Abstract
Chitosan films are increasingly being applied in the biomedical field owing to their biocompatibility, biodegradability, non-toxicity, mucoadhesive nature, hemostatic properties, antibacterial and biological activities. This study aimed to enhance the mechanical properties of chitosan films by doping niosomal sage nanoparticles (NS-SagNPs) at various concentrations (100–300 μg). The NS-SagNPs were prepared by a thin-film hydration process with an average particle size of 21.5 nm. The doped chitosan films were fabricated through a simple casting method. FTIR and DSC measurements confirmed the successful incorporation of NS-SagNPs in the chitosan films. The mechanical properties of the doped films were improved and the most significant improvement was found in tensile strength and elasticity when the NS-SagNPs loading was increased to 300 μg. Based on these results, chitosan films doped with NS-SagNPs have the advantageous feature of sage and show enhanced mechanical properties compared with pure chitosan, rendering them more suitable for biomedical applications.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
A variety of polymeric films has been employed for biomedical applications; in particular, natural polymers have attracted considerable interest because of their remarkable biological properties [1–5]. Chitosan is a natural carbohydrate that is non-toxic, biodegradable, and biocompatible; most importantly, it possesses antibacterial activity against a wide range of gram-positive and gram-negative bacteria [6–10]. Chitosan can stimulate fibroblast proliferation and collagen deposition [9, 10], facilitating the re-epithelization process [11–13]. Chitosan films have good mechanical strength, allowing them to be used in many medical applications [14–18], such as tissue engineering and wound care. However, the elasticity, stress resistance, and flexibility of the chitosan films require further improvements [19–21]. Chitosan has been combined with different inorganic nanomaterials, such as halloysite nanotubes [22, 23], bentonite [24, 25], montmorillonite [26], and laponite [27], to achieve better physical properties for wound care applications. However, these inorganic nanoparticles may have potential safety issues. Therefore, the possibility of incorporating natural extracts to enhance the mechanical properties of chitosan has been explored, and a wide range of biologically active materials, such as antioxidants, anti-inflammatory agents, and vitamins, has been introduced as additives.
Sage (Salvia officinalis L.) is grown worldwide and used in cooking. Its leaves are rich with natural phenolic and flavonoid compounds having antioxidant [28, 29], antibacterial [30], and antifungal [29] effects. Therefore, sage is also used to treat excessive sweating, dyspepsia, and throat and skin inflammation [31]. In the present study, the extract of the sage leaf was employed as an additive to create bioactive chitosan dressings. The sage extract was prepared in nano-form [32, 33] and encapsulated by a carrier to enhance biodistribution and bioavailability. Niosome (NS) was selected as the carrier molecule because its spherical, microscopic, and lamellar structure can ensure the stability of the loading agents [34, 35]. This study prepared chitosan films with nano sage extract of different concentrations encapsulated by NS in a simple and unique way. The obtained films contain anti-inflammatory and antioxidant agents in nano-formulation. Further, they have enhanced mechanical properties compared with the pure chitosan film, offering them great potential to be excellent wound dressing materials.
2. Materials and methods
2.1. Materials
Chitosan (low molecular weight), cholesterol (purity ≥ 99.70%, M.W. 386.65), Tween 80, and ethanol were purchased from Sigma-Aldrich (Chemie, Steinheim, Germany). Phosphate buffer tables (pH = 7.4 at 25 °C) and acetic acid (purity ≥ 99%) were purchased from Bio Shop (Mainway, Burlington, ON L7L 6A4, Canada). The sage extract was obtained from the Department of Biochemistry, Faculty of Applied Medical Sciences, October 6 University, Giza, Egypt. All chemical compounds were used without further purification.
2.2. Samples preparation
2.2.1. Determination of polyphenols in sage using HPLC
HPLC analysis was performed on a Varian ProStar System (Agilent Technologies, Inc., Santa Clara, CA, USA) with a ProStar 230 solvent delivery system, a Rheodyne 7125 injector, and a Pro Star 330 UV photodiode array detector. Chromatographic separation was performed on a Zorbax ODS column (250´4.6 mm, i.d. 5 mm; Agilent Technologies).
