 | 1.97 |
| cp8 | 1.99 | 2.28 | 3.14 | 3.98 | 4.36 | −0.88 | 2.55 | 4.73 | 4.16 | 5.44 | 625 | 1.98 |
| cp12 | 1.43 | 1.94 | 2.46 | 2.93 | 3.45 | −0.93 | 2.16 | 3.78 | 3.02 | 4.45 | 632 | 1.96 |
| cp15 | 1.24 | 0.57 | 1.06 | 1.03 | 1.72 | −2.65 | 2.38 | 4.01 | 3.34 | 5.36 | 635 | 1.95 |
The color of Pal/α-Fe2O3 was also analyzed by reflectance spectroscopy (Baryshev et al 2004, Wang et al 2018). The level of reflectivity (R%) at the same wavelength meant the saturation and lightness of the pigment color. The higher the reflectivity, the higher the color saturation and lightness. As shown in figure 2(a), the reflectance of composite pigments cp1, cp2, and cp8 was significantly higher than that of other pigments, indicating their excellent color saturation and lightness. Figure 2(b) was the absorption edge of 190 and the composite pigments. The absorption edge (λ) of the composite pigment was derived from the Kubelka–Munk function of F(R) = (1 − R)2/2 R (Wang et al 2020). The redness of red iron oxide pigment was closely related to the bandgap (Guan et al 2016). The bandgap (Eg ) of the composite pigments was calculated by the formula of Eg = 1240/λ. The absorption edge and bandgap of each composite pigment were displayed in table 1. The absorption edges of all composite pigments were blue-shifted, while the largest and the smallest bandgap were of cp1 and cp15, respectively. The bandgap decreased with the iron oxide content increasing, which was due to the change in the redness value (a) of the composite pigment. The changing trend of the chromatic redness value (a) of the composite pigment was basically consistent with the changing trend of the bandgap (table 1). The increase in the bandgap and redness value of the composite pigment was due to the quantum confinement effect induced by the small size region (Liu et al 2015).
Figure 2. UV–vis diffuse reflectance spectra (a) and absorption edge of composite pigments (b).
Download figure:
Standard image High-resolution image3.2. Characterization of the composite pigments
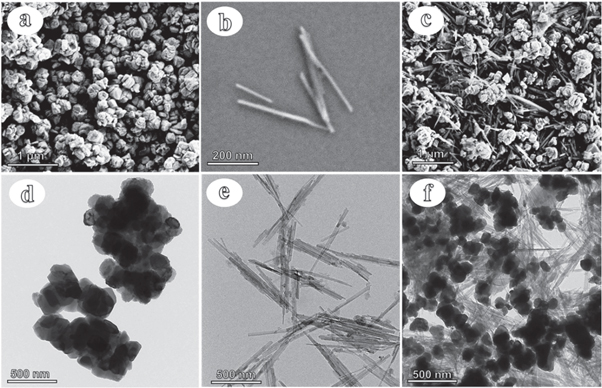
Figure 3 showed the SEM and TEM morphologies of the red iron oxide pigment (190), Pal, and Pal/α-Fe2O3 hybrid pigment. The red iron oxide pigment displayed an irregular spherical morphology with a size of 100–150 nm (figures 3(a) and (d)). The Pal showed a typical rod-like structure with rods length of 0.5–5 μm, and sizes of 30–60 nm (figures 3(b) and (e)). For Pal/α-Fe2O3 hybrid pigment, red iron oxide can be evenly coated on the Pal rod (figures 3(c) and (f)), which increased the distance between pigment particles and thereby improves the dispersion of pigment (Lu et al 2019). We reasoned that Pal plays an important role in controlling the structure and color performances of composite pigments, which was the major factor to improve the color performances of red iron oxide composite pigments (Wheeler et al 2012).
Figure 3. SEM images of 190 iron oxide pigment (a), Pal (b), and the composite pigment of cp8 (c). TEM images of 190 iron oxide pigment (d), Pal (e), and the composite pigment (f).
