Abstract
In this work, sepiolite hybrid pigments were prepared with four kinds of cationic dyes with different structures and functional groups, Basic orange 2 (BO2), Mytheme blue (MB), Basic red 18 (BR18) and Basic red 2 (BR2), by the vacuum method. The obtained samples were respectively named as BO2/Sep, MB/Sep, BR18/Sep and BR2/Sep. The sepiolite hybrid pigments were characterized and tested by XRD, FT-IR, Microporous physical/chemical adsorption analyzer, thermal analyzer, et al. The effects of cationic dyes structure and functional groups types on the positions and loading capacity of cationic dyes in sepiolite hybrid pigments were investigated. The results indicated that positions and loading capacity of cationic dyes in sepiolite hybrid pigments are not only related to the size of the cationic dyes but also related to the functional groups and electrical properties of the cationic dyes. The smaller the size of the cationic dyes, the greater the positive charge, and the stronger the functional groups interact with the sepiolite, the greater the amount that enters the sepiolite channel and is adsorbed. The loading capacity of cationic dyes in hybrid pigments dropped as the order MB/Sep, BO2/Sep, BR18/Sep, BR2/Sep. In addition, the position and interaction of cationic dye in sepiolite determine the stability of hybrid pigments. The resistance of hybrid pigments to the acid decreased as the order MB/Sep, BR18/Sep, BO2/Sep, BR2/Sep. In the base medium, the order is MB/Sep, BO2/Sep, BR18/Sep, BR2/Sep. The weatherability of the hybrid pigments was also better than pure cationic dyes.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
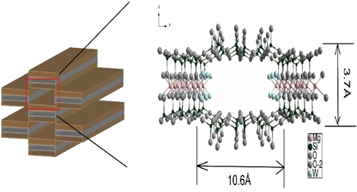
Palygorskite and sepiolite, as typical one-dimensional nanomaterials, are layered silicates formed by associating aluminum-oxygen/magnesium-oxygen octahedron and silicon-oxygen tetrahedron. They are mainly aggregates of nanorods and nanofiber bundles [1]. Both of them are 2:1 (TOT-type) layer silicates: their structures are formed by two continuous silica tetrahedral sheets (SiO4) linked by a discontinuous octahedral sheet (MO6), with M primarily aluminum and magnesium ions [2]. The primary difference between the two clay minerals is that the size of sepiolite channels (3.7 × 10.6 Å) is larger than that of palygorskite (3.7 × 6.4 Å) [3]. In addition, the content of Mg in the octahedron of sepiolite is relatively higher.
Sepiolite and palygorskite were widely found in ancient Maya ruins [4–6]. As the base of blue pigment, this pigment is called Maya Blue. The Maya Blue contains no mineral-based color and is stable in extreme conditions of humidity [3]. Maya Blue has been widely discussed by scholars because of its peculiar hue, characteristic brightness, excellent stability to chemical and biochemical attack [7–9].
Inspired by Maya Blue, Ouellet-Plamondon et al [10] took the adsorption of clay minerals to transform soluble dyes into insoluble pigments by liquid-phase method and successfully obtained hybrid pigments with excellent stability. While preparing bright blue cobalt blue hybrid pigment, the introduction of sepiolite not only greatly reduced the cost of cobalt blue pigment, but also improved its stability [11]. Cationic red X-GRL and methylene blue were employed as color giving agents and palygorskite was chosen as the substrate to prepare 'Maya Red' pigment and Maya Blue-like pigment by Fan et al [12] and Zhang et al [13], respectively. Both hybrid pigments with strong stability were obtained. The solid-phase synthesis method was utilized to compound palygorskite with tetrachloroindigo and quinalizarin respectively to prepare Maya-like blue hybrid pigments. The chemical stability of the two hybrid pigments both had been improved [14]. 'Maya Powder' hybrid pigments were prepared by compounding palygorskite with thioindigo and Ciba Brilliant Pink, respectively. The results showed that the mixture of palygorskite and two indigo sulfur derivatives displayed remarkable stability toward organic solvents, acids, and alkalis [15]. For sepiolite/basic red 46 hybrid pigments, the sepiolite was believed to protect the basic red 46 molecules from the attack of acid, base, heat and ultraviolet light [16]. The stable 'Maya Violet' pigment was fabricated via adsorption of methyl violet by palygorskite, and then deposition of a layer of SiO2 on the surface by Zhang et al [17]. The PAL/MV@SiO2 pigment showed superior stability against chemical attacks and SiO2 layer formed on the surface of PAL/MV contributed greatly to the enhanced stability. The sepiolite was also used as an adsorbent for the investigation of the adsorption of acid blue 25 (AB25) and methylene blue (MB). The adsorbed amounts of AB25 and MB showed an obvious difference [18]. Palygorskite-based nanocomposites were formed with methyl red and Sudan red. By comparing, methyl red/palygorskite showed better stability and Sudan red/palygorskite performed worse [19]. The preparation of clay minerals-dye nanocomposite pigments is an important strategy for the development of high-performance pigments. These studies demonstrated clay minerals could significantly improve the stability and dispersion of pigments. And clay minerals are selective for dyes while preparing hybrid pigments.