2.2.2. Preparation of niosaomal sage nanoparticles (NS-SagNPs)
NS-SagNPs were synthesized using a thin-film hydration process. Tween 80 (10 μl) and cholesterol (3 mg) were dissolved at a ratio of 2:1 in ethanol in a round-bottom flask. The ethanol was evaporated at 62 °C under a reduced pressure to generate a dry, thin coating using a rotary evaporator operating at 50 rpm. The thin film was then hydrated with PBS (pH = 7.4) containing 2 mg of sage extract to reach a final mass concentration of 0.04 mg ml−1. The produced multilamellar niosomes were sonicated for 5 min, resulting in the formation of tiny vesicles. Finally, a high-speed (10000 rpm × 30 min) cooling centrifuge (VS-18000M, Korea, 220 V/50 Hz) was used, and niosomes were precipitated in PBS [36, 37].
2.2.3. Preparation of chitosan films doped NS-SagNPs
A chitosan solution was prepared by dissolving 1 g of chitosan powder in 100 ml distilled water. Acetic acid (1% v/v) was added to facilitate the dissolution. The prepared solution was added to a glass plate and dried as a control film. The chitosan solution was then mixed with 100 μg, 200 μg, or 300 μg NS-SagNPs to form a mixture with three different concentrations of NS-SagNPs. Finally, the mixture was added to a glass plate and left to dry.
2.3. Characterization
2.3.1. Transmission electron microscope (TEM)
The morphology of niosomes was characterized using a TEM (JEOL JEM.1230, Japan) working at an accelerating voltage of 100 kV [38]. Sage extract encapsulated by NS was negatively stained with 1% phosphotungstic acid and then incubated for one minute on a porous carbon-coated grid before analysis.
2.3.2. Fourier transform infrared (FTIR) spectroscopy
FTIR measurements were performed on an FT-IR spectrometer (Thermo Scientific, NICOLET 6700, England) and an FT/IR-4100 (A Basic Vector, Germany). The samples were scanned in the wavelength range from 4000 to 400 cm−1. The number of scans was 10 [39].
2.3.3. Differential calorimetry scanning (DSC)
DSC studies were conducted to assess the thermal stability of the chitosan films doped with various concentrations of NS-SagNPs. The films were placed in a DSC sample holder, and the calorimetric curves in the temperature range of 25 °C–400 °C were recorded at a heating rate of 10 °C min−1 in a nitrogen environment (30.0 ml min−1) on a DSC-60 system (Shimadzu, Japan). Pure chitosan film without doping was used as a control.
2.3.4. Mechanical properties
The mechanical properties of pure chitosan film and films doped with various concentrations of NS-SagNP were evaluated using a tensile testing machine (Z010, Zwick Roell, Germany). The tensile strength (TS) and elastic modulus (EM) of each film were measured, and the average of three measurements was calculated for each film to evaluate the stress and strain values.
2.3.5. X-ray diffraction
Chitosan films were analyzed using a Philips X'pert Multipurpose X-ray diffraction system (MPD) operating at 40 kV and 40 mA. The device uses a Cu target (size 12 mm × 0.4 mm) to produce collimated X-ray energy of 8.047 keV. Measurements were carried out in a step mode at a step size of 0.5° in an angular range from 2θ = 5 ° to 80 ° with a step time of 20 s. Diffraction data were collected using a PW 3011/10 proportional detector with a graphite monochromator [40, 41].
2.4. Statistical analysis
Statistical analysis was performed by one-way analysis of variance (ANOVA) and Duncan's test using SPSS 17.0. Statistical significance was set at P ≤0.05 (*). Mean ± standard deviation (SD) was used to represent all data.
3. Results
3.1. Determination of polyphenolic compounds in sage
Seven phenolic acids (vanillic, caffeic, syringic, rosmarinic, salvianolic K and salvianolic I acids, and methyl rosmarinate) and six flavone glycosides (6-hydroxyluteolin-7-glucoside, luteolin-7-glucuronide, luteolin-7-glucoside, luteolin-3-glucuronide, apigenin-7-glucuronide, and apigenin-7-glucoside) were successfully identified in the sage according to the retention times using HPLC (tables 1 and 2).
