Abstract
Steam oxidation is the main limited factor for the heat-resistant steel in fossil power plant. Disclosing the oxidation mechanism and kinetics could be helpful for the daily inspection to ensure the safety operation. To investigate the water vapor oxidation behavior of Super304H, the oxidation test was conducted at 600 ℃ for 25, 50, 100, 150 and 200 h in flowing steam. The structure and compositions of the oxide layers were investigated by line-scanning of scanning electron microscopy (SEM) equipped with energy dispersive spectroscopy (EDS). Surface morphology was observed after the oxidation test. The oxide phases were identified by XRD. The results showed that the oxide layer was duplex structure consisted of a Fe3O4 - Fe2O3 outer layer and an inner oxide layer which contained nickel oxide, Cr2O3, and FeCr2O4. As the oxidation time increased, the thickness of the total oxide layer and inner layer both increased. After 100 h oxidation, oxide layer exfoliation occurred and resulted in rapid growth of new oxide layer in the exfoliation region.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Heat resistant steel is the most important structure materials in fossil power plant. In recent years, increasing operating temperature is widely regarded as effective strategy to achieve higher efficiency in the power plant, but also proposes higher requirements in high temperature properties to these steels, especially the oxidation resistance [1–3]. The long term oxidation would lead to the wall loss and long-term overheating resulting from the oxides exfoliation [4, 5]. The oxidation behavior of heat resistant steels such as T22, SA32654, P92, 441 has been widely studied in laboratory and in service application, as shown in table 1 [6–8]. Results suggested that the oxide layer was consisted with an inner Cr rich Fe-Cr spinel oxide layer and an outer Fe rich porous oxide layer. The oxidation rate greatly depends on the compositions, grain size and the stability of the oxide layer. Thus, elements alloying have been developed to improve the oxidation resistance and results suggested that Mo, W, Ce could be helpful in improving the strength in elevated temperature but not so efficient in improving the oxidation resistance [9–11]. At the same time, many researchers turned to develop surface modification techniques including peening and protective coatings [12, 13]. Thermal sprayed coatings with Ni, Cr, Al, which are dense oxide layer forming elements or high temperature stable elements, are widely studied for oxidation protection. Results indicated the effectiveness of these coatings as protective coatings and the function could be enhanced with the increase of Cr and Al, achieving half oxidation rate with 50% Cr [14, 15]. The principle of these strategies is to increase the chromium content to form a dense inner Cr2O3 coating to segregate inner substrate from the oxidation atmosphere to improve the oxidation resistance [16]. Inspired by this principle, 18Cr–9Ni–3Cu–Nb–N austenite steel (Super304H) has been developed as superheater and reheater tubes materials in fossil power plant [17]. The steel has high content of Cr to provide excellent oxidation resistance. Moreover, the addition of copper and nitrogen could increase the creep strength at elevated temperatures [18].
Table 1. Oxidation behavior of heat-resistance steel.
| Materials | Oxidation details | Kinetic and structure | References |
|---|---|---|---|
| T22 | Static supercriticalwater (SCW) at 550 °C–600 °C for 1000h | Follow parabolic law;The inner layer is a compact Cr-rich spinel, the outer layer is a porous Fe-rich oxide. A thin transition layer is observed. | [6] |
| T22 | steam oxidation at 544 °C to 585 °C for 200kh | An inner layer of Fe–Cr spinel and an outer layer of magnetite, with a thickness ratio of approximately 1:1. Exfoliation of flakes of scale occurs by separation along an interface between laminates in the inner layer, and involves the full thickness of the magnetite layer and part of the spinel layer including multi-laminations. Regrowth of the outer magnetite layer is rapid, and is accompanied by thickening of the immediately subjacent spinel laminate such that a thick magnetite-spinel pair is re-established on the outside of the scale. | [7] |
| P92 | Static supercriticalwater (SCW) at 550–600 °C for 1000h | Near cubic rate equations; The inner layer is a compact Cr-rich spinel, the outer layer is a porous Fe-rich oxide; A thin transition layer is observed | [6] |
| ferritic 441 316L | Ar2 + H2O at 85 °C for 20 min | A few microns thick main layer of Cr2O3 mixed with (Mn,Cr)3O4. More spinel phase, with Fe,Mn and Cr are present on the outer part. A thin layer of SiO2 forms a barrier. | [8] |