Many stable sepiolite-based hybrid pigments have been prepared. However, the performance difference of these hybrid pigments is obvious and the effects of different cationic dyes on sepiolite hybrid pigments are rarely reported. Therefore, it is necessary to explore the effect of cationic dyes with different structures on the sepiolite-dye interface reaction and the properties of nanocomposite pigments. In this paper, sepiolite was employed as a substrate and kinds of cationic dyes with different structures and functional groups were chosen as the color giving agent. Through the comparative study of four hybrid pigments, the effects of cationic dyes with different structures on the structure and performance of hybrid pigments were revealed. What's more, the mechanisms of action and the loading location and the amounts of different dyes in sepiolite were also investigated. The work aims to provide effective guidance and help while selecting the best matching cationic dyes to prepare sepiolite hybrid pigments with excellent stability and less cost.
2. Experiment
2.1. Experimental materials
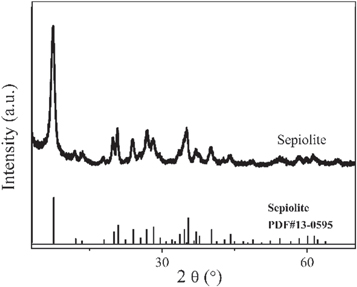
Raw sepiolite(sepiolite content>99%), obtained from Spain, was crushed and filtered by passing through a 200-mesh sieve. The XRD pattern of sepiolite (figure 1) shows the characteristic peaks which are consistent with the JCPDS card (No.13-0595) of sepiolite. The crystal structure of sepiolite is shown in figure 2. The sepiolite channel size is 3.7Å *10.6 Å. BO2, MB, BR18 and BR2 were provided by Shanghai Macleans Biochemical Technology Co., Ltd., whose basic information is shown in table 1. Concentrated hydrochloric acid (AR), sodium hydroxide (AR) and absolute ethanol (AR) were bought from Beijing Chemical Works.
Figure 1. XRD pattern of sepiolite.
Download figure:
Standard image High-resolution imageFigure 2. Crystal structure of sepiolite.
Download figure:
Standard image High-resolution imageTable 1. Cationic dyes information.
| Name | BO2 | MB | BR18 | BR2 |
|---|---|---|---|---|
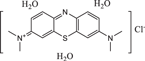
| Chemical formula | C12H13ClN4 | C16H18ClN3S·3H2O | C19H25ClN5O2 + | C20H19ClN4 |
| constitutional formula |
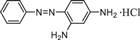
|
|
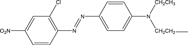
|
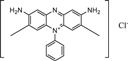
|
| Size | 6.213 Å*2.195 Å | 6.013 Å*2.409 Å | 6.008 Å*4.056 Å | 10.167 Å*4.267 Å |
2.2. Synthesis of hybrid pigments
1 g of sepiolite was heated at 180 °C for 1 h in the vacuum environment and then stirred for 2 h in 25 ml of concentrated cationic dye aqueous solution (3.2 g L−1) at 50 °C. The cationic dyes were BO2, MB, BR18 and BR2. The mixtures were centrifuged at 9000r min−1 for 10 min. The supernatant was left as a reserve. The solid was dried at 60 °C for 24 h in an oven. Then the obtained hybrid pigments were filtered by passing through a 200-mesh sieve and heated at 150 °C for 2 h. The samples prepared by BO2, MB, BR18 and BR2 were respectively named as BO2/Sep, MB/Sep, BR18/Sep and BR2/Sep.
2.3. Characterization
FT-IR spectra of samples were obtained on a Spectrum 100 FT-IR spectrometer (Perkin-Elmer, America), which recorded the waves ranging from 400–4000 cm−1 with a KBr background.
The XRD patterns of samples were obtained using a D8 Advance x-ray powder diffractometer (Bruker, Germany) with filtered Cu Kα radiation operated at 40 kV and 40 mA). The patterns of XRD were recorded from 3 ° to 70 ° and the resolution step size was 0.02 °.
The morphology of samples was characterized by a transmission electron microscope (TEM, Tecnai G2 F20, FEI). For TEM samples, a drop of solution was put on a carbon-coated copper grid and dried in the open atmosphere.
The Zeta potential data was recorded by a Zeta potential analyzer (Zetasizer Nano ZS90, Malvern, UK), and 0.01 g of sample was dispersed in 10 ml distilled water with sonication for 30 min.
The total specific surface area of the samples, cumulative pore volume (CPV) and pore size distribution of the micropores were calculated according to the N2 physisorption method and the density functional theory (DFT) method. The instrument used was Autosorb iQ-MP microporous physical/chemical adsorption analyzer (Quantachrome, USA).
The thermogravimetric analysis was performed on a thermal analyzer (STA 6000, PerkinElmer Instrument Co., Ltd., USA). The temperature increased from 30 °C to 900 °C based on the heating rate of 10 °C min−1 under N2 atmosphere with a flow rate of 20 ml min−1.