Table 1. The results of HPLC-UV PDA determination of individual phenolic acids obtained from sage water extract.
| Phenolic acids/(mg per 100 g of dry matter) | |||||||
|---|---|---|---|---|---|---|---|
| Extraction time (min) | Vanillic acid | Caffeic acid | Syringic acid | Rosmarinic acid | Salvianolic K acid | Salvianolic I acid | Methyl rosmarinate |
| 30 | 11.10 ± 0.27 | 88.23 ± 0.93 | 55.12 ± 0.78 | 2174.02 ± 13.03 | 29.10 ± 0.72 | 16.21 ± 0.54 | 15.10 ± 0.53 |
| 60 | 10.20 ± 0.65 | 103.20 ± 4.02 | 63.10 ± 0.95 | 2445.31 ± 20.59 | 33.11 ± 0.65 | 16.21 ± 0.87 | 99.01 ± 4.47 |
| 90 | 10.10 ± 0.37 | 115.31 ± 7.32 | 65.12 ± 0.55 | 2872.11 ± 21.96 | 35.12 ± 0.59 | 12.12 ± 0.98 | 85.36 ± 1.24 |
Table 2. The results of HPLC-UV PDA determination of flavone glycosides obtained from sage water extract.
| Flavone glycosides/(mg per 100 g of dry matter) | ||||||
|---|---|---|---|---|---|---|
| Extraction time (min) | 6-hydroxyluteolin-7-glucoside | luteolin-7-glucuronide | luteolin-7-glucoside | luteolin-3-glucuronide | apigenin-7-glucuronide | apigenin-7-glucoside |
| 30 | 150.41 ± 1.02 | 235.22 ± 12.00 | 98.01 ± 4.45 | 471.32 ± 11.48 | 103.27 ± 0.73 | 41.55 ± 2.04 |
| 60 | 173.22 ± 0.54 | 312.48 ± 10.02 | 122.56 ± 5.21 | 625.33 ± 9.73 | 122.20 ± 0.30 | 38.40 ± 1.95 |
| 90 | 205.13 ± 0.89 | 340.41 ± 11.56 | 234.23 ± 9.73 | 684.7 ± 11.21 | 122.03 ± 0.10 | 40.50 ± 3.20 |
3.2. Morphological analysis of films
3.2.1. Digital micrograph
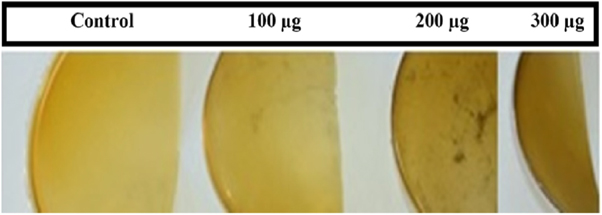
A smooth and flat surface was observed for all tested films. Chitosan doped with NS-SagNPs (100 μg and 300 μg) had a homogeneous morphology, while some agglomerates of NS-SagNPs were distributed randomly in the film with 200 μg NS-SagNPs (figure 1).
Figure 1. Photographs of four different films: pure chitosan (Control), chitosan NS-SagNPs (100 μg, 200 μg, and 300 μg).
Download figure:
Standard image High-resolution image3.2.2. TEM
The TEM image revealed nearly spherical and homogeneous NS-SagNPs with an average particle size of 21.5 nm (figure 2).
Figure 2. TEM image of the NS-SagNPs.
Download figure:
Standard image High-resolution image3.3. Fourier transform infrared (FTIR) measurements
The functional groups of pure chitosan and chitosan films doped with different concentrations of NS-SagNP were identified using FTIR (figure 3). The spectra of pure chitosan showed a characteristic peak at 1628 cm−1 corresponding to CONHR amide I stretching, broad adsorption of hydrogen-bonded OH stretching and NH2 asymmetric stretching (3400–3600 cm−1), CO stretching (1030 cm−1), a polysaccharide region (400–950 cm−1), and a C–H peak (2878 cm−1). All these distinct peaks were detected in doped films with a minor shift.
Figure 3. Characteristic FTIR spectra of chitosan films.
Download figure:
Standard image High-resolution image3.4. Differential calorimetry scanning analysis
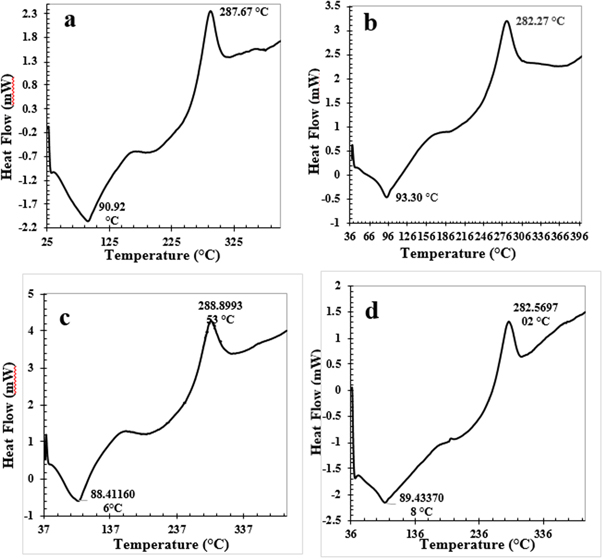
DSC was used to determine the thermal stability of chitosan films. Figure 4(a) shows a sharp endothermic peak at 90.92 °C, and an exothermic peak at 287.67 °C. The endothermic peaks were also strong in doped chitosan films, and the peak position of 100, 200, and 300 μg doping were located at 93.3 °C, 88.41 °C, and 89.43 °C, respectively; while the exothermic peaks were at 282.27 °C, 288.90 °C, and 282.60 °C, respectively (figures 4(b)–(d)).
