Abstract
Ag2S doped chalcogenide glassy systems have been characterised on the basis of AC conductivity and electric modulus formalism. Various nanophases such as Ag2Se, Te0.5 Se3.5 etc. and dislocation (defects) have been identified and their roles in the conduction process have been established. XRD analysis provides that incorporation of more Ag2S content in the present system should play important role to enhance the dislocation and to decrease the crystallite sizes. The Fourier transform infrared spectra (FT-IR) confirm the characteristic vibration of Ag–S at 500–650 cm−1, stretching vibrations of the O–H bond near 3400 cm−1 and bending vibrations of the adsorbed H2O molecules on the surface of Ag2S near 1600 cm−1. Composition dependent optical phonon frequency (ν0) and Debye temperature (θD) have been estimated from FT-IR and it is noteworthy that θD increases with Ag2S content in the compositions up to x = 0.1, but decreases for x = 0.2. This result suggests higher kinetic energy of the constituent atoms/molecules, which may refer to higher electrical conductivity due to polaron hopping. Correlated barrier hopping (CBH) model in its modified version has been found most suitable model to explore the conduction mechanism. Short time relaxation process may be considered to be trivially associated with conduction of polaron. universal scaling approach proposed by Ghosh and Pan has been adopted to interpret electrical relaxation process from time-temperature superposition principle. AC conductivity spectra at various temperatures exhibit a perfect overlap into a single master curve. This feature must be an indication of the temperature independent relaxation process. On the other hand, conductivity spectra of all the compositions at a particular temperature do not exhibit perfect overlapping into a single master curve. This result indicates that the relaxation dynamics of charge carriers (polarons) is strongly dependent on compositions.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
AC conductivity experiments have been extensively performed on the chalcogenide glassy systems to explore the conduction process of thermally activated AC conduction [1]. Many researchers [1, 2] have paid their interest to involve on the analysis of hopping conductivity in such glassy systems. It is well known that the chalcogenide glassy systems have been characterized by the presence of localized states in the mobility gap due to the absence of long-range order [3, 4]. Therefore, measurement of the frequency-dependent electrical conductivity of them is a powerful experimental method to obtain information about the existence and location of these states [4]. The hopping conduction has a fundamental feature that shows the frequency dependence of the AC conductivity in terms of a power law [3, 4]. For this purpose, a model [5] has been proposed for describing the character of defect centers in disordered materials. Kastner et al [6] anticipated the presence of defect states (D+, D−) in the mobility gap as a common characteristic of chalcogenides, where the hopping of an electron pair occurs from twin occupied D+ states to a close D−center over the barrier unscrambling such two sites. In this way, the model of correlated barrier hopping (CBH) [7] of bipolarons was developed for the explanation of the AC conduction mechanism in such systems. The extensive studies about the local structure of chalcogenides are found useful to understand their physical, structural and chemical properties [8]. Such studies also provide more insights about probable structural combinations and local structural transitions to synthesize newly designed devices by using novel compositions. The stiffness transition in multicomponent chalcogenide glassy systems may be governed by the rigidity percolation model [6]. This model predicts the structural properties of covalent network based on some mechanical constraints and the corresponding chemical compositions. In covalent network, the bond angles and the interatomic separations are governed by such mechanical constraints [5, 6]. The amorphous semiconducting nature of chalcogenide glassy systems makes them suitable to be applicable in many technological applications such as non-volatile memory optical data storage (CD-RW, DVD etc.), Phase Change Random Access Memory etc. [9–12]. Some of the advantages of these glassy systems, like good transparency in infrared region, low phonon energy, low optical losses, good thermal stability etc, enhance their applicability in fabrication of optical fibres [13–16]. These glassy systems can be considered as a promising material in various applications like infrared monitoring (such as lenses, windows, IR vision systems etc.), chemical sensors in different fields (such as medicine, biology, chemical etc), power fibre optics and telecommunication systems (such as high speed switches, frequency convertors amplifiers etc) [17].
Ag2S is particularly important as it exhibits several attractive properties such as narrow band gap of about 1.5 eV, low cost, good chemical stability and good optical properties [18]. Ag2S can also be applied in various devices such as photovoltaic cells, infrared detectors, superionic conductors, photoelectric switches, gas sensors etc. [18]. In spite of this, Ag2S has some limitations because of its requirement of complex preparation process and very low yield [18]. To overcome its limitations, Ag2S can be mixed with other suitable elements to prepare composite materials. Here, preparation of some new chalcogenide glassy system, xAg2S-(1−x) (0.5Se–0.5Te) has been reported and its microstructure has been explored using XRD and FT-IR studies. XRD analysis reveals crystallite size, dislocation density, lattice strain and crystalline volume fraction in the present system. Recent work [19] shows that Se-Te-In chalcogenide glassy system may be used as phase change memory device. Information related to various functional groups and molecules and their characteristic Debye temperatures have been computed from FT-IR analysis. In the present communication, conductivity spectra of Ag2S-Se-Te system in a wide frequency and temperature zones have been reported. Dielectric relaxation studies help to know the nature and origin of dielectric losses, which may be helpful for the structural analysis of them. Correlation between AC conductivity mechanism and microstructure of the present system may explore new scientific aspects not only for their uses in various device applications but also for academic interest [10, 11, 19].