Download figure:
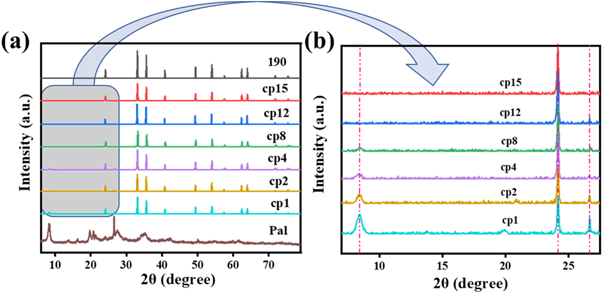
Standard image High-resolution imageThere are four common types of iron oxide (Fe2O3), namely α-Fe2O3, β-Fe2O3, γ-Fe2O3, and ε-Fe2O3. α-Fe2O3 is the most common and stable type of iron oxide, with a uniform hexagonal crystal structure, and could be used as the red pigment (Song et al 2014). Figure 4 showed the x-ray diffraction patterns of 190, Pal, and composite pigments. The characteristic peaks of α-Fe2O3 crystal were observed at 24.14° (012 planes), 33.15° (104 planes), 35.61° (110 planes), 40.85° (113 planes), 49.48° (024 planes), 54.09° (116 planes), 57.59° (018 planes), 62.45° (214 planes) and 63.99° (300 planes), showing a high purity of α-Fe2O3 in red iron oxide pigment 190 (Liu et al 2013). Pal displayed the characteristic peaks at 8.41° (110 planes) and 13.86° (200 planes) (Giustetto and Wahyudi 2011, Boudriche et al 2014). After being hybridized with Pal, the characteristic peaks of α-Fe2O3 crystal in the composite pigment were maintained unchanged. However, as the mass ratio of Pal in complex pigments decreased, its characteristic peak gradually decreased. When the ratio of α-Fe2O3 in the composite pigment was increased to 1:4, the crystal structure of the composite pigment has become consistent with that of α-Fe2O3. This was possibly due to the decrease of Pal in composite pigments and the damage of Pal rod structure caused by mixing and grinding during the preparation of composite pigments (Bruni et al 1999, Ruiz-Hitzky et al 2013).
Figure 4. XRD patterns of Pal, cp1, cp2, cp4, cp8, cp12, cp15 and α-Fe2O3:(a) 2θ in range of 7° to 78°, (b) 2θ in range of 7° to 27°.
Download figure:
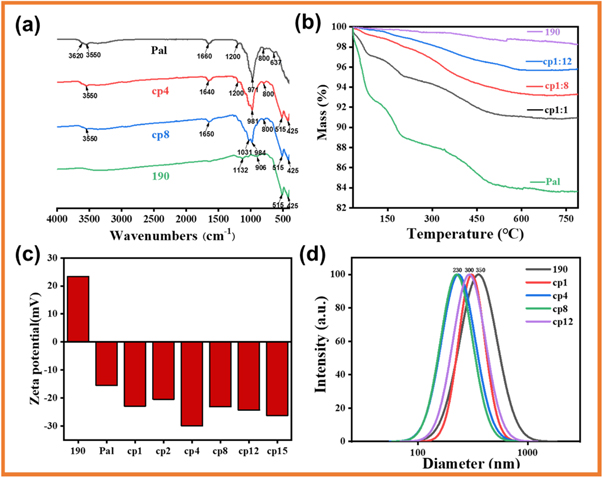
Standard image High-resolution imageFT-IR spectra showed two characteristic bands at 515 cm−1 and 425 cm−1 for red iron oxide pigment 190, which were attributed to Fe-O-Fe stretching vibration and Fe-O-Fe bending vibration, respectively (figure 5(a)) (Frost et al 2001). These two characteristic bands could also be observed from cp4 and cp8. Similarly, the characteristic bands of Pal at 3550 cm−1 (stretching vibration of (Fe/Mg)OH or (Al/Mg)OH), 1660 cm−1, and 1200 cm−1 (stretching vibration of Si-O-Si groups connected two) also appeared in cp4 and cp8 (Yan et al 2012). The slight shift of 1660 cm−1 indicated the strong interaction between Pal and α-Fe2O3 (Frini-Srasra and Srasra 2008).