2.4. Stability tests
0.1 g of four hybrid pigments was dispersed respectively in 8 ml of 1 mol L−1 HCl, 1 mol/L NaOH and absolute ethanol. The mixtures were shaken in a thermostatic shaker (SHA-B, Guohua, Changzhou, China) at 120 rpm and 30 °C for 12 h and then centrifuged at 9000 rmp for 10 min. The released dyes in the supernatant were measured by a spectrophotometer (722S, INESA, Shanghai, China).
The weatherability was conducted according to the following procedure. Firstly, 0.2 g of four hybrid pigments were respectively flattened with a circle groove. Then the samples were irradiated under UV light (395 nm, 50 W, 550 mW cm−2) for 5 days continuously. Finally, the chroma of samples was recorded by a colorimeter (NR60CP, 3NH, Shenzhen, China) after 0 h, 1 h, 2 h, 4 h, 8 h, 24 h, 48 h, 72 h, 96 h and 120 h, respectively.
3. Results and discussion
3.1. Positions and loading capacity of cationic dyes in sepiolite hybrid pigments
3.1.1. XRD of heated samples
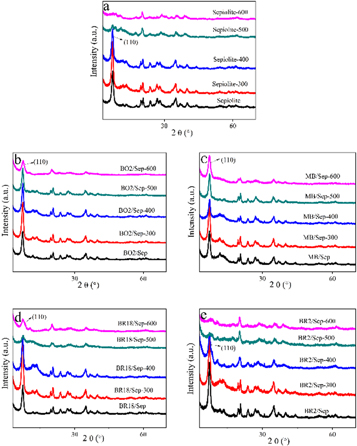
The sepiolite and four hybrid pigments were respectively heated at room temperature, 300 °C, 400 °C, 500 °C and 600 °C for 30 min to observe the strength of the d110 crystal face and other characteristic peaks [20]. In figures 3(a) and (c), the XRD patterns of sepiolite did not change below 400°C. However, when heated beyond 500 °C, the characteristic peak of the d110 crystal face of the raw sepiolite disappeared and the strength of other characteristic peaks dropped at the same time (figure 3(a)). But the XRD patterns of MB/Sep hybrid pigment remained basically unchanged after heated at 500 °C and 600 °C (figure 3(c)), which illuminated that MB entered the channels of sepiolite and braced the structure of sepiolite. When the samples were heated under high-temperature conditions, the sepiolite structure was conserved and would not collapse, similar to the phenomenon observed by Ovarlez et al [20, 21]. In figures 3(b) and (d), the temperature at which the structure of the hybrid pigments changed was also delayed, so BO2 and BR18 could also brace the sepiolite channels. What's more, the strength of the characteristic peak of the d110 crystal face and other characteristic peaks in BR18/Sep hybrid pigment were weaker after 500 °C compared with BO2/Sep, which showed that the bracing effect of BR18 was weaker than BO2. The XRD spectra of BR2/Sep hybrid pigment (figure 3(e)) were basically the same as that of raw sepiolite after heated. Therefore, BR2 could little brace the sepiolite structure.
Figure 3. XRD patterns of sepiolite and four hybrid pigments before and after heated (a) sepiolite; (b) BO2/Sep; (c) MB/Sep; (d) BR18/Sep; (e) BR2/Sep.
Download figure:
Standard image High-resolution imageTo investigate the positions of the four cationic dyes in sepiolite, the sizes of cationic dyes were considered. The sizes of BO2 and MB were both smaller than that of sepiolite channels (3.7 Å*10.6 Å) and MB was smaller than BO2. Therefore, MB was easier to enter the sepiolite channels and braced sepiolite structure better. The size of BR18 was larger than MB and BO2, but the size of the terminal group in BR18 was comparable with that of sepiolite channels by rotation. Therefore, the BR18 could partially insert into the sepiolite channels or grooves and partly brace the structure of sepiolite. The size of BR2 was larger than that of sepiolite channels, leading to the inability of BR2 to enter the sepiolite channels, and BR2 may little support the structure of sepiolite.
As a result, the cationic dyes with smaller sizes than sepiolite channels would more easily enter the channels and the cationic dye with comparable size compared with sepiolite channels could also partially insert channels by rotation. These cationic dyes completely entering or partially inserting the channels could brace the sepiolite structure when heated at high-temperature conditions.
3.1.2. BET analysis
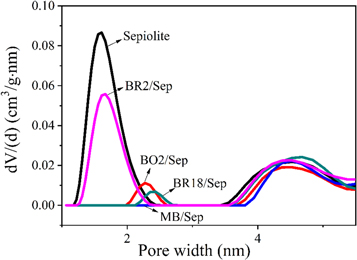
To further investigate the positions and amounts of four cationic dyes in sepiolite, the specific surface area, micropore specific surface area, total pore volume and total micropore volume of sepiolite and four hybrid pigments are shown in table 2 and figure 4. A lower specific surface area meant higher loading amounts, and a lower pore volume showed the amount of cationic dye in sepiolite channels or pore openings was higher. Table 2 shows that the specific surface area of sepiolite was 327.225 m2 g−1 and the specific surface area of four hybrid pigments was obviously smaller than that of sepiolite. The specific surface area in various hybrid pigments reduced according to the following order: BR2/Sep, BR18/Sep, BO2/Sep, MB/Sep. The decrease suggested that the four cationic dyes were significantly adsorbed in the sepiolite to different degrees, with the highest adsorption of MB/Sep. Furthermore, the micropore specific surface area of sepiolite was 112.099 m2 g−1, but MB/Sep, BR18/Sep and BO2/Sep hybrid pigments had a micropore specific surface area of 0. The micropore specific surface area of BR2/Sep was less than half that of sepiolite, which demonstrated that MB, BO2 and BR18 mainly entered or inserted the sepiolite channels and blocked pore openings, while BR2 could only block pore openings and fail to enter the channels.