2. Experiment
Chalcogenide glassy system, xAg2S-(1−x) (0.5Se–0.5Te) with x = 0.05, 0.1 and 0.2 was prepared by melt quenching method. Attempts have been made to prepare chalcogenide glassy system for a wide range of compositions. It is observed that particular concentrations of 0.5Te and 0.5Se are appropriate to form present glassy system for x = 0.05, 0.1 and 0.2. It may be predicted that the amount of free volume for concentrations of 0.5Te and 0.5Se of present glassy system should accommodate such Ag2S doping due to limited open structure [7]. High purity (Aldrich 99.9%) Ag2S, Se and Te in powdered form, were taken in suitable atomic weight percentage (as per the composition) and mixed in a mortar. The mixture was sealed in quartz ampoules in a high vacuum (10–4Torr) in order to avoid oxidation in the present glassy system at high temperature. The ampoules were then placed inside furnace at 250 °C. The temperature of the furnace was set to rise at the rate of 3 °C min−1. After 1 h,the temperature was increased to 500 °C and it is kept fixed for 2 h. Then the temperature was raised to 850 °C. The ampoules were kept at that temperature for 6 h and shaken continuously. The ampoules, containing the melted mixture, were then taken out of the furnace and quickly placed on crushed ice for quenching of the melt to take place to get glassy samples in bulk form. Pellets of the as prepared samples are formed to carry out AC and DC measurements. To get good electrical contact between electrode and the glassy sample, silver paste is coated on both the sides of the pellets.To carry out AC measurements in the frequency range from 42Hz to 5MHz at different temperatures (303K–423K), Hioki made LCR Hi-Tester (Model No. 3532–50) was used. X-ray diffraction of the as- prepared samples reveal the polycrystalline nature and the formation of different nanocrystallites. FT-IR analysis is carried out to identify various functional groups of the as- prepared samples.
3. Results and Discussion
3.1. XRD Analysis
3.1.1. Crystalline size, dislocation density and lattice strain
X-ray diffractograms of as-prepared samples are illustrated in figure 1, which exhibit various peaks for [h k l] values for various nanophases [20]. Investigations on various peaks in figure 1 exhibit [1 0 0] and [2 0 1] for Te0.5Se0.5[JCPDS Card no. 01-078-1061], [2 2 0] for Te0.5 Se3.5[JCPDS Card no. 01–087–2414], [1 1 0] for Ag2S (JCPDS Card no. 75–1061), [1 4 1] for S3Se5 (JCPDS Card no. 71–1118), [5 1 0] for Ag4.53Te3 (JCPDS Card no. 86–1953), [4 4 0] for Ag5Te3 (JCPDS Card no. 86–1168) and [4 2 2] for TeS (JCPDS Card no.78–1062) nanocrystallites. The different crystallites in figure 1 show the short range order of constituent atoms. Various peaks in figure 1 directly indicate their polycrystalline behaviour. To estimate crystallite size and corresponding lattice strain, developed in the present systems, various peak positions and nature of broadening of peaks have been exercised. Here, sulphur (S) nanoparticles are absent as (113), (222), (027), (046) and (318) peak positions [21] are not found in figure 1. This result may conclude that sulphur (S) in Ag2S should take part in the bonding of the network structure.
Figure 1. XRD diffractograms of system, xAg2S—(1−x) (0.5Se—0.5Te) for x = 0.05, 0.1 and 0.2.
Download figure:
Standard image High-resolution imageXRD data can be exploited to find out the broadening of peaks (βT) due to combined effect of crystallite sizes (βD) and corresponding micro strain (βε ) using the following relation [20]:
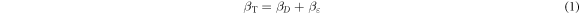
Scherrer equation [22] can be employed to compute crystallite size in the form of :
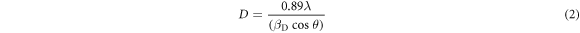
Here, βD (broadening of peak) is the measured full width at half maxima (FWHM) in radians, λ = 0.15406 nm (wavelength of the x-ray source), D is the crystallite size (in nm) and θ is the peak position in radians.
At the same time, XRD peak broadening due to micro strain [23] can be presented as:
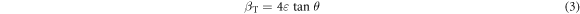
where, ε is the micro-strain developed in the samples under study.
Due to the course of formation of glass matrices, crystallographic irregularities or defects are developed in the form of dislocation, which are supposed to be highly responsible for the electrical transport via hopping of polarons. This dislocation density (δ) can be presented as [7]:
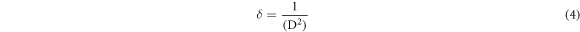
Estimated values crystallite sizes of different phases with their miller indices, ε and δ are included in table 1.
Table 1. Crystallite size and its average value, dislocation density and lattice strain of Chalcogenide glassy alloys xAg2S-(1−x) (0.5Se–0.5Te) for x = 0.05, 0.1 and 0.2. Other essential parameters such as peak position and full with at half maxima (FWHM) are also included. Estimated errors are also included here.