| S32654 | In air at 900 °C for 1–5 h | Inner FeCr rich oxides and outer Mo oxide, Fe Cr oxides promote the diffusion of Mo | [9] |
| ferritic stainless steel+W/Ce | in air at 1000 °C for 0–5 h | The mass gain of F4 steel at 950 °C and 1000 °C were 1.1 mg cm−2 and 1.35 mg cm−2, respectively, and they were lower than other steels Compact oxide layer | [10] |
| Fe-20Cr-25Ni-Nb | He+H2O at 1000 °C for 4h | The oxidation kinetics follows between the linear and parabolic rate law before the transition point, while it is very close to the parabolic rate law after the transition point. Fe2Nb Laves phases at the matrix/oxide interface can induce the micro-cracks in the oxide scale and promotes the formation of the re-generated Cr2O3 phases. | [11] |
Previous laboratory research on austenitic stainless steels suggested that steam oxidation of Super304H steels led to the development of double layer oxide scales over 500 °C [19]. The inner oxide layer consisted of Fe–Cr spinel particles embedded in a metallic Fe–Ni network [20]. The outer layer consists of Fe2O3 on top of Fe3O4 after the oxidation in water vapor at 700 °C, while a self-healing chromium-rich layer has been formed at the oxide/metal interface and significantly reduces the oxidation rate in the temperatures range from 499 to 628 °C [21]. Though the structure of oxide film of 18-8 stainless steel has been widely studied, however, little search focus on the water vapor oxidation behavior of Super304H. As the representation of new austenitic stainless steel, Super304H revealed excellent oxidation resistance in comparison with other SUS316 steels because of the smaller grain. In recent years, Super304H has been widely used for high-parameter steam turbine which makes it more important to investigate the oxidation behavior of Super304H in water vapor. However, most of the oxidation experiments were carried out in air or confined space filled with water such as high pressure actors, which made the results just helpful in understanding the oxidation mechanism. The lack of steam would lead to a relative slower oxidation rate and the static steam could not simulate the oxide layer spallation of Super304H served in flow steam.
Thus, a simulated oxidation system with flowing steam has been made to study the oxidation behavior of Super304H. In our previous study, the short-term water vapor oxidation of Super304H has been studied less than 4 h to investigate the initial oxidation mechanism [22]. However, the long term oxidation of Super304H in flowing steam is still inadequate which could be helpful for the tube maintenance during service [23]. In this paper, the microstructural evolution and oxidation kinetic of the oxide films of Super304H after long term exposure in water vapor. The results should be useful for the understanding of the long term oxidation mechanism and application of Super304H in power plant.
2. Experimental detail
The specimens used in this study were commercial Super304H tubes, as-received from thermal power plant produced by Sumitomo Metal Industries Ltd. The stand compositions and mearsured composition s were listed in table 2. The as-received Super304H presents hardness about 176 HV and yield strength of 304 MPa. The samples were prepared with a size of 5 × 5 × 2 mm3. Firstly, samples were grounded by SiC sand paper down to a 1400 grit and followed polished by diamond grinding paste to a mirror surface. Then, pickling and cleaning process were employed. The length axis of steel samples were designed paralleled to the pure water vapor gas flow and exposed to a horizontal furnace at 600 °C for 25, 50, 100, 150, and 200 h, respectively. The oxidation system was consisted with a furnace, a steam generator and a thermal couple. The steam was produced by a steam generator and furtherly heated to 600 °C in the furnace and the samples were hold in the last half where a thermal couple was equipped to monitor the steam temperature.
Table 2. Compositions of Super304H.
| Elements | C | Mn | Si | Cr | Mo | W | V | S | P | Ni | Nb | N | Cu | Al | B | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ASME content (at. %) | 0.08 | 0.66 | 0.2 | 18.3 | 0.004 | 0.017 | 8.72 | 0.52 | 0.10 | 3.02 | 0.008 | 0.005 | rest | |||
| EDS results | 20.34 | 8.57 | 2.75 | 68.34 |
The mass variation of the specimens with different oxidation time was determined by an analytical balance with a sensitivity of 1 × 10-5g. After oxidation tests, the surface and cross sections of the samples were investigated by FEI Sirion IMP scanning electron microscope (SEM) system equipped with an energy dispersive spectroscope (EDS). The phase structure of the oxide film were determined by X-ray diffraction (Bruker-axs D8 with a Cu kα radiation (0.15418 nm).
