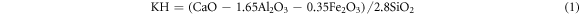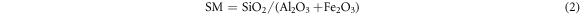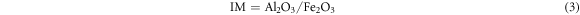Abstract
This work reported a process for the preparation of Portland cement clinker from sulfuric acid leaching residue of coal fly ash. Aluminum and iron in fly ash were effectively leached by sulfuric acid leaching, and the silica was all concentrated in the acid leaching residue. The chemical analysis showed that the content of SiO2 in the leaching residue was 79.1% and the leaching residue contained a small amount of Al2O3, CaO and Fe2O3, which could replace clay as the raw material for the preparation of Portland cement clinker. The effects of main process conditions on the quality of prepared cement clinker were studied. The results showed that the burnability of the raw material with clinker rate value KH = 0.92, SM = 2.1, IM = 1.2 and leaching residue content of 26.98% was excellent and good. The mineralogical analysis and microscopic examination showed that the good quality clinker was obtained under the conditions of sintering temperature of 1450 °C and holding time of 60 min, and the tricalcium silicate phase was well developed. Based on this study, acid leaching residue of coal fly ash was viable as an effective, alternative raw material in Portland cement production.

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
This article was updated on 18 May 2022 to correct one of the funding numbers.
1. Introduction
As an important building material, cement is widely used in emerging industries and engineering construction, such as industrial construction, civil construction, transportation engineering, hydraulic engineering, seaport engineering, national defense construction and so on. In 2020, China's cement output was 2.4 billion tons, with a year-on-year increase of 2.5% [1]. The cement raw material is obtained by mixing and grinding calcium material, siliceous material, aluminum material, iron material and correction raw material in appropriate proportions. The cement clinker is prepared by sintering and grinding the raw material. At present, the main raw materials are limestone, clay, iron powder, gypsum, admixture, etc Large amounts of non-renewable natural mineral resources are consumed every year [2].
The comprehensive utilization of industrial solid waste to produce cement is a global research hotspot. Zhang [3] prepared Portland cement from red mud after calcification−carbonization treatment at 1450 °C for 60 min, and the strength of obtained clinker was more than 425 cement and close to 525 cement. Hong [4] used silicon calcium slag which contained about 40% calcium oxide and 12% silica to replace some of the cement raw material to prepare cement clinker. The effect showed that adding silicate calcium slag into cement raw materials could improve the burnability of raw material and reduce the sintering temperature of clinker. Kuang [5] prepared Portland cement from stone coal acid-leaching vanadium slag at 1450 °C for 30 min, and the cement clinker met the Chinese standards. Puertas et al [6] explored the reactivity and burnability of cement raw mixes ceramic wastes as alternative raw materials. The results showed that the new raw mixtures containing this kind of waste were technically feasible and had higher reactivity and burnability than conventional mixtures. Young et al [7] reported a type of high-magnesium and low-silicon iron ore tailings (IOT) utilized as a raw material replacing clay to produce cement clinkers by conventional sintering process. The Research suggested that the IOT had huge potential as cement raw materials instead of partial natural resources. Schoon et al [8] showed the fines fractions generated out of concrete recycling could be used as an alternative raw material for Portland clinker production.
Coal fly ash (CFA) is a kind of industrial waste with large production and storage capacity [9]. Since the main chemical composition of fly ash is similar to that of clay, it can be used to replace clay for the production of cement clinker [10, 11]. The application of fly ash in cement production can be divided into two ways [12], one is the use of fly ash as cement mixed material to improve the performance of cement clinker by using its potential chemical activity [13, 14]. The other uses fly ash as cement raw material. Wu et al [15], Kleib et al [16] and Wu et al [17] studied the preparation of alinite cement, Portland cement, and sulfoaluminate cement by using municipal solid waste incineration (MSWI) fly ash as alternative raw material, respectively, and all of which could produce good quality cement clinker under suitable conditions. Li et al [18] prepared sulphoaluminate-alite cement from high alumina fly ash. XRD analysis indicated that the optimal mineralogical phase could be obtained at 1300 °C, and C4A3S reached the strongest intensity. Darweesh [19] studied the effect of the fly ash content and clinkering temperature on the quality of cement clinker. The results indicated the optimum fly ash content and clinkering temperature for synthesizing cement were 35 wt% and 1350 °C, respectively. Komljenović et al [20] performed the chemical, mineralogical and thermal characterization of fly ash in order to determine the possibility of its use as the raw material for the cement industry. In summary, it is feasible to prepare Portland cement from fly ash.