| x | Pos. [2θ in °] | FWHM [rad] | Crystallite size (nm) (±1.0) | Average crystallite size (nm) (±1.0) | Phases | h | k | l | Strain (±0.001) | Average strain (±0.001) | Dislocation density (nm−2) (±0.001) | Average dislocation density (nm−2) (±0.001) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.05 | 23.23 | 0.2303 | 34.8286 | 32.41 | Te0.5Se0.5 | 1 | 0 | 0 | 0.04106 | 0.237 | 0.0008244 | 0.00134 |
| 28.49 | 0.2047 | 39.5993 | Te0.5 Se3.5 | 2 | 2 | 0 | 0.00563 | 0.0006377 | ||||
| 30.39 | 0.1498 | 54.3481 | Ag2S | 1 | 1 | 0 | 0.06676 | 0.0003386 | ||||
| 40.74 | 0.307 | 27.2980 | S3Se5 | 1 | 4 | 1 | 0.003868 | 0.001342 | ||||
| 43.58 | 0.4546 | 18.6119 | Ag4.53Te3 | 5 | 1 | 0 | 0.56312 | 0.0028868 | ||||
| 50.48 | 0.42063 | 20.6488 | Te0.5Se0.5 | 2 | 0 | 1 | 0.949866 | 0.0023454 | ||||
| 54.14 | 0.27938 | 31.5790 | Ag5Te3 | 4 | 4 | 0 | 0.02689 | 0.0010028 | ||||
| 0.1 | 28.58 | 0.46792 | 17.3270 | 29.35 | Te0.5 Se3.5 | 2 | 2 | 0 | 0.01844 | 0.126 | 0.0033308 | 0.00226 |
| 30.42 | 0.1407 | 57.8673 | Ag2S | 1 | 1 | 0 | 0.06501 | 0.0002986 | ||||
| 43.56 | 0.42257 | 20.0210 | Ag4.53Te3 | 5 | 1 | 0 | 0.49287 | 0.0024948 | ||||
| 54.17 | 0.24173 | 36.5027 | Ag5Te3 | 4 | 4 | 0 | 0.02438 | 0.0007505 | ||||
| 71.86 | 0.6449 | 15.0429 | TeS | 4 | 2 | 2 | 0.03198 | 0.0044191 | ||||
| 0.2 | 30.45 | 0.1623 | 50.1701 | 28.05 | Ag2S | 1 | 1 | 0 | 0.07823 | 0.205 | 0.0003973 | 0.00316 |
| 43.59 | 0.5283 | 16.0163 | Ag4.53Te3 | 5 | 1 | 0 | 0.68069 | 0.0038983 | ||||
| 54.16 | 0.2558 | 34.4941 | Ag5Te3 | 4 | 4 | 0 | 0.0256 | 0.0008404 | ||||
| 71.89 | 0.84126 | 11.5338 | TeS | 4 | 2 | 2 | 0.038557 | 0.0075171 |
It is observed from table 1 that average crystallite size decreases with compositions. Micro-strain is found to decreases up to x = 0.1 and then to increase beyond it and dislocation density increases with compositions. This enhancement of dislocation/defect may be responsible to instigate the degree of structural alterations as well as grain boundary effect [7, 23]. So incorporation of more Ag2S content in the present system suggests to enhance the dislocation and to decrease the crystallite sizes [7, 23]. Higher order defects may insist polaron conduction in a higher order via hopping of polarons in the present system [7, 23] up to a limit with x = 0.1. The gradual fall in the values of lattice strain (ε) up to x = 0.1 suggests to more stable network structure, which can be justified from the nature of diffractograms in figure 1. Sample with x = 0.2 shows higher micro-strain, which may be associated with different type of defects/dislocation. It is also noted from table 1 and figure 1 that the phases, formed with Se (i.e., Te0.5Se0.5, S3Se5 and Te0.5 Se3.5) are found to disappear with the increase of x. The crystallite sizes of Ag containing phases (i.e., Ag2S, Ag4.53Te3 and Ag5Te3) are found to first increase and then decrease with x. It is clear from literature [24–27] that Se has a tendency to form long chain agglomerated structure. Hence, the nature of formation of nanocrystallites clearly indicates the reason for lowering of sizes of nanocrystallites, formed in the glassy matrices with Ag2S content. Higher dislocation density for x = 0.2 may cause the possibility of higher collision of charge carriers/polarons which may decrease their mobility and hence the electrical conductivity. Present dislocation defect may be treated as Frenkel defect as here atom may be displaced from its lattice point to interstitial site in order to develop a vacancy at the lattice point.
3.1.2. Crystalline volume fraction
Crystalline volume fraction XXRD of the present glassy system has been determined by equation [28]:
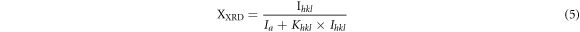
where, Ihkl is the area of a diffraction peak for a phase of a particular [hkl] value, Ia is the maximum area of peak of the considered XRD diffraction pattern and Khkl is the calibration constant value, which can be computed from the relation [28]:
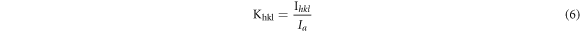
The estimated average values of crystalline volume fractions of the present glassy system with x = 0.05, 0.1 and 0.2 are presented table 2. On increasing the value of x from 0.05 to 0.1 in the glass composition, the crystalline volume fraction percentage is found to decrease from 39.4% to 24.7% and then increase to 27.3% for x = 0.2 as shown in table 2. Higher value of crystalline volume fraction percentage for x = 0.2 is expected to be associated with different type of defects/ dislocation as discussed earlier.
Table 2. Wavenumber (ν), optical phonon frequency (ν0), Debye temperature (θD) and relaxation times (τ) of xAg2S-(1−x) (0.5Se–0.5Te)for x = 0.05, 0.10 and 0.20. Estimated errors are also included here.
| Composition | ν(cm–1) from FT-IR (±1.0) | ν0 (x 1013 S−1) from FT-IR (±0.001) | θD (K) From FT-IR (±1.00) | τ (x 10–13 S) from FT-IR (±0.001) |
|---|---|---|---|---|
| x = 0.05 | 640 | 1.92 | 921 | 0.082 |
| x = 0.10 | 680 | 2.00 | 960 | 0.079 |
| x = 0.20 | 535 | 1.60 | 768 | 0.099 |
3.2. FT-IR Spectra analysis
The Fourier transform infrared spectra (FT-IR) of all samples are presented in figure 2. The characteristic vibration of Ag–S appears [29–31] at 500–650 cm−1 in figure 2. 3400 and 1600 cm−1 in figure 2 can be attributed to the stretching and bending vibrations of the O–H bond of the adsorbed H2O molecules on the surface of Ag2S [29–31]. It is also expected that other vibrations in the lower and higher wave number zones in figure 2 are responsible for asymmetric stretching modes of the bridges of Te-Se bonds [29–31].
Figure 2. FT-IR transmittance spectra of the system, xAg2S-(1−x) (0.5Se—0.5Te) for x = 0.05, 0.1 and 0.2.