Table 2. Specific surface area and pore volume of sepiolite and four hybrid pigments.
| Sample | SBET(m2 g−1) | Smicro(m2 g−1) | Vtotal(cm3 g−1) | Vmicro(cm3 g−1) |
|---|---|---|---|---|
| Sepiolite | 327.225 | 112.099 | 0.594 | 0.043 |
| BO2/Sep | 173.903 | — | 0.523 | 0 |
| MB/Sep | 162.602 | — | 0.502 | 0 |
| BR18/Sep | 182.995 | — | 0.533 | 0 |
| BR2/Sep | 258.391 | 52.333 | 0.558 | 0.026 |
Figure 4. The pore size distribution plots of sepiolite and four hybrid pigments.
Download figure:
Standard image High-resolution imageAccording to table 2 and figure 4, the total pore volume of sepiolite was 0.594 cm3 g−1 and the total pore volumes of four hybrid pigments were smaller than sepiolite whose decreasing order was the same as the specific surface area. In addition, except BR2/Sep, the cumulative micropore volume of MB/Sep, BO2/Sep and BR18/Sep were 0, which showed that BR18, BO2 and MB could enter the sepiolite channels or grooves, whereas BR2 only adsorbed on the outer surface of the sepiolite or covered the pore openings.
Among them, BO2 and MB could enter the sepiolite channels and BR18 could insert sepiolite channels. But BR2 could not enter the sepiolite channels due to its large molecular size. The loading capacity of cationic dyes in sepiolite hybrid pigments dropped according to the order MB/Sep, BO2/Sep, BR18/Sep, BR2/Sep.
3.1.3. TGA analysis
For sepiolite, the mass loss between 150 °C–300 °C of samples was mainly attributed to the removal of zeolite water [22]. According to the DTG image of raw sepiolite (figure 5(d)), the zeolite water loss rate of raw sepiolite was maximal approximately at 256 °C. By comparing the removal temperature of the zeolite water maximal loss rate in the hybrid pigments, the position and amounts of the cationic dyes in the sepiolite were obtained.
Figure 5. TG and DTG diagrams of sepiolite, four cationic dyes and four hybrid pigments (a) TG images of sepiolite and four cationic dyes; (b) TG images of sepiolite and four hybrid pigments; (c) DTG images of sepiolite and four cationic dyes; (d) DTG images of sepiolite and four hybrid pigments.
Download figure:
Standard image High-resolution imageFor MB/Sep, the mass loss rate was maximal at 315 °C, which was higher than the temperature (300 °C) of zeolite water loss in sepiolite [22]. This meant that the zeolite water was essentially replaced by MB and MB effectively entered the sepiolite channels. What's more, the mass loss rate of MB was maximal at 285 °C (figure 5(c)), yet in MB/Sep, the MB entered the sepiolite channels and adsorbed on the inner surface of the sepiolite. As a result, the MB was encapsulated in channels and the thermal stability of MB was enhanced. And the mass loss at 315 °C was attributed to the decomposition of MB. The mass loss rate in BO2/Sep and BR18/Sep was maximal at 218 °C and 236 °C (figure 5(d)), respectively, which were also attributed to the removal of zeolite water from the hybrid pigment. Because the cationic dyes entering the sepiolite channels interfered with the stability of the zeolite water, resulting in the temperature of zeolite water loss dropping. And BR18 only partially inserted the sepiolite channels and produced less interference, the temperature of maximal zeolite water loss rate in BR18/Sep hybrid pigment was higher than BO2/Sep. In addition, the mass loss at 292 °C for BO2/Sep and BR18/Sep were both attributed to the decomposition of cationic dyes (figures 5(c) and 8(d)). For BR2/Sep hybrid pigment, the highest mass loss rate was at 283 °C (figure 5(d)), which was attributed to the removal of zeolite water but higher than 256 °C. This was because the BR2 could only adsorb on the outer surface of the sepiolite and cover the sepiolite channels, the zeolite water in the sepiolite channels needed to overcome the resistance of the cover to escape the channels. As a result, the temperature of zeolite water maximal loss rate in the BR2/Sep hybrid pigment was greater than that of the sepiolite [7].
The results showed that the cationic dyes entering the sepiolite channels would obviously influence the temperature of sepiolite zeolite water maximum loss rate. The more cationic dyes that enter the sepiolite channels, the worse the stability of zeolite water. The cationic dye with a larger size than sepiolite channels would cover the channels and couldn't enter the channels, which raised the temperature of sepiolite zeolite water maximum loss rate. Therefore, the loading capacity of cationic dyes in sepiolite channels decreased as the order MB/Sep, BO2/Sep, BR18/Sep, BR2/Sep.