3. Results
3.1. Surface morphology of the oxide layer
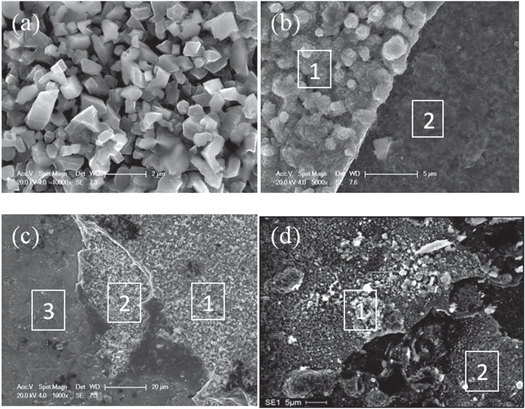
The surface morphologies of the samples oxidized for 50,100,150, and 200h are shown in figure1 and the compositions of marked areas are presented in table 3. For the sample oxidized for 50 h, the grains of oxide with a size of about 1 μm were observed and formed porous structure. EDS analysis revealed that these oxide grains consisted of 55 at%O, 44 at%Fe and 1 at%Cr, indicating that the top surface oxides were Fe3O4. As shown in figure 1(b), (c) and (d), further increase in oxidation time led to obvious spallation. The compositions of top layer (marked as 1) was rich in Fe, O with small amount of Cr, which were identical to samples oxidized 50 h. However, the chemical compositions of spallation areas (marked as 2) were quite different with 10 at% Cr which was similar to that the inner layer. It suggested that the peeled layer should be the outer layer. In sample oxidized for 150 h, three different areas could be observed. The Cr content of the area marked as 3 was 20 at% indicated that the spallation of the outer oxide layer led to quickly regrowth of the oxide layer in the underlying layer or substrate.
Figure1 Surface morphologies of S304H oxidized for (a) 50h, (b)100h, (c) 150h, (d)200h.
Download figure:
Standard image High-resolution imageTable 3. EDS results of the surface of Super304H oxidized for various time.
| Sample | Area | O | Fe | Cr | Ni |
|---|---|---|---|---|---|
| 50H | 54.0 | 44.5 | 1.5 | ||
| 100h | 1 | 53.8 | 45.0 | 1.1 | |
| 2 | 53.2 | 38.4 | 8.4 | ||
| 150h | 1 | 53.8 | 46.2 | ||
| 2 | 36.3 | 58.9 | 4.9 | ||
| 3 | 55.7 | 24.2 | 20.2 | ||
| 200h | 1 | 54.9 | 32.1 | 10.8 | 2.1 |
| 2 | 54.1 | 33.2 | 10.9 | 1.8 |
3.2. Oxidation kinetic
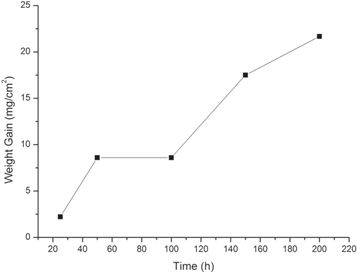
Figure 2 shows the oxidation kinetic curve of Super304H steel oxidized at 600 °C. The oxidation kinetic was neither the reported linear law nor the parabolic law [24]. Initially, the mass gain rate increased from 2.2 to 8.6 mg cm−2 as the oxidation time increased from 25 h to 50 h. But further increase of the oxidation time did not lead to increase of mass gain rate, which suggested that mass loss occurred and compensated the oxidation weight gains. The area where mass loss occurred would be quickly re-oxidized which have been observed from figure1(c) (d). That is the reason for the mass gain rate increased again and reached 21.7 mg cm−2, when the oxidation time increased from 100 h to 200 h,
Figure 2. Oxidation kinetic curve of Super304H at water vapor oxidation at 600 °C.