Download figure:
Standard image High-resolution imageIt is apprehended to predict the optical phonon frequency (ν0) from first absorption peak of IR vibration spectra [29–31]. By applying the relation: c = λν0, where c = 3 × 1010 (cm/s), ν0 is the optical phonon frequency (Hz), λ is the wavelength (cm). The Debye temperature (θD) of the present glassy system could be estimated using the relation: hν0 = KB θD, where h is the Plank's constant and KB is Boltzmann constant. The computed values of optical phonon frequency and Debye temperature are presented in table 3. It is clear from table 3 that the values of optical phonon frequency (ν0) and Debye temperature (θD) are composition dependent. The optical phonon frequency is supposed to be the characteristic vibration of Ag–S. It is observed from table 3 that Debye temperature (θD) increases with Ag2S content in the compositions up to x = 0.1, but it decreases for x = 0.2. This result indicates higher kinetic energy of the constituent atoms/molecules of the present system, which may refer to higher electrical conductivity due to polaron hopping. Approximating polaron transport [7, 29, 32] in the present system as a part of Debye-type relaxation [7, 29, 32], the relaxation time can be estimated by the relation: τ = 1/(2πν0). The approximated values of relaxation times are also included in table 3. The relaxation time is found to decrease with Ag2S content up to x = 0.1, which suggests higher rate of polaron hopping [7, 29, 32]. This interpretation makes it clear for higher electrical conductivity, which will be discussed afterward. But beyond x = 0.1, it is found to increase, which directly suggest to reduce the conductivity due to lower rate of polaron hopping [7, 29, 32].
Table 3. Crystalline volume fraction and its average value of chalcogenide glassy alloys, xAg2S - (1−x) (0.5 Se − 0.5 Te ) for x = 0.05, 0.1 and 0.2. estimated errors are also included here.
| x | Phases | h | k | l | Area (Ihkl) in au. (±1.0) | Khkl | Crystalline volume fraction XXRD (±0.01) | Average Crystalline volume fraction (±0.01) |
|---|---|---|---|---|---|---|---|---|
| 0.05 | Te0.5Se0.5 | 1 | 0 | 0 | 872.39 | 0.57943 | 0.43379 | 0.39426 |
| Te0.5 Se3.5 | 2 | 2 | 0 | 1457.1 | 0.96779 | 0.49973 | ||
| Ag2S | 1 | 1 | 0 | 1505.6 | 1 | 0.5 | ||
| S3Se5 | 1 | 4 | 1 | 1121.8 | 0.74509 | 0.47911 | ||
| Ag4.53Te3 | 5 | 1 | 0 | 1141.8 | 0.75837 | 0.48147 | ||
| Te0.5Se0.5 | 2 | 0 | 1 | 362.20 | 0.24057 | 0.22741 | ||
| Ag5Te3 | 4 | 4 | 0 | 212.36 | 0.14105 | 0.1383 | ||
| 0.1 | Te0.5 Se3.5 | 2 | 2 | 0 | 532.12 | 0.17451 | 0.16935 | 0.24673 |
| Ag2S | 1 | 1 | 0 | 3049.3 | 1 | 0.5 | ||
| Ag4.53Te3 | 5 | 1 | 0 | 1963.9 | 0.64405 | 0.45522 | ||
| Ag5Te3 | 4 | 4 | 0 | 363.38 | 0.11917 | 0.1175 | ||
| Ag5Te3 | 5 | 4 | 2 | 234.53 | 0.07691 | 0.07646 | ||
| TeS | 4 | 2 | 2 | 507.07 | 0.16629 | 0.16182 | ||
| 0.2 | Ag2S | 1 | 1 | 0 | 2320.8 | 1 | 0.5 | 0.27325 |
| Ag4.53Te3 | 5 | 1 | 0 | 1735.3 | 0.74772 | 0.47959 | ||
| Ag5Te3 | 4 | 4 | 0 | 271.47 | 0.11697 | 0.11539 | ||
| Ag5Te3 | 5 | 4 | 2 | 155.10 | 0.06683 | 0.06653 | ||
| TeS | 4 | 2 | 2 | 496.93 | 0.21412 | 0.20473 |
3.3. Power Law model and interpretation of conductivity spectra
Thermally activated process of charge carriers may lead to the electrical conductivity of disordered solids in terms of hopping process under the influence of an electric field. The AC conductivity data of amorphous semiconductors can be well-analysed using Jonscher's universal power law :[33]
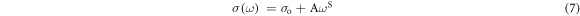
where, σo is the conductivity at low frequency region (DC conductivity) which is found to be temperature dependent, A is the pre-factor and S is the frequency exponent. The AC conductivity spectra at higher frequency region have been analysed and one such spectra for x = 0.05 is illustrated in figure 3 at various temperatures. It is observed in figure 3 that the AC conductivity increases linearly with frequency at the high frequency region [7, 29, 32]. It is also noted that it shows thermally activated mode and consequently increases with temperatures. AC conductivity data in Fig. 3 have been fitted with least square straight lines using equation (7). Slopes from these linear fittings provide the values of S. To explore the nature of conduction process in the present system, S is projected with respect to temperature in figures 4(a)–(c) for x = 0.05, 0.2 and 0.3 respectively. Remarkably, it is noted that S decreases with temperature for all the samples under study. To extract necessary information regarding the conduction process, the experimental data in figure 4 should be analysed by fitting them with suitable theoretical model. In search of such model in trial basis, correlated barrier hopping (CBH) [7, 32, 34–36] model has been found suitable in its modified version to explore the conduction mechanism in this system. CBH Model [7, 32, 34–36] should anticipate hopping methods of charge carriers (or polarons) in pairs between localized sites at the Fermi level via transport of current in a system with negative temperature dependence of S. In modified CBH model [7], S can be presented as:
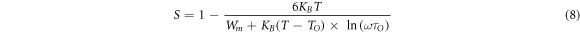
where, KB is the Boltzmann Constant, T is the absolute temperature, To is the temperature at which S becomes unity and Wm is the maximum barrier height and τ0 is the relaxation time. Solid lines in figure 4 indicate the best fitted curve using equation (8). All the fitting parameters are included in table 4.