3.2. Binding modes of cationic dyes and sepiolite
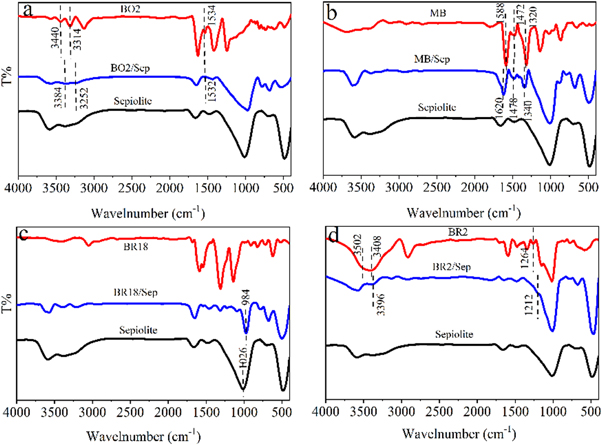
To further explore the binging modes between cationic dyes with different structures and sepiolite, the sepiolite, cationic dyes and the four hybrid pigments were tested by Fourier transform infrared. Figure 6(b) showed FT-IR spectra of MB, sepiolite and MB/Sep hybrid pigment. For MB, the bands at 1588 cm−1,1472 cm−1 and 1320 cm−1 were attributed to the stretching vibration of C=N, benzene ring skeleton and CAr–N [23], respectively. In MB/Sep hybrid pigment, the bands at 1588 cm−1,1472 cm−1 and 1320 cm−1 shifted to 1620 cm−1, 1478 cm−1 and 1340 cm−1. The phenomenon indicated that the length and electron cloud of the C=N and CAr–N were changed since the electrostatic attraction was formed between MB and sepiolite. The surface of sepiolite served as the electron donor. The N+ group in the MB molecule was electrostatically adsorbed with sepiolite, which increased the electron cloud density within the MB. For BO2(figure 6(a)), the bands at 3440 cm−1 and 3314 cm−1 belonged to the anti-symmetric stretching vibration and symmetric stretching vibration of NH2, respectively. In BO2/Sep hybrid pigment, the bands at 3440 cm−1 and 3314 cm−1 shifted to 3384 cm−1 and 3252 cm−1. The results illustrated that the NH2 group in the BO2 formed the hydrogen bond with sepiolite. In addition, the band at 1534 cm−1 corresponded to the bending vibration of NH2, which remained basically unchanged in BO2/Sep hybrid pigment. Therefore, the BO2 molecule mainly interacted with sepiolite through hydrogen bond. Figure 6(c) showed FT-IR spectra of BR18, sepiolite and BR18/Sep hybrid pigment. For sepiolite, the band at 1026 cm−1 was attributed to the stretching vibration of the Si–O. For BR18/Sep hybrid pigment, the band at 1026 cm−1 shifted to 984 cm−1, which demonstrated the length and electron cloud of Si–O were changed because of the electrostatic attraction between BR18 and sepiolite. The R–N+ in BR18 was electrostatically adsorbed with the base site of sepiolite. The electrostatic attraction could limit the stretching vibration of the Si–O bond and decrease the frequency of the vibration. For BR2 (figure 6(d)), the bands at 3502 cm−1, 3408 cm−1 and 1264 cm−1 were ascribed to the anti-symmetric stretching vibration of NH, symmetric stretching vibration of NH and stretching vibration of C–N, respectively. In BR2/Sep, the bands at 3502 cm−1 and 3408 cm−1 both shifted to 3396 cm−1, and the band at 1264 cm−1 shifted to 1212 cm−1. The result meant that there were hydrogen bonds formed between NH2 and sepiolite. In addition, there was also some electrostatic adsorption between BR2 and sepiolite due to R–N+ in BR2.
Figure 6. FT-IR spectra of sepiolite, cationic dyes and corresponding hybrid pigments. (a) BO2, BO2/Sep, sepiolite; (b) MB, MB/Sep, sepiolite; (c) BR18, BR18/Sep, sepiolite; (d) BR2, BR2/Sep, sepiolite.
Download figure:
Standard image High-resolution imageBased on FT-IR spectra of samples, it can be concluded that no new chemical bond appeared in four hybrid pigments. The absorption bands of the four cationic dyes also appeared in hybrid pigments, which meant the formation of the four hybrid pigments. In addition, the cationic dyes (MB and BR18) with stronger electropositivity mainly interacted with sepiolite through the electrostatic interaction. And the cationic dyes (BO2 and BR2) with naked hydrogens mainly compounded with sepiolite by hydrogen bonds.