Download figure:
Standard image High-resolution image3.3. Composition and phase structure
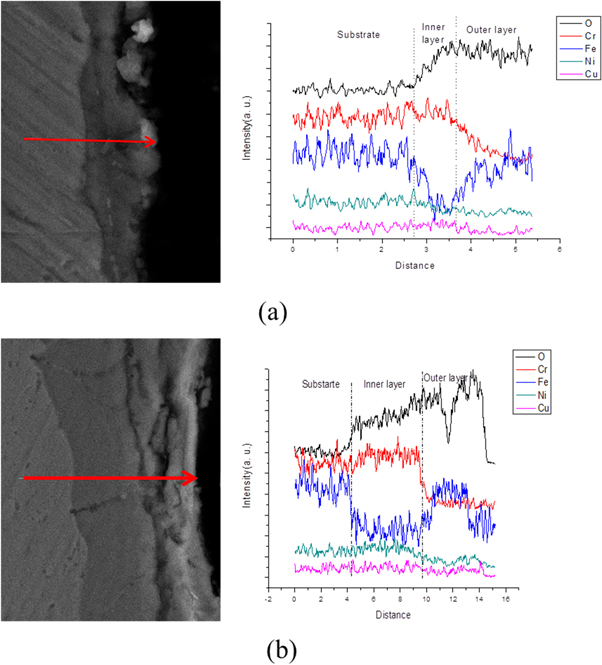
Cross-sectional images and EDS results of oxide layers are shown in figure 3. EDS line scan was conducted along the line in (ai) image to observe the composition variations from substrate to the surface. O content is an indicator to distinguish the substrate from the oxide layer. The dark region in (ai) image revealed higher oxygen content while the gray region with lower oxygen is the substrate. According to the content variation of Fe and Cr, the oxide layers exhibited double-layer structure with Cr rich inner layer and Fe rich outer layer, which was also reported by other groups in 18-8stainless steels [25, 26]. The boundary where the distribution lines of Fe and Cr varied significantly was the interface of two different oxide layers.
Figure 3 Line scanning of samples oxidized at 600 °C for (a) 25h, (b) 50h.
Download figure:
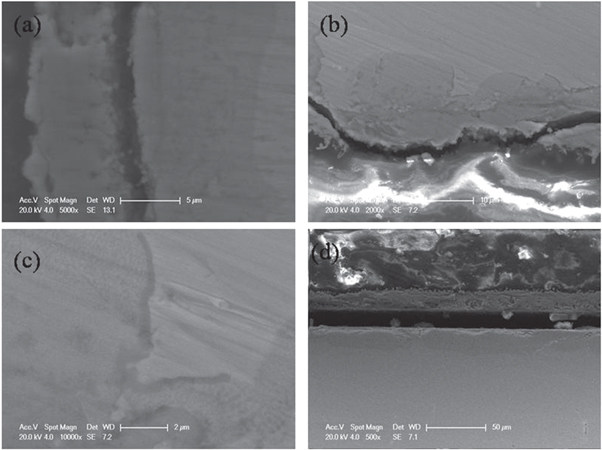
Standard image High-resolution imageTo study the compositions and structures of the double oxide layers, cross-sections of other three samples oxidized for 100, 150, and 200 h were also observed by SEM. As shown in figure 4, the double-layer structure could be observed. The interface between the oxide layer and substrate was curvilinear rather than straight line in samples oxidized less than 200 h. The location where thin oxide layer located was the metal grain boundaries [20]. In addition, the outer layer exhibit porous structure while the inner layer was denser morphology. Some voids could be observed in the outer layer from the cross-section images which would be sensitive to the crack generation and led to the exfoliation of the outer oxide layer. The strip structure with a width of 200 nm observed in samples oxidized for 150h was the self-healing scale which acted as a barrier between the inner oxide layer and the substrate [21]. EDS analysis was used to obtain the compositions of the inner oxide layer (close to the substrate) and outer oxide layer. As shown in table 4, O, Cr, Fe, Ni, Cu had been detected in the inner layer while only Fe and O were observed in outer layer. The compositions of the inner layer in samples oxidized for100, 150 and 200 h was almost identical. The content was 45 at%O, 18 at%Cr, 27at%Fe, 8 at% Ni, and 3 at% Cu in inner layer. The composition of the outer layer in three samples were 54 at% O and 45 at% Fe. The existence of copper, which was alloying elements in steel, indicated that the inner layer was developed by inward diffusion of corrosion gases while the outer layer was formed by outward diffusion of iron from the substrate. The proportion of Fe, O was close to 4:3, which was corresponded to Fe3O4. The results of the point composition analysis agreed well with the results of line scanning analysis.
Figure 4. Micrographs of Super304H oxidized at 600 °C for (a)100, (b)150, (c)150, (d)200h.