Al, Fe can be effectively leached by acid leaching method with HCl, NH4HSO4 and H2SO4. Valeev et al [21] investigated the hydrochloric acid leaching of aluminum from fly ash and the Al extraction efficiency could be higher than 90% under optimum conditions. The extraction rate of alumina from fly ash could reach 86.7% in NH4HSO4 leaching at 180 °C for 240 min [22]. After acid leaching of fly ash with sulfuric acid, most of the metals were converted into soluble sulfate and entered the leaching solution, and the main chemical composition of the leaching residue was SiO2 [23]. In this paper, the feasibility of substituting ordinary raw materials with acid leaching residue of fly ash was studied in Portland cement production. The effects of sintering temperature and holding time on the phase formation were investigated.
2. Experimental
2.1. Experimental materials
The experimental raw material was fly ash from Shuozhou Second Power Plant of China, and the composition of fly ash was analyzed by XRF spectral qualitative analysis combined with chemical total analysis, the result was shown in table 1. The main chemical composition in fly ash were SiO2, Al2O3, Fe2O3 and CaO. The physical phase composition of fly ash was analyzed by XRD technique, and the result was shown in figure 1. The main physical phases of fly ash were mullite (3Al2O3·2SiO2), sillimanite (Al2O3·SiO2) and quartz (SiO2).
Table 1. The main chemical composition of coal fly ash (%).
| SiO2 | Al2O3 | Fe2O3 | CaO | SO3 | MgO | C |
|---|---|---|---|---|---|---|
| 41.5 | 40.9 | 2.26 | 3.30 | 0.253 | 0.330 | 2.47 |
Figure 1. XRD patterns of fly ash.
Download figure:
Standard image High-resolution imagePrevious research results showed that the aluminum and iron in the fly ash were efficiently leached by the sulfuric acid leaching at high temperature. With the leaching temperature of 230 °C, the sulfuric acid mass fraction of 20%, the reaction time of 120 min and the liquid-solid ratio of 10:1, the leaching efficiency of Al from the fly ash was 89.43%, and the leaching efficiency of Fe from the fly ash was 69.78%. The main chemical composition of the acid leaching residue obtained after the sulfuric acid leach treatment was shown in table 2. The main chemical composition of acid leaching residue was SiO2, with a content of 79.1%, which contained a small amount of non-leached Al2O3, CaO and Fe2O3. The XRD patterns of leaching residue was shown in figure 2. The mullite phase in fly ash was destroyed by sulfuric acid, so SiO2 and Al2O3 in leaching residue existed in their own crystal form.
Table 2. The main chemical composition of acid leaching residue (%).
| SiO2 | Al2O3 | Fe2O3 | CaO | SO3 | MgO | C |
|---|---|---|---|---|---|---|
| 79.4 | 7.53 | 1.19 | 1.94 | 1.59 | 0.218 | 1.49 |
Figure 2. XRD patterns of acid leaching residue.
Download figure:
Standard image High-resolution imageThe reagents used in the experiments were listed in table 3.