Figure 3. High frequency conductivity spectra of glassy alloy, xAg2S-(1−x) (0.5Se–0.5Te) for x = 0.05. Solid lines indicate best-fitted straight lines.
Download figure:
Standard image High-resolution imageFigure 4. S versus T plots of xAg2S-(1−x) (0.5Se–0.5Te) for (a) x = 0.05, (b) x = 0.1 and (c) x = 0.2. Solid lines indicate the fitting of plots with modified CBH Model.
Download figure:
Standard image High-resolution imageTable 4. Different parameters obtained from CBH model (modified form) fitting and almond West formalism of chalcogenide glassy system, xAg2S-(1−x) (0.5Se–0.5Te). The value of bulk conductivity at room temperature for all samples are included. Estimated errors are also included here.
| Composition (x) | CBH (modified form) | ALMOND -WEST | log10 [σ (Ω−1cm−1)] (±0.01) | |||
|---|---|---|---|---|---|---|
| Wm (eV) (±0.01) | τ0 (s)x 10–6 (±0.001) | T0 (K) (±1.0) | EH (eV) (±0.01) | N (±0.01) | ||
| 0.05 | 0.07874 | 5.0091 | 279.42 | 0.498 | 2.46 | −4.50 |
| 0.10 | 0.11433 | 2.7909 | 265.70 | 0.3824 | 1.80 | −4.25 |
| 0.20 | 0.09874 | 7.6066 | 321.78 | 0.900 | 1.68 | −5.75 |
CBH model [7, 32, 34–36] in terms of AC conductivity can be projected as:
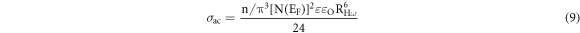
where, N(EF) is the concentration of pair states, RHω is the hopping distance at frequency ω and n/ is the number of polarons involved in the hopping process. Here, bipolar hopping process may be anticipated the dominant conduction mechanism.
3.4. Almond west formalism and conduction pathways
AC conductivity spectra of the present system have also been analysed at various temperatures to explore hopping frequency and conduction pathways of charge carriers (polarons). One such conductivity spectra for x = 0.05 is presented in figure 5(a) at various temperatures.
Figure 5. (a) AC conductivity spectra of the system,xAg2S-(1−x) (0.5Se–0.5Te) for x = 0.05 at different temperatures; (b) AC conductivity spectra of the system xAg2S-(1−x) (0.5Se–0.5Te) with x = 0.05, 0.1 and 0.2 at 363K; (c) AC conductivity with reciprocal temperature for x = 0.05 at four frequencies.
Download figure:
Standard image High-resolution imageObservation of figure 5 provides the following points: (i) a plateau region at low frequencies corresponds to DC conductivity, which increases with temperature. This nature suggests a thermally activated process of low frequency conductivity. (ii) At high frequency, the conductivity shows dispersion, which is governed by Jonscher power law [33]. In this high frequency region, AC conductivity can be made by the relaxation processin the ionic environment, which may arise from the correlated motion of charge carriers (polarons) [7, 34–36].Other samples have also exhibited similar nature of AC conductivity.
Experimental data in figure 5(a) can be analyzed to get vivid information of the conduction process using Almond West formalism [37]:
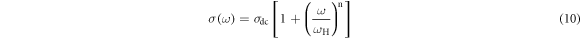
where, σdc is low frequency AC conductivity, ωH is the hopping frequency of charge carriers, n is the fractional power law exponent and ω is the frequency of the applied electric field. The solid lines in figure 5(a) represent the best fitted curve of AC conductivity spectra using equation (10). Estimated values of n from the fitting using equation (10) are listed in table 4. I is noted that n is found to be greater than unity (n >1). It is observed from others woks [37, 38] that n values, estimated from power law model is closely associated with the dimensionality of conduction pathways. Sidebottom [39] successfully interpreted different values of n and established that the value of n ∼2/3 for a three dimensional motion of Ag+ ions in the iodomolybdate glass-nanocomposite when an electric field is applied. This consideration does not comply significantly on composition as well as temperature. It follows that the numerical value of n may be such that the dimensionality may be fractal [39]. It is noted that the fractal dimension of quasicrystals [40] is found to be 2.72, which suggests a quasiperiodic long-range order with a hierarchy of atomic clusters [41]. It is evident from table 4 that n values of the present system are much greater than unity and comparable with the fractal dimension of quasicrystals [40]. The above-mentioned discussion may suggest the percolation type motion ofpolarons can be predicted [42] for the present system.The value of bulk conductivity at room temperature for all samples are included in table 4.
AC conductivity spectra of the present system at a fixed temperature (363 K) are presented in figure 5(b). Figure 5(b) shows that AC conductivity is found to increase first and then decrease with Ag2S content in the compositions. The sample with x = 0.1 exhibits the maximum AC conductivity. The nature of variation of AC conductivity may be validated the values of relaxation times as presented in table 3. Lowering of relaxation times should instigate to higher rate of polaron hopping [7, 29, 32]. But the sample for x = 0.2, relaxation time increases, which directly suggest to reduce the conductivity due to lower rate of polaronhopping [7, 29, 32]. Plateau region of conductivity spectra in figure 5(b) is found to shift downwards as we move from x = 0.1 to x = 0.2. This result indicates that conductivity decreases. It is clear from table 3 that average crystalline volume fraction percentage increases in the above-mentioned composition range, which may directly indicate that less amount of free volume due to limited open structure [7] should instigate a constraint on polaron hopping. As a consequence conductivity decreases.