Download figure:
Standard image High-resolution imageTable 4. EDS results of the inner outer layer of Super304H oxidized at 600 °C.
| Inner layer (at%) | O | Cr | Fe | Ni | Cu | Outer layer(at%) | O | Fe | |
|---|---|---|---|---|---|---|---|---|---|
| 100h | 45.2 | 18.4 | 23.4 | 9.1 | 3.8 | 53.6 | 46.4 | ||
| 150h | 43.7 | 18.0 | 27.9 | 7.9 | 2.4 | 54.4 | 45.6 | ||
| 200h | 46.4 | 16.7 | 28.2 | 8.6 | 53.4 | 46.6 |
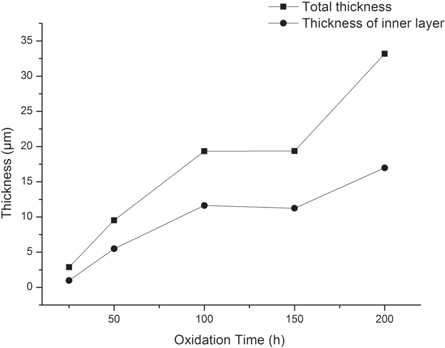
In figure 5, exposure time versus thickness is used to give a qualitative picture of the growth of the inner oxide layer and the whole oxide layer. The thickness of inner layer and whole layer remained unchanged when the exposure time increased from 100 h to 150 h. As the oxidation time further increased up to 200 h, the thickness increased significantly and the largest thickness growth rate was obtained. The reason c could be attributed to the reduction of the Cr content in substrate beneath the self-healing layer caused by the assumption of the initial protective scale. Less Cr bellow the self-healing layer led to quick regrowth of the exposed substrate after spallation of the outer oxide layer.
Figure 5. Thickness of the total layer and inner layer versus oxidation time.
Download figure:
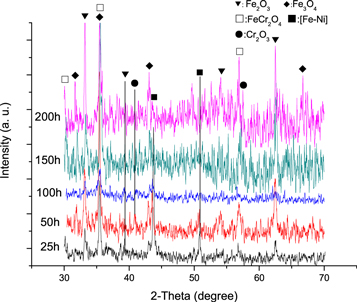
Standard image High-resolution imageFigure 6 shows the XRD patterns of Super304H oxidized at 600 °C for 25, 50, 100, 150, and 200 h. According to the XRD patterns, the phases of the oxide samples were identified as Fe-Ni, Fe2O3 andFe3O4phase with small amountofCr2O3 and FeCr2O4. The diffraction peaks originating from Fe-Ni have been observed in samples oxidized for 25 and 50 h because of the penetration depth of the diffracted X-ray is larger than the thickness of the oxide layers. It was worth noting that after 25 h oxidation, diffraction peaks of Fe3O4 were observed, which indicated that the initial Fe2O3 partially converted to Fe3O4. As the exposure time increased, the characteristic diffraction peaks of Fe3O4 exhibited stronger intensity which suggested that more Fe3O4 phase were formed. FeCr2O4 with spinel structure had been detected in all samples and a relatively weak Cr2O3 diffraction peak was also observed. No nickel oxide was identified because of the low nickel oxide content. Combined with the EDS results obtained from cross-section of samples, it can be concluded that the oxide layer consisted of an outer magnetite layer and an inner Fe-Cr-Ni oxides layer. The inner iron chromium oxide layer was composed of NiO,Fe2O3, FeCr2O4and Cr2O3.
Figure 6. XRD pattern of Super304H oxidaized at 600 °C for various time.
Download figure:
Standard image High-resolution image3.4. Oxidation behavior of Super304H stainless steel in supercritical water
To obtain a protective chromium oxide film on the steel substrate in water vapor atmosphere, Fe-Cr alloys should possess minimum 25 wt% of chromium [27, 28]. However, for Super304H, the content of chrome was 19 wt%, which means it is difficult to form a intact inner chromium oxides to segregate the substrate from the oxidation atmosphere. In our previous studies, when the sample was oxidized at 700 °C for 4 h at water atmosphere, a duplex-structure oxide layer had been formed with an outer Fe3O4layer and composite inner oxide layer, which contained NiO, Fe2O3, Cr2O3and FeCr2O4. In the long term oxidation samples, similar structure was observed except the formerly reported nickel oxide, which was attributed to the insufficient content. The existence of copper in the inner layer indicated that inner oxide layer was formed by H2O(g). It is suggested that the corrosion is the reaction between H2O and Fe as follow: Fe + 2H2O = Fe(OH)2 + H2, or, 3Fe + 4H2O = Fe3O4 + 4H2. The reaction between Fe and H2O should be responsible for the accelerated corrosion of stainless steel in water vapor compared with in air [29]. The dense self-healing Cr2O3 scale located closer to the substrate and acted as barrier to retard the diffusion of water vapor and alloy elements. The oxidation rate could be slowed down by Cr2O3 self-healing scale even though it did not completely separate the oxide layer from the substrate because of the insufficient chromium of Super304H [30]. In addition, the formation of Cr rich oxide layer led to dramatically drop of the chromium content in the underlying substrate and resulted in a chromium depleted zone [26].