Table 3. Reagents used in the experiments.
| Name | Purity | Manufacturer |
|---|---|---|
| CaO | AR | Sinopharm Reagent Co., Ltd |
| Al2O3 | AR | Sinopharm Reagent Co., Ltd |
| Fe2O3 | AR | Sinopharm Reagent Co., Ltd |
| NaOH | AR | Sinopharm Reagent Co., Ltd |
| Ethanol absolute | AR | Xilong Scientific Co., Ltd |
| Glycerol | AR | Xilong Scientific Co., Ltd |
| Benzoic acid | AR | Xilong Scientific Co., Ltd |
2.2. Sample preparations
The production of cement clinker was controlled by clinker rate value. Lime saturation ratio (KH) indicated the degree to which silicon oxide in clinker was saturated by calcium oxide into tricalcium silicate; Silica rate (SM) represented the mass percentage of SiO2 to Al2O3 and Fe2O3 in the clinker, reflecting the quality and burnability of the clinker; Alumina rate (IM) referred to the mass percentage of Al2O3 to Fe2O3 in the clinker, too high IM would make the liquid phase viscous and the material was hard to burn. The calculation formulas of clinker rate value were as follows [24, 25]:
Typically, the rate value parameters are controlled at KH values of 0.90–0.95, SM values of 1.7–2.7, and IM values of 0.8–1.7 [3, 5]. Here, KH, SM, and IM were set to 0.92, 2.10, and 1.20, respectively. Analytical pure CaO, Al2O3 and Fe2O3 were selected to supplement the missing components in the raw material. The numerical optimization tool (Solver, Excel®) [3, 26] was used to calculate the composition of each raw material, with the maximum amount of leaching residue as the target for the planning solution and the results represented that the maximum leaching residue content in the raw material was 26.98%. The potential mineral composition of the clinker and the composition of the raw materials were listed in table 4.
Table 4. Composition of the raw material and potential mineral composition (%).
| SiO2 | Al2O3 | Fe2O3 | CaO | C3S | C2S | C3A | C4AF |
|---|---|---|---|---|---|---|---|
| 21.68 | 5.63 | 4.69 | 66.78 | 61.29 | 16.03 | 6.99 | 14.26 |
The process flow of comprehensive utilization of valuable elements in fly ash by sulfuric acid leaching was shown in figure 3.
Figure 3. Comprehensive utilization process of fly ash.
Download figure:
Standard image High-resolution imageThe experimental method for the preparation of cement clinker from acid leaching residue of fly ash was as follows: First, the component raw materials were mixed and milled to all passed through the 0.08μm sieve, distilled water was added to knead into a ball, and the diameter of the raw material pellets were controlled at about 1cm. Then, the dried raw pellets at 100 °C for 10h were put into the box resistance furnace and heated up to 900 °C with a heating rate of 6 °C min−1 and held for 30 min. Next, the temperature was raised to the target value at the same rate. The raw material was removed immediately after a period of sintering and allowed to cool rapidly to room temperature in the air. Finally, the prepared clinker was milled again to all pass through the 0.08μm sieve and bagged for storage.
2.3. Characterization
The phase of clinkers produced were analyzed by x-ray diffraction (PW3040/60, Panalytical, Netherlands). Free lime (f-CaO) content was detected by the glycerol-ethanol method. Furthermore, the microstructure of cement clinkers was studied on metalloscope (Axis-10, Carl Zeiss, Germany).
3. Results and discussion
3.1. Burnability of raw material
The f-CaO content reflected the burnability of the cement raw material, and the lower the f-CaO content indicated that a better burnability of the raw material. The f-CaO content of the clinker at different sintering temperatures was shown in figure 4.
Figure 4. f–CaO content of cement clinker at different temperatures.
Download figure:
Standard image High-resolution imageIt could be seen from figure 4 that the f-CaO content decreased with the increase of sintering temperature. The f-CaO content was high at the low temperature and decreased sharply as the sintering temperature increased. When the sintering temperature was 1400 °C, the f–CaO content was 1.89%, and continuing to increase the temperature to 1450 °C, the f-CaO content was lower than 1%. Although the content of f–CaO at 1500 °C was lower than that at 1450 °C, the change was small.