The AC conductivity at four frequencies with reciprocal temperature for x = 0.05 is presented in figure 5(c). It is observed that at lower temperatures the AC conductivity is spreading out due to dominant nature of AC conductivity over the DC conductivity. As the temperature is increased, the AC conductivity approaches to the DC conductivity. It is also noteworthy that the AC conductivity increases with the increase in frequency. Similar nature is observed for other compositions.
Estimated values of hopping frequency (ωH), obtained from the fitting of experimental data in figure 5(a) using equation (10) are presented in figure 6(a) with reciprocal temperature. It shows that ωH is thermally activated and increases with compositions up to x = 0.1. After x = 0.1, it is found to decrease. Nature of variation of ωH with temperature and compositions is similar to that of AC conductivity as presented in figure 5.
Figure 6. (a) Plot of ωH versus 1000/T of the system xAg2S-(1−x) (0.5Se–0.5Te) with x = 0.05, 0.1 and 0.2 at 363K; (b) Plot of EH versus x of the system,xAg2S-(1−x) (0.5Se–0.5Te) with x = 0.05, 0.1 and 0.2.
Download figure:
Standard image High-resolution imageExperimental data in figure 6(a) can be analyzed by the following Arrhenius equation:
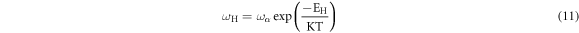
where, ωα is the pre-exponential factor of the hopping frequency and EH is the activation energy corresponding to hopping frequency. Experimental data in figure 6(a) are best fitted with straight line fits using equation (11). Slopes of solid straight line fits have provided the values of EH, which are presented in table 4. The values of EH are illustrated in figure 6(b) with compositions. It can be observed from figure 6(b) and table 4 that EH first decreases and then increases with the increase of Ag2S in the compositions.
The nature of variation of EH is found to be opposite to hopping frequency as well as AC conductivity of the present system. Attempt has been made by Anderson and Stuart [43] in the ionic system, where the ions has to jump from one void to another. In this process [43], the total activation energy in ion diffusion mechanism through an amorphous solid has two parts: one is the strain energy and the other is the electrostatic interactions. Theoretical approach [44] reveals that the strain energy increases with ion size, whereas change in electrostatic energy is higher for smaller ions. As a whole, the additive of the two terms is the manifestation of minimum value for intermediate sized ions [44]. This approach [44] should suggest for lowering of activation energy for intermediate composition. The ions hopping from one interstitial void to another is strongly dependent on the radius of the window interconnecting two voids (doorway radius) [44]. In the present system, as the size of electron/ polaron is much lower than ion, the electrostatic interactions should contribute a lot in the activation energy corresponding to hopping frequency. Lowering of EH suggests to increase corresponding hopping frequency as well as electrical conductivity as the nature of dislocation is expected to be favourable for polaron hopping process in the present system.
3.5. AC conductivity scaling
Dynamic processes involving charge carriers in disordered materials have received considerable interest in the past several years [7, 29, 45–48]. The temperature-dependence study of conductivity spectra at different temperatures led to a scaling law known as the time-temperature superposition principle [46]. In this consideration [7, 29, 46], the AC conductivity axis is scaled with respect to DC conductivity (σdc) and the frequency axis is scaled with respect to hopping frequency (ωH). Proper scaling of the conductivity and frequency demands that the conductivity isotherms collapse to a single master curve, which may indicate that the dynamic processes of charge carriers (polarons) can be interpreted with the help of a common mechanism. Barton, Nakajima and Namikawa (BNN) [47] established a relation between the DC conductivity (σdc) and dielectric loss peak frequency (ωc) as σdc = pε0Δεωc, where p is the numerical constant almost equal to 1 and Δε = ε(0)—ε∞ is the dielectric loss strength. According to BNN, the master curve can be expressed as:
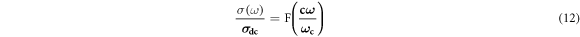
where, F is the temperature-independent function, c depends on the charge-carrier concentration and temperature and ωc is the characteristic cross-over frequency. It is observed [48] that characteristic cross-over frequency (ωc) is numerically equal to hopping frequency (ωH). Ghosh and Pan [45] have established universal scaling approach of the conductivity with respect to composition in a limited composition range, where the structure does not change appreciably. In their approach [45], the hopping frequency played an important role in scaling process of the conductivity spectra for the glassy system. The change in hopping length with composition in terms of the expression of the hopping frequency must instigate to make correlation between successive hops [45].
Figure 7(a) shows temperature scaling i.e., the AC conductivity scaling of the present system with x = 0.05 at different temperatures. It can be seen from figure 7(a) that the conductivity spectra of the system at various temperatures exhibit a perfect overlap into a single master curve. This feature must be an indication of the temperature independent relaxation process. Similar features have been observed for other compositions. On the other hand, AC conductivity scaling of all the samples under study at a fixed temperature is illustrated in figure 7(b). It can be observed from figure 7(b) that the scaling does not result in overlapping of conductivity spectra of the system into a single master curve. This imperfect scaling process should indicate that the relaxation process depends on compositions/concentrations as the structure should change with Ag2S doping in the system. Thus, from the AC conductivity scaling it can be concluded that the nature of relaxation mechanism in the conductivity formalism is strongly dependent on compositions but it is independent on temperatures.
Figure 7. (a) AC conductivity scaling of the system, xAg2S-(1−x) (0.5Se–0.5Te) with x = 0.05 at different temperatures; (b) Composition scaling of the system, xAg2S-(1−x) (0.5Se–0.5Te) with x = 0.05, 0.1 and 0.2 at 373K.