Figure 5. (a) FTIR spectra of α-Fe2O3, cp8, cp4, and Pal, (b) TGA curves of Pal, cp1, cp8, cp12 and 190 α-Fe2O3, (c) Zeta potential of 190 α-Fe2O3, Pal, cp1, cp2, cp4, cp8, cp12 and cp15 and (d)Particle size distribution of 190 α-Fe2O3, cp1, cp4, cp8, and cp12.
Download figure:
Standard image High-resolution imageTG measurements showed that the red iron oxide pigment 190 has almost no mass loss (1.5%) even when heated to 750 °C (figure 5(b)). TG curve of Pal showed three stages of mass loss. The mass loss from room temperature to 100 °C came from the physically adsorbed water and a small part of zeolite water in Pal; the mass loss from 100 °C to 400 °C was related to the coordination water and most of the zeolite water in Pal; the loss of quality from 400 °C to 540 °C came from the release of structural water in Pal (Boudriche et al 2012). When the temperature rose to 540 °C, the mass loss reached 15%. At high temperatures, some hydroxyl groups in Pal were dehydroxylated, resulting in a slight mass loss (Gueli et al 2018). As the content of 190 in the composite pigment increased, the quality loss gradually decreased and mainly came from Pal. Therefore, the thermal stability of composite pigments was somewhat lower than pure α-Fe2O3.
As a colorant, the most important performance index of red iron oxide pigment was tinting power and shade, especially the tinting power reflecting the efficiency and ability of the pigment (Jose et al 2019). The dispersity and particle size of pigment were the main factors affecting these two properties. In general, the tinting strength of pigment increased with the decrease in particle size and the increase in uniformity and dispersion. As a white powder, Pal will reduce the concentration and tinting strength of the red iron oxide pigment after it is added to the red iron oxide pigment. However, the tinting strength of composite pigments is improved instead of undiminished by adding Pal. We added an experiment by only ball-milling 190 red iron oxide, without adding Pal. Then the tinting strength of the 190 iron oxide red pigment was monitored after ball milling. ΔL, Δa, and Δb were determined to be 0.57, 0.31, and 2.04, respectively. Although the a* of iron oxide red pigment 190 has been improved, its tinting strength was slightly less than that of cp8 Pal/α-Fe2O3 composite pigment. Therefore, we believe that Pal plays a key role in the performance of pigment tinting strength. Pal was negatively charged due to the abundant hydroxyl groups on its surface, while α-Fe2O3 is positively charged due to the presence of oxygen vacancies on its surface (Galan 1996). Therefore, α-Fe2O3 particles could coat to Pal surface through electrostatic interaction. This could be verified through the zeta potential test (figure 5(c)). 190 showed positive potential, while Pal and all composite pigments were negative potentials, indicating that α-Fe2O3 is successfully dispersed on Pal's surface.
In addition, Pal not only acted as the adhesion base of α-Fe2O3 in composite pigments, but also could increase the dispersion of α-Fe2O3 particles through steric hindrance, reduce the average particle size, and make the particle size distribution more uniform. The average particle size of composite pigments was determined by DLS measurements (figure 5(d)). The average particle size of 190 was about 350 nm, while cp1 and cp12 had particle sizes of 300 nm, cp4 and cp8 had particle sizes of 230 nm. At the same time, the particle size distribution of compound pigment was much narrower than that of 190, indicating that the particle size distribution of compound pigment became more uniform. This increased the tinting strength and tinting power of pigment.