The oxidation kinetic curve of Super304H at 600 °C in water vapor atmosphere was neither linear law nor the reported parabolic law [20, 31].At the first period, it exhibited highest oxidation rate. As the oxidation time increased from 50 h to 100 h, there is no increase of the mass. Combined with the SEM results of the surface morphologies, the oxide layer spallation should be responsible for the invariant mass gain ratio. The oxidation mass gain was offset by mass loss of exfoliation layer. However, further increase of oxidation time will lead to mass gain because of the quickly regrowth of the oxide layer in the exfoliation region. The compositions of the spallation area were similar to that of the outer oxide layer, while the unspalled area was similar to that of the inner layer. It could be concluded that the outer oxide layer was easy to peel off and led to regrowth of the oxide layer in the exfoliation region, which could interpret the continuous mass gain as the oxidation time further increased from 100h to 150h [32–34]. The observed spallation of the oxide layer is one of the main limited factors for the application of austenite stainless steels [35–37]. In the literature, it suggested that oxide layer spallation occurred when internal stress exceeded the adhesion force or the yield strength of the oxide layer [34]. The most important contribution to oxide layer stress is thermal stress which was generated by the large differences in thermal expansion coefficients between the metal and the metal oxide. The outer layer with large curvature would be preferred exfoliated as shown in our previous results produced by finite element modelling. The thickness evolution of total oxide layer and inner layer was quite different from the mass gain. From the cross section images, the thickness of the total layer and the inner layer increased continually with increasing oxidation time. But the increase rates gradually slowed down, especially in the inner layer. The decreased growth rates of the oxide layer suggested that the inner layer could protect the substrate from oxidation by retarding the diffusion process [33]. In exfoliation region, the inner layer would be subjected to the water vapor directly and would be quickly oxidized because of Cr depleted. Thus, as the peeling –oxidation process repeated, the wall loss of the tube happened and the peeled off oxides would block the bends to induce overheat. Based on above discussion, the oxidation of Super304H stainless steel can be similarly divided into five stages:
- (i)the inner NiO, Cr2O3, and FeO nuclei;
- (ii)a nickel and chromium dense oxide layer formed and retard the formation of Fe oxides;
- (iii)Fe oxides dually grown with outward diffusion of Fe due to the incomplete inner oxide layer;
- (iv)porous and crack generated in the outer layer because of the dissolution of some oxides and the thermal stress and transformation stress;
- (v)spallation occurred and the beneath substrate suffering a quickly re-oxidation as step (i).
4. Conclusions
The oxidation behavior of Super304H at 600 °C from 25 h to 200 h was investigated at water vapor atmosphere. The oxide layer exhibited duplex structure which consisted of an outer Fe3O4 layer, and inner oxide layer which contained Fe-Cr-Ni oxides. Self-healing Cr2O3 formed adjoining the substrate and separated the oxide layer from the substrate. The thickness of the total layer and the inner layer both increased as the oxidation time prolonged, but the growth rate gradually slowed down because of the formation of protective Cr2O3 oxide layer. After 100 h oxidation, spallations occurred at surface and resulted in a quick oxidation of underlying regions, which were Cr depleted. Taking the effect of spallation into consideration, the oxidation kinetic curves did not follow the linear laws or the parabolic law. The results would be helpful for the maintenance of steam tube in power plant. However, the synergistic effect between oxidation and aging is more complex and closer to the service conditions. Thus, more efforts should be paid on the effects of steam oxidation on the phase stability and mechanical properties evolution of Super304H to guarantee the safety of power plant.
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).