3.3. Morphology of hybrid pigments
3.3.1. TEM analysis
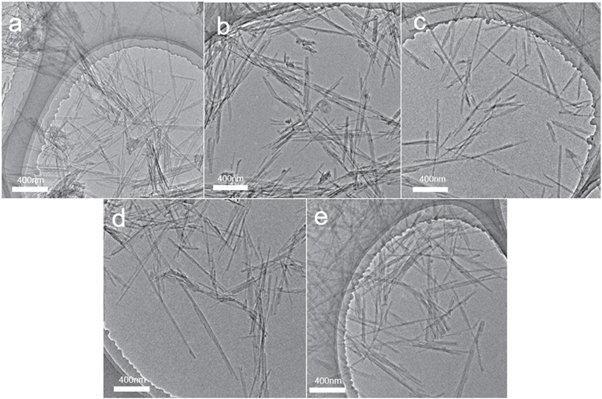
The morphology and aggregation state of the sepiolite and four hybrid pigments were analyzed by TEM. In figure 7, sepiolite primarily appeared in the form of nanorod crystals and the fiber structure of sepiolite in four hybrid pigments was not changed. In the TEM images of BO2/Sep, BR18/Sep and BR2/Sep (figures 7(b), (d) and (e)), the dispersion of hybrid pigments were comparable to that of the sepiolite and the nanorod crystals of the samples were dispersed well without evident accumulation. The nanorod crystals of the MB/Sep were basically not in contact with each other and dispersed better than the other three hybrid pigments (figure 7(c)).
Figure 7. TEM images of sepiolite and four hybrid pigments. a:sepiolite; b:BO2/Sep; c:MB/Sep; d:BR18/Sep; e:BR2/Sep.
Download figure:
Standard image High-resolution image3.3.2. Zeta potential
To further investigate the dispersivity of the four hybrid pigments, the absolute values of Zeta potential of sepiolite and four hybrid pigments were measured. From table 3, the absolute value of the Zeta potential of sepiolite was 9.71. The absolute values of Zeta potential of BO2/Sep, BR18/Sep and BR2/Sep hybrid pigments were comparable with sepiolite and not much different. From the TEM images (figure 3), the dispersibility of three hybrid pigments in water also was equivalent with that of sepiolite. For MB/Sep hybrid pigment, the absolute value of Zeta potential was the largest among the four hybrid pigments, which illuminated that the MB had the largest loading on the sepiolite surface and the dispersion of the sample was the highest, which was consistent with the TEM results.
Table 3. Absolute values of Zeta potential of sepiolite and hybrid pigments.
| Sepiolite | BO2/Sep | MB/Sep | BR18/Sep | BR2/Sep | |
|---|---|---|---|---|---|
| Absolute value of Zeta potential(mV) | 9.71 | 10.27 | 20.49 | 10.23 | 11.57 |
3.4. Stability
3.4.1. Chemical stability
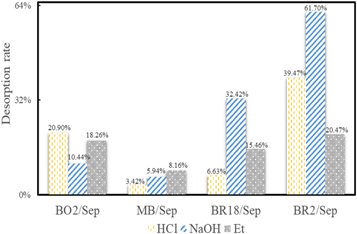
To evaluate the chemical stability of hybrid pigments, the four hybrid pigments were attacked by 1 mol l−1 HCl, 1mol/L NaOH and ethanol at 30 °C for 12 h. The supernatants gained after the samples were centrifuged were measured by a spectrophotometer (figure 8). The hybrid pigments prepared by cationic dyes with different functional groups have different desorption rates in the supernatants after inorganic/organic attack. A lower desorption rate of cationic dye in the supernatant indicated higher stability of the hybrid pigment.
Figure 8. Desorption rates of four hybrid pigments after inorganic/organic attack.
Download figure:
Standard image High-resolution imageIn the acidic medium, the supernatants of MB/Sep showed distinctively lower desorption rate and the BR18/Sep was not much different from MB/Sep, but that of BR2/Sep had the highest desorption rate. It was because H+ basically didn't interfere with electrostatic interaction between cationic dyes and sepiolite. And MB could enter the sepiolite channels and BR18 could partially insert into the sepiolite channels, so the resistance of the two hybrid pigments to the acidic was better. Although BO2 could also enter the sepiolite channels like MB, the –NH2 group in BO2 was prone to generate –NH2·HCl in HCl medium and H+ would adsorb oxygen on the surface of sepiolite. These two effects would weaken the hydrogen bonds between BO2 and sepiolite. As a result, the supernatants of BO2/Sep showed a higher desorption rate than MB/Sep. The resistance of BR2/Sep to the acidic was the worst because H+ also interfered with the formation of Sep/BR2 and BR2 couldn't enter the channels. In ethanol medium, the same trends were also observed and the resistance of four hybrid pigments to the acidic decreased according to order: MB/Sep, BR18/Sep, BO2/Sep, BR2/Sep.
In the NaOH medium, MB/Sep also showed the best chemical stability but was worse than in the acidic medium, and that of BR18/Sep significantly deteriorated. What's more, the stability of BO2/Sep was better in the alkaline medium than in the acidic medium and BR2/Sep was still the worst. For MB/Sep, OH− in the medium and the negative electrostatic surface of sepiolite would generate competitive adsorption to the R–N+ groups in MB. Therefore, the combination of MB and sepiolite was disturbed and the stability of MB/Sep was worse than in the acidic medium. Similarly, the formation of BR18 and sepiolite was also disturbed by OH−. But BR18 could not completely enter the channels like MB and the resistance of BR18/Sep to the NaOH significantly deteriorated. Based on FT-IR, the BO2 and BR2 mainly interacted with sepiolite through hydrogen bonds, which were also disturbed by OH−. The interference in BR2/Sep was stronger due to two –NH2 groups in the BR2 (one –NH2 group in the BO2). What's more, BO2 could enter the channels and be protected by sepiolite. Therefore, the resistance of four hybrid pigments to the NaOH decreased as the order: MB/Sep, BO2/Sep, BR18/Sep, BR2/Sep.