3.3. Application of compound pigments in iron-red coating and ceramics
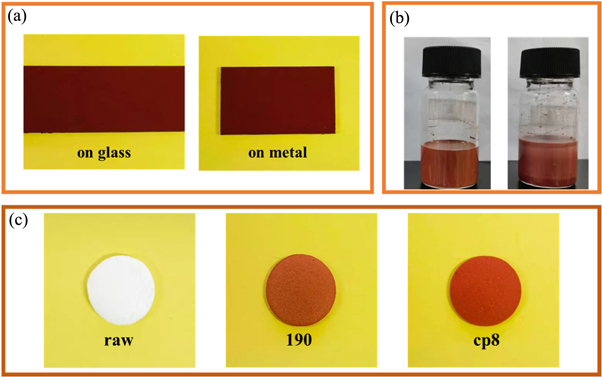
Iron oxide red was a commonly used pigment, especially for building exterior walls, antirust coatings, and ceramic products. In this work, cp8 was used as a raw material to prepare water-based paint that can be coated on the surface of metal and glass. Figure 6(a) was the actual sample picture of the coating, showing that the coating exhibits a bright red color. The color of the paint only slightly changes in acid, alkali, and organic solvents, indicating that the pigment had good corrosion resistance and stability (table S3). The tape test method was used to measure the adhesion of the coating on the glass sheet and the iron sheet. According to the American Society of Testing Materials standard, the adhesion of the coating on the two substrates has reached the 4B standard. Under the same conditions, the paint with cp8 as the raw material was completely suspended for six months, and there was no change compared with the original paint, while the paint made of 190 iron oxide red had obvious delamination, which indicated that the water-based coating with the composite pigment cp8 had better storage stability (figure 6(b)).
Figure 6. (a) Digital photograph of coating on glass and metal, (b) cp8 waterborne coatings and 190 waterborne coatings after 6 months of storage under natural conditions, and (c) ceramic chips with different compositions.
Download figure:
Standard image High-resolution imageIn addition, the waterborne coating with cp8 as the raw material could maintain a relatively stable color and light performance in different solvents or sodium chloride solution (table S3), indicating that Palygorskite could improve the color performance at the same time, ensure the resistance of the composite pigment, and would not reduce the viscosity and stability of the paint (figure 6(b)). Afterward, the effect of pH on the stability of the coatings was evaluated by measuring the Colorimetric coordinates of the coatings in solutions of different pH. The results (table S4) demonstrated that the changes of L*a*b* for the coating were slightly between different pHs. This showed that the coating could maintain good stability at different pHs.
Then we added cp8 to the ceramics during the preparation process to give the ceramic a red color and made the color not fade after high-temperature calcination. The XRD characteristic pattern of the composite pigment did not change significantly at all three calcination temperatures, and the characteristic peaks belonging to α-Fe2O3 did not disappear (figure S1). This showed that the crystal form and structure of the red iron oxide crystals in the composite pigment did not change significantly after high-temperature calcination, indicating that the composite pigment had good high-temperature resistance. From the digital picture of the ceramic sheet, the ceramic color after high-temperature firing was bright and smooth (figure 6(C)). The redness value a* of cp8 ceramic was 30.2, which was much higher than the redness value of 25.4 for 190 ceramic. Moreover, the color, hardness, and smoothness of the ceramic chip made of cp8 are better.
4. Conclusion
In this paper, Pal and red iron oxide powder were successfully used to prepare high-performance, low-cost iron oxide composite pigments. The tinting strength performance of composite pigments had been significantly improved. As the base of composite pigments, Pal could improve the performance of composite pigments by reducing its particle size and improving the dispersibility of α-Fe2O3. The optimal ratio of Pal to α-Fe2O3 (mass ratio) of the composite pigment was 1:4 and 1:8. The simple synthesis method improves the performance of the composite pigment while greatly reducing the production cost. The water-based coating prepared by the composite pigment could be well coated with various materials and has good corrosion resistance and weather resistance.
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).