Download figure:
Standard image High-resolution image3.6. Study of dielectric properties
3.6.1. Permittivity and loss tangent
The frequency dependent dielectric constant ε/ (real) and dielectric loss ε// (imaginary) in a wide frequency range (42Hz—5MHz) at various temperatures (343K—423K) have been studied for understanding the nature and origin of losses occurring in these materials [29, 32, 49]. Moreover, the incorporation of Ag2S content in the chalcogenides may instigate to enrich its conductivity level, which may in turn improve its dielectric properties in terms of substantial decrease in activation energy for hopping of polaron [29, 32, 49]. This feature makes them capable of being used for integrated circuits (ICs) device applications [29]. ε/ and ε// spectra are presented in figures 8(a) and (b) respectively for x = 0.2. ε/ and ε// spectra of other samples under study have shown similar nature.
Figure 8. Spectra of (a) dielectric constant (ε/) and (b) dielectric loss (ε//) of the system, xAg2S-(1−x) (0.5Se–0.5Te) for x = 0.2 at various temperatures.
Download figure:
Standard image High-resolution imageDielectric constant (ε/) of the present system decreases on increasing frequency in the region of lower frequencies and it becomes independent on frequency in higher frequency region for the entire range of temperature as shown in figure 8(a). It is also noted in figure 8(a) that ε/ increases with temperature, which shows it's thermally activated nature. Higher values of dielectric constant (ε/) at low frequency and high temperature is because of the diverse constituents such as electronic, ionic, orientation and interfacial polarization [32, 49]. Electronic polarization does not exist here as it takes place at ∼1016 Hz. Displacement of positive and negative ions may be responsible for ionic polarisation, which may exists at ∼1013 Hz. So, it should not be present here due to the above-mentioned facts. Permanent dipole moments should instigate dipolar polarization (orientational polarization), which is associated with the rotation to the dipoles at ∼1010 Hz frequencies. Movement of charge of carriers by interfaces is the possible reason for space charge polarization, which exists at frequencies ranging between 1 and 103 Hz. As the applied field is increased, the dipoles exhibit slow rotation, which may take more time compared to electronic and ionic polarizations. In the higher frequency region, the permanent dipolewill not be capable of being following the orientational polarization. So, a competition may take place between orientational polarization and space charge polarization in the higher frequency, where the former tries to go down and the later should have a tendency to go up. As a consequent, dielectric constant(ε/) becomes a constant value at a higher frequency. The nature of temperature dependent dielectric loss (ε//) of a system has been explained by Stevels [50] to interpret it as an outcome of conduction losses, dipole relaxation losses and ion vibrational losses. This process [32, 49, 50] depends on the migration of charge carriers (polarons) over long distances via loss of thermal energy in the lattice.
To validate dielectric loss data, dielectric loss tangent (tanδ = ε///ε/) has been estimated for all samples under study and one such set of results for x = 0.2 is presented in figure 9(a) at various temperatures. The results in figure 9(a) show that tanδ increases as the frequency decreases and increases sharply at a particular frequency. It is also observed that tanδ increases with temperature, indicating thermally activated nature. The rate of loss of thermal energy [32, 49] is proportional to σ/ω (σ is the conductivity and ω is the angular frequency). Conduction loss [32, 49] is also found to increase with temperature and plays a dominant role. At higher temperature, all types of losses contribute to the dielectric loss of the present system. For this reason, tanδ exhibits a sharply pronounced valueat a particular frequency (call it critical frequency).But the values of tanδ at high frequency region may strongly depend on ion vibrations [32, 49, 50] in the lattice of the glassy matrices under study.
Figure 9. (a) Spectra of dielectric loss tangent, point of intersection between drawn straight lines and frequency axis provides the values of critical frequency (ωc); (b) hopping frequency (ωH) with respect to critical frequency (ωc) for all samples under study at various temperatures.
Download figure:
Standard image High-resolution imageCritical frequency (ωc) has been estimated from the point of intersection between drawn straight lines and frequency axis in figure 9(a) at various temperatures for x = 0.2. Similarly, ωc has been computed for x = 0.05 and 0.1 respectively at various temperatures. The reason for dielectric loss has already been discussed. In search of other possible reasons for dielectric loss, contribution of hopping frequency (ωH) may be considered as a matter of fact that discussion of hopping frequency (ωH) and corresponding activation energy (EH) in figure 6 should impel us to think over in that direction. To investigate its contribution in dielectric loss process, hopping frequency (ωH) has been projected in figure 9(b) with respect to critical frequency (ωc) for all samples under study at various temperatures. It is observed from figure 9(b) that hopping frequency (ωH) does not show any considerable changes with respect to critical frequency (ωc) for all samples at various temperatures. It may be directly concluded from this result that hopping frequency (ωH) does not contribute any role in the dielectric loss process. It depends on conduction loss [32, 49, 50] and ion vibrations [32, 49, 50] as discussed earlier.
3.6.2. Electric modulus spectra and relaxation times
Electrode polarization effects [29, 32] in dielectric spectra of disordered solids may cause a difficulty in the computation of various dielectric parameters, particularly at low frequencies.Efforts [38, 51] have been made in the past to correct the electrode polarization effect. Electric modulus formalism [38, 51] can be used for investigating complex electrical response of a system by nullifying the electrode polarization effect as the reciprocal of complex permittivity is taken into account. The complex electrical modulus can be presented as [51]:
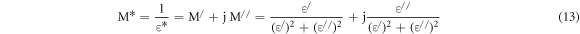
where, M* is the complex modulus, ε*is the complex dielectric permittivity, M/ is the real and M// is the imaginary parts of the electric modulus.