Regardless of the types of medium, the stability of MB/Sep hybrid pigment was the best and that of BR2/Sep was the worst in four hybrid pigments. Based on the above discussion, the resistances of hybrid pigments to the organic/inorganic attack were closely related to functional group types of the cationic dyes and the locations of cationic dyes in sepiolite. And it can be concluded that H+ could weaken hydrogen bonds in hybrid pigments and OH− could interfere not only with hydrogen bonds but also with electrostatic interaction between cationic dyes and sepiolite. What's more, the sepiolite could protect cationic dyes which completely entered or partially inserted channels from inorganic/organic attack.
3.4.2. Weatherability
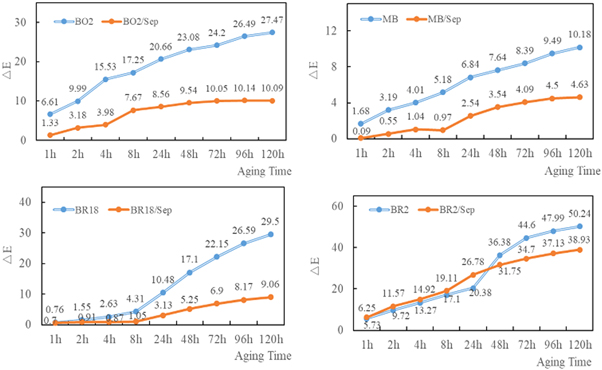
To evaluate the weatherability of four hybrid pigments, the chroma of samples exposed under an ultraviolet lamp for 120h was measured. The color difference (ΔE) was calculated by the chroma. The color difference reflected the color stability of the hybrid pigments, the smaller the color difference (ΔE), the better the weatherability of the hybrid pigments. Except for BR2/Sep, the color difference of the hybrid pigments was always lower than that of the pure cationic dyes. In addition, with the extension of the UV lamp irradiation time from 1 to 120 h, the difference between hybrid pigments and pure dyes showed an increasing trend (figure 9). It can be observed from figure 9 that the weatherability of four hybrid pigments was superior to that of the corresponding pure cationic dyes after 120 h, which meant the weatherability of the pigments could be improved by the hybrid of sepiolite and cationic dyes.
Figure 9. Color difference (ΔE) of four cationic dyes and hybrid pigments under UV light. (a) BO2, BO2/Sep;(b) MB, MB/Sep;(c) BR18, BR18/Sep;(d) BR2, BR2/Sep.
Download figure:
Standard image High-resolution imageThe improvement degree of the weatherability of cationic dyes after composited with sepiolite was expressed by the color difference changing rate (figure 10). A lower color difference changing rate indicated a higher improvement degree for the cationic dye. After 120 h, the color difference changing rate of BR18/Sep was lowest in all hybrid pigments, which showed the highest improvement degree. The improvement mainly was attributed to BR18 partially inserting the sepiolite channels and sepiolite protected BR18 from UV lamp. For MB/Sep, the weatherability of the MB self was better than the other three pure cationic dyes according to figure 9, as a result, although MB was encapsulated in the sepiolite channels, the improvement degree was not the highest. In addition, the BR2 could not enter the sepiolite channels and only adsorb on the outer surface of the sepiolite, so the improvement rate of hybrid pigments in weatherability was lower.
Figure 10. Color difference changing rate (%) between pure cationic dyes and hybrid pigments. (Color difference changing rate = (ΔE hybrid pigment-ΔE cationic dye)/ΔE cationic dye).
Download figure:
Standard image High-resolution imageIn general, the weatherability of four hybrid pigments obviously was improved compared to pure cationic dyes. The cationic dyes which could completely enter or partially insert the channels performed better in weatherability. In addition, the weatherability of hybrid pigment was closely related to weatherability of cationic dyes. The improvement degree of hybrid pigments in weatherability decreased according to the order: BR18/Sep, BO2/Sep, MB/Sep, BR2/Sep.