3.2. Effect of sintering temperature
To study the effect of sintering temperature on the quality of the produced clinker, the raw material was sintered at different temperatures (1250 °C, 1300 °C, 1350 °C, 1400 °C, 1450 °C, 1500 °C) for 60 min. XRD patterns of the sintered samples were shown in figure 5. Quantitative analysis was done according to the XRD results, and the contents of each phase at different temperatures were shown in figure 6.
Figure 5. XRD patterns of clinker at different temperatures.
Download figure:
Standard image High-resolution imageFigure 6. The contents of each phase of clinker at different temperatures.
Download figure:
Standard image High-resolution imageIt could be seen from figure 5 that the diffraction peaks of Ca(OH)2 and CaO were still sharp with higher peaks at 1250 °C. With the increase of sintering temperature, the diffraction peaks were gradually flattened and almost disappeared at 1350 °C. C2S began to appear when the sintering temperature reached 1250 °C, and C3S began to form when the temperature increased to 1300 °C. It could be seen from figure 6 that the main phases in the clinker prepared at 1350 °C were C3S, C2S, C3A and C4AF, which had met the phase composition of Portland cement. The further increase of temperature made C3S well developed, and the C3S diffraction peak was the sharpest with the highest peak at 1450 °C. The phase composition of the clinker changed little when the sintering temperature increased to 1500 °C.
The apparent morphology of cement clinker prepared at different sintering temperatures were shown in figure 7. It could be seen from figure 7 that when the sintering temperature was 1250 °C, the obtained clinker was light yellow with white spots on the surface, indicating that C2S began to form, accompanied by the formation of C3A and C4AF. At 1300 °C, the color of clinker deepened to gray, on which black spots began to appear. The color turned to brownish yellow at 1350 °C and the black part became more, which implied that C3S was produced in large quantities. The clinker appeared powdering at 1300 °C and 1350 °C, which indicated that C2S reached the maximum at this stage. During the cooling process of some C2S, there was no time to cool down and crystal transformation occurred. The clinker prepared at 1400 °C and 1450 °C were both black, but the volume shrinkage of clinker prepared at 1450 °C was greater, the hardness was higher, and the structure was denser. The clinker prepared at 1500 °C began to melt.
Figure 7. The appearance of the clinker at different temperatures.
Download figure:
Standard image High-resolution imageThe microstructure of cement clinker prepared at 1400 °C and 1450 °C were shown in figure 8. The pictures were taken after the cooled pellets were corroded in 1% nitric acid alcohol solution for 6s. C2S was shown as round granular, and there were often two groups of crossed parallel lines on the surface. C3S was shown as pseudo hexagon flake or short prismatic. It could be seen from figure 7 that C2S and C3S existed in the clinker prepared at 1400 °C, and their contents and sizes were similar. C3S in the clinker prepared at 1450 °C was more pronounced, with clearer edges and larger dimensions.
Figure 8. The microstructure of cement clinker at different temperatures.
Download figure:
Standard image High-resolution imageThe f-CaO content in the clinker is required to be less than 1.5% (mass fraction) [27]. The Portland cement clinker meeting the standard requirements could be prepared under the sintering temperature of 1450 °C and 1500 °C. The main contributor to the strength of cement clinker is C3S, whose content, crystal form and crystal morphology have a great influence on the clinker. The content of C3S in ordinary Portland cement ranges typically from 55% to 65% [28, 29]. Normally, C3S formation is effectively complete at a sintering temperature of about 1450 °C [30]. Therefore, the optimum sintering temperature for the preparation of Portland cement clinker from acid leaching residue was 1450 °C.
3.3. Effect of holding time
To study the effect of holding time on the quality of the produced clinker, the raw material was sintered at 1450 °C for different time (10 min, 30 min, 60 min, 90 min, 120 min). XRD patterns of the sintered samples were shown in figure 9. Quantitative analysis was done according to the XRD results, and the contents of each phase at different holding time were shown in figure 10.