Figure 4. Exothermic peaks of (a) pure chitosan and chitosan film doping with (b) 100 μg NS-SagNPs, (c) 200 μg NS-SagNPs, (d) 300 μg NS-SagNPs.
Download figure:
Standard image High-resolution image3.5. Mechanical properties
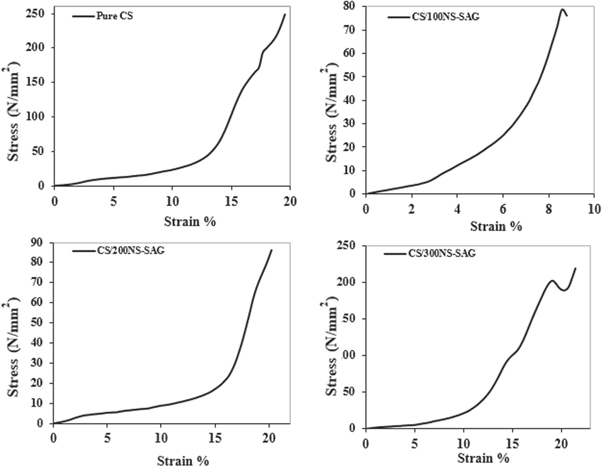
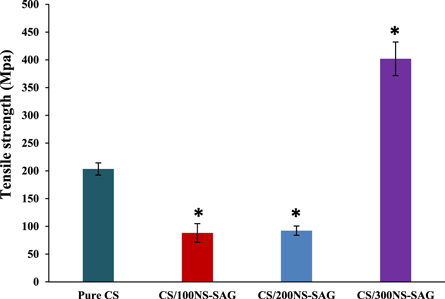
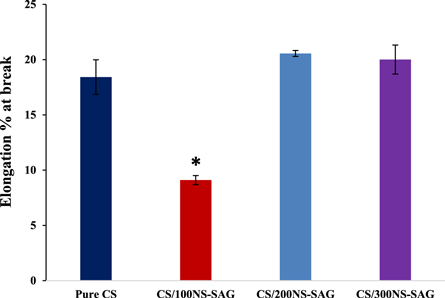
The stress-strain curves of chitosan and chitosan doped with 100, 200, and 300 μg NS-SagNPs are shown in figure 5. Tensile strength, elastic modulus, and elongation at break were used to assess the mechanical characteristics. In figure 6, a slight decrease in the tensile strength was found for the chitosan NS-SagNP films (100 μg, 200 μg), compared to the pure chitosan film, while a significant increase was detected for the chitosan NS-SagNP film (300 μg). Figure 7 shows the change in film elastic modulus, where all doped films exhibited an increase in elastic modulus compared with the pure chitosan film. The increase is more significant in the films doped with 100 μg or 300 μg NS-SagNP. Compared with pure chitosan, the elongation at break (figure 8) was significantly reduced in the chitosan NS-SagNP film (100 μg) and increased when the NS-SagNP doping increased to 200 μg and 300 μg.
Figure 5. The stress-strain curves of chitosan and chitosan doped with 100, 200, and 300 μg NS-SagNPs films.
Download figure:
Standard image High-resolution imageFigure 6. Tensile strength of chitosan NS-SagNPs films.
Download figure:
Standard image High-resolution imageFigure 7. Change in films elastic modulus.
Download figure:
Standard image High-resolution imageFigure 8. Elongation at break of the films.