4. Mechanism of action
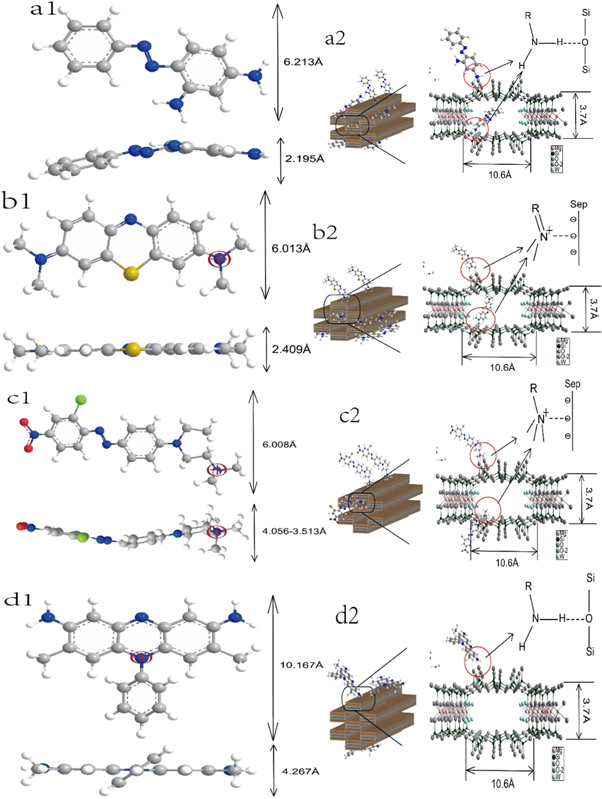
Based on XRD, TEM, TG and BET discussions, it can be concluded that four cationic dyes existed in different positions of sepiolite. The size of cationic dyes was considered and calculated in Chembio 3D software (figures 11(a1)–(d1)). Combining the FT-IR and functional groups of cationic dyes, the mechanism for the action between cationic dyes and sepiolite could be revealed (figures 11(a2)–(d2)).
Figure 11. Molecular structures of four cationic dyes and mechanisms of action of cationic dyes. (a1) BO2, (a2) BO2/Sep; (b1): MB, (b2) MB/Sep; (c1) BR18, (c2) BR18/Sep; (d1) BR2, (d2):BR2/Sep
Download figure:
Standard image High-resolution imageThe sizes of MB and BO2 are smaller than that of sepiolite channels (3.7 × 10.6 Å). Therefore, MB and BO2 could enter the sepiolite channels (figures 11(a1)–(d1)). The size of one end of BR18 by rotation is comparable to the size of sepiolite channels (figure 11(c1)). Thus, one end of BR18 can insert the sepiolite channels. However, the size of BR2 is larger than that of sepiolite channels (figure 11(d1)). As a result, BR2 can't enter the sepiolite channels. Based on FT-IR, it can be observed that the MB and BR18 interacted with sepiolite mainly through electrostatic adsorption (figures 11(b2), (c2)). The BO2 and BR2 interacted with sepiolite mainly through the hydrogen bonds between the –NH2 group and sepiolite (figure 11(a2), (d2)).
In general, MB could enter the sepiolite channels and adsorb on the inner and outer surface. MB/Sep hybrid pigment showed excellent resistances of organic and inorganic. The BR2 was difficult to enter the sepiolite channels due to the large size. Therefore, the sepiolite could not protect BR2 from organic/inorganic attack and the chemical stability of BR2/Sep hybrid pigment was the worst. The BO2 was encapsulated in sepiolite channels, the stability of BO2/Sep was superior to that of BR2/Sep. In addition, in HCl medium, the –NH2 group in BO2 readily formed –NH2·HCl group with HCl, which would weaken hydrogen bonds. However, in NaOH medium, –NH2·HCl was prone to generate –NH2 groups which strengthened hydrogen bonds. Therefore, the stability of BO2/Sep hybrid pigments in NaOH medium was better. For BR18, the σ-bond between R–N+ could rotate (the rotation range was from 4.056 Å to 3.513 Å). As a result, the BR18 could partially insert the sepiolite channels. In NaOH medium, OH− could interfere with the electrostatic adsorption between BR18 and sepiolite, so the BR18/Sep hybrid was more stable in HCl medium.
5. Conclusions
In summary, four sepiolite hybrid pigments were prepared successfully. Through the comparative study of four hybrid pigments, the effects of cationic dyes with different structures and functional groups types on the stability and structures of sepiolite hybrid pigments were revealed. The greater the degree to which cationic dyes enter the sepiolite, the sepiolite provided more protection. And the stability of the pigment and the loading capacity of cationic dyes in sepiolite hybrid pigments were higher. Among them, MB and BO2 with smaller sizes than sepiolite channels could enter the channels and BR18 whose size was comparable to that of sepiolite channels could partially insert into the channels by rotation. These hybrid pigments showed better stability and a more obvious improvement degree for weatherability in hybrid pigments compared with pure cationic dyes. The BR2 with a large size could not enter into the sepiolite channels, which performed less well. The loading capacity of cationic dyes in sepiolite hybrid pigments decreased as the order MB/Sep, BO2/Sep, BR18/Sep, BR2/Sep. In addition, the functional groups types of cationic dyes also were important factors affecting the performance of hybrid pigments. The electrostatic interaction (in MB/Sep and BR18/Sep) was easily weakened by the H+ and OH−. The hydrogen bond (in BO2/Sep and BR2/Sep) was easily attenuated by the H+. Hence, the above work provides guidance for the selection of cationic dyes with appropriate structures and functional groups to prepare various stable sepiolite hybrid pigments.
Acknowledgments
The research and publication fees are supported by the National Natural Science Foundation of China with the project name of Key Parameter Test and Intelligent Evaluation System of Gas-bearing Shale (award number: 41927801).
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).