Figure 9. XRD patterns of clinker at different holding time.
Download figure:
Standard image High-resolution imageFigure 10. The contents of each phase of clinker at different holding time.
Download figure:
Standard image High-resolution imageIt could be seen from figure 9 that the material phases in the clinker prepared at 1450 °C were C3S, C2S, C3A and C4AF, and their diffraction peaks did not change much in the holding time interval of 10–120 min. Combined with figure 10, it could be seen that the highest C3S content was found in the interval of holding time of 30–60 min. Extending the holding time, the content of each phase changed little.
The apparent morphology of cement clinker prepared at different holding time were shown in figure 11. The clinker prepared at 10 min showed obvious pores and the structure was not compact enough, and the pores decreased relatively when the holding time was extended to 30 min. At a holding time of 10–30 min, small white spots could be seen on the surface of the clinker. The surface of clinker prepared at 60–120 min was dense, no obvious pore structure could be seen, and the volume of clinker was relatively reduced. It could be clearly perceived that the hardness of the 60 min clinker was higher relative to that of the 10–30 min during grinding, which indicated that the structure of the clinker prepared at 60 min was dense and the phases were well developed. The clinker prepared at 120 min appeared molten and irregularly shaped.
Figure 11. The appearance of the clinker at different holding time.
Download figure:
Standard image High-resolution imageThe microstructure of cement clinker prepared at 10–120 min were shown in figure 12. It could be seen from figure 12 that with the extension of the holding time, the phase in the clinker continued to develop and gradually increased in size. There were not enough C3S in the clinker prepared at 10–30 min, indicating that C2S was producing to C3S. The phase in the clinker prepared at 60–90 min was well developed with a large size and clear edges, and C3S was the main phase. Continued to extend the holding time to 120 min, the contour of C3S became coarse and no longer clear, and C2S partly transformed into finger-like and dendritic. In summary, the optimum holding time for the preparation of Portland cement clinker from acid leaching residue was 60 min.
Figure 12. The microstructure of cement clinker at different holding time.
Download figure:
Standard image High-resolution imageThe chemical composition of the Portland cement clinker obtained under optimal conditions of sintering temperature 1450 °C and holding time 60 min was shown in table 5. The content of MgO and SO3 were very little, far less than the national standard requirements of 5% and 1.5% [27, 31].
Table 5. Composition of the Portland cement clinker obtained under optimal conditions (%).
| CaO | SiO2 | Al2O3 | Fe2O3 | MgO | TiO2 | SO3 |
|---|---|---|---|---|---|---|
| 65.6 | 21.2 | 6.81 | 4.83 | 0.55 | 0.459 | 0.209 |
4. Conclusions
In this work, we explored the feasibility and optimal process conditions for the preparation of Portland cement clinker from sulfuric acid leaching residue of coal fly ash. The following conclusions could be drawn:
- (1)The raw material was configured with clinker rate value KH = 0.92, SM = 2.1, IM = 1.2 and leaching residue content of 26.98%. The f-CaO content was less than 2% in the cement clinker prepared at 1400 °C and less than 1% in the cement clinker prepared at 1450 °C. This indicated that the raw material had good burnability.
- (2)The optimum conditions for the cement preparation process were sintering temperature of 1450 °C and holding time of 60 min. The mineralogical analysis and microscopic examination showed the clinker prepared under optimal conditions had well developed C3S crystals with the best quality and the highest quantity.
Acknowledgments
The present study was financially supported by the National Natural Science Foundation of China (No. U1710257), Scientific and Technological Innovation Programs of Higher Education Institutions in Shanxi (No. 2019L0656), Doctoral Research Foundation of Taiyuan University of Science and Technology, China (No. 20142001), Open Foundation Program of Key Laboratory for Ecological Metallurgy of Multimetallic Mineral, Ministry of Education, China (No. 2020002) and Supported by Fundamental Research Program of Shanxi Province, China (No. 202103021224281).
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).