Download figure:
Standard image High-resolution image3.6. X-ray diffraction (XRD) for chitosan films
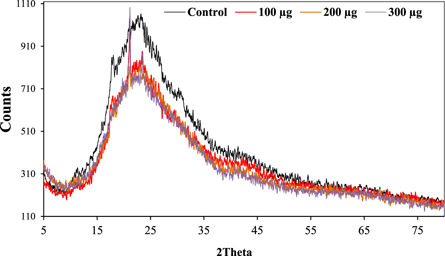
The XRD pattern of the chitosan films showed a main characteristic peak at 2θ = 23.39°. An additional peak at 2θ = 21.17 and some very sharp peaks emerged in the chitosan films with 100 or 300 μg NS-SagNPs loading (figure 9), indicating that some crystalline structures were present.
Figure 9. XRD pattern of the chitosan films.
Download figure:
Standard image High-resolution image4. Discussion
Chitosan films doped with different concentrations of NS-SagNPs (100–300 μg) were characterized using different techniques. TEM confirmed the successful preparation of spherical and homogeneous NS-SagNPs. A smooth and flat surface of the chitosan film doped with the highest NS-SagNP concentration (300 μg) was shown in the digital micrograph, suggesting the enhancement of the mechanical properties (stress-strain curves in figure 5) may be related to the morphology.
FTIR is a well-known technique to characterize the structures of a wide range of compounds [42]. All the characteristic peaks of chitosan were found in the doped films, indicating that the main chemical structure of chitosan films was maintained. The intensity of some characteristic peaks of pure chitosan changed slightly after doping because of the intermolecular rearrangement and variations in the configuration of the main chain [43, 44].
Moreover, chitosan films containing NS-SagNPs at different concentrations exhibited new peaks attributed to the phenolic compounds in the sage. The intensity difference between these peaks in the NS-SagNPs (300 μg) film compared to the other films was probably due to the synergistic effect of phenolic compounds in sage and the interactions between NS and the chitosan chain structure.
The sharp endothermic peak in the DSC curve of pure chitosan is found at 90.92 °C and can be related to the loss of adsorbed water. The exothermic peak at 287.67 °C is due to the decomposition of amine units. These data are comparable to those reported in a previous study [45]. The shift of the sharp endothermic and exothermic peaks in the doped films might have resulted from the interaction of NS-SagNPs with chitosan polymer.
Tensile strength, elastic modulus, and elongation at break were measured as the mechanical characteristics of the films. The strong and intermolecular interaction between NS-SagNPs and chitosan would result in a more compact structure, which was responsible for the significant increase in the tensile strength for the film doped with 300 μg NS-SagNPs and high elastic modulus for films doped with 100 and 300 μg NS-SagNPs. These observations agree with the previous reports by Salari et al 2018 and Shapi'I & Othman 2016 [46, 47]. Compared with the pure chitosan film, the films doped with 200 μg or 300 μg NS-SagNPs demonstrated higher elongation at break (figure 8). The NS-SagNPs doped at these concentrations showed a reinforcement effect that improved the motion of the chitosan matrix and the film elasticity, which was consistent with the previous studies [48, 49]. In addition, XRD analysis showed the crystalline structure of the NS-SagNPs in the doped films, confirming these particles have been successfully incorporated into chitosan.
Finally, characterization of the doped films indicated that some interactions occurred between the chitosan chains and NS-SagNPs. The barriers and the mechanical and thermal properties of the films were affected by the loading of NS-SagNPs. In general, the physicochemical properties of chitosan films are reinforced by increasing the concentration of NS-SagNPs. The elasticity of the films can also be improved compared to those obtained by Nguyen et al, 2020 [39]. Considering that sage has anti-inflammatory and antioxidant properties, chitosan film doped with NS-SagNPs can be a promising candidate for many biomedical applications, particularly as wound dressing materials.
5. Conclusion
In this study, we successfully doped chitosan films with niosomal sage nanoparticles of different concentrations using a simple and unique method. The structural integrity of the chitosan matrix was reinforced through doping. The presence of NS-SagNPs in the chitosan films was confirmed by FTIR and DSC measurements. Increasing the NS-SagNP doping concentration significantly improved the tensile strength. When the NS-SagNP doping increased to 300 μg, both tensile strength and elasticity could be enhanced. This film contained anti-inflammatory and antioxidant agents in nano-formulation and exhibited enhanced mechanical properties compared with the pure chitosan film, showing great potential to be excellent wound dressing materials.
Data availability statement
The data that support the findings of this study are available upon reasonable request from the authors.
Compliance with ethical standards
Conflict of interest, the authors declare that they have no conflict of interest.