The assimilation of the electric modulus spectra of the present system in a wide frequency and temperature range may compel us to explore complete dielectric properties. Figure 10(a) displays the imaginary part of the electric modulus M//spectra for x = 0.2. It can be seen from figure 10(a) that at low frequency region, M//approaches to zero, which implies the suppression of electrode polarization effect. At higher frequencies,impact of relaxation process makes M//maximum [29, 52, 53]. As the frequency increases, M//is found to decrease, which may suggest the short-range mobility of charge carriers in the system.
Figure 10. (a) Imaginary electric modulus M// spectraat various temperatures for x = 0.2; (b) relaxation time (τ) with 1000/T for x = 0.05, 0.1 and 0.2.
Download figure:
Standard image High-resolution imageIt is evident from figure 10(a) that M// peaks have been shifted towards higher frequency region as the temperature increases. This is an indication of thermally activated relaxation process in the system. Similar nature of electrical relaxation is also observed for other samples under study. M// peak positions in 10(a) corresponds topeak relaxation frequency (ωmax). In this approximation [29, 52, 53], ωmax indicates the limiting frequency up to which the charge carriers (polarons) may hop over long distances. Beyond ωmax region, charge carriers are supposed to be confined to potential wells, where they are freely accessible [29, 52, 53]. It may be concluded from this discussion, that the transition of mobility of charge carriers from long range to short range order takes place at ωmax.
To investigate the nature of M// , the following expression [52–54] can be considered:
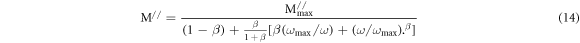
where, 0 < β <1, M// max is the peak or maximum value of M//, ωmax is angular frequency corresponding to M// max and β stands for Kohlrausch-Williams-Watts (KWW) stretched coefficient [54].The experimental data in figure 9(b) have been analysed by best fitted curves using equation (14). The values of M// max, ωmax and β have been estimated from this fitting.The activation energy (Eτ ) related to the relaxation time τ was calculated by using the equation [29, 53]:
To analyse the nature conduction mechanism, it is approximated that the present systems undergo Debye type relaxation [29, 32], which requires the essential condition: ωmax x τ = 1, where, τ is the relaxation times. The estimated relaxation time τ with reciprocal temperature is projected in figure 10(b). Figure 10(b) exhibits thermally activated nature of τ. As the temperature increases, τ is found to decrease, which shows its semiconducting behaviour. It should also be pointed out that τ decreases with composition up to x = 0.1 and then increases, which can be validated from the nature of AC conductivity and hopping frequency as presented in figures 5 and 6 respectively.
To investigate thermally activated relaxation times in figure 10(b), theoretical expression of relaxation time [29, 49, 53] can be used in its form as:
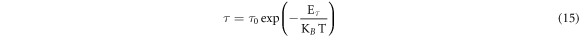
where, τ0 is pre-exponential factor, KB is the Boltzmann constant and T is the absolute temperature. Experimental data in figure 10(b) have been best fitted by linear fits using equation (15). Activation energy (Eτ ) corresponding to relaxation time has been estimated from the slopes of the best fitted solid straight lines and presented in table 5. Eτ is found to increase and becomes maximum for x = 0.1. After x = 0.1, Eτ is found to decrease, indicating higher values of relaxation times. Smaller values of relaxation times and higher values of corresponding activation energy are the indication of higher conductivity due to higher rate polaron hopping in the system. Short time relaxation process may be considered to be trivially associated with motion of polaron. A detailed understanding of these relaxation processes may be related to the least vibration of dipoles and conducting species at lower frequencies. At higher frequencies, the dipoles may vibrate rapidly and hence it requires more activation to attain relaxed state. It is essential to mention that relaxation times have also computed earlier FT-IR spectra. These values of relaxation times (table 3) are found to be much higher than those obtained from dielectric relaxation process. The reason for such discrepancy is that computation of relaxation times from FT-IR study entirely depends on the vibrations due to only optical phonon frequency (ν0). But in the dielectric relaxation process, all types of lattice vibrations are counted and the results are supposed to be more accurate.
Table 5. Variation of activation energy related to dielectric relaxation process, chalcogenide glassy alloys xAg2S-(1−x) (0.5Se − 0.5Te). Estimated errors are also included here.
| x | Eτ (eV) (±0.10) |
|---|---|
| 0.05 | 1.20 |
| 0.1 | 1.48 |
| 0.2 | 1.38 |
3.6.3. Electric modulus scaling approach
Like AC conductivity scaling data, attempts have been made to scale the conductivity relaxation spectra (modulus spectra) at various temperatures [29, 49, 53]. In this scaling process, M// values have been divided by M// max and ω values have been divided by ωmax to acquire modulus scaling. One such scaling spectra for x = 0.05 has been illustrated in figure 11(a) at various temperatures. All other samples show similar nature of temperature scaling. Perfect overlapping of all the scaled data in figure 11(a) explores temperature independent relaxation process [29, 52, 53] in the present system. M// scaling spectra at a fixed temperature (343K) for all the samples under study are projected in figure 11(b), which indicates imperfect overlapping nature of M// scaling spectra.The imperfect overlapping nature of the plots in figure 11(b) clearly indicates composition dependent relaxation process in the present system. So, it can be concluded from the discussion that conductivity relaxation process in the present system is independent of temperature, but it depends on compositions. It is also noteworthy that the nature of conductivity scaling process is same as that of AC conductivity scaling. In this regard, point should be noted that two methods (AC conductivity approach and Modulus approach) are independently employed here to explore conduction process. The outcomes of these two distinguished processes are of similar interpretations of the experimental results.
Figure 11. (a) Temperature scaling of M// of system for x = 0.05; (b) Composition scaling of M//for x = 0.05, 0.1 and 0.2 at 343K.
Download figure:
Standard image High-resolution image3.7. Mobility and carrier density
Carrier mobility (μ) and carrier density (N) [7, 29, 32] of a glassy system play important role for assimilation of polaron transport. They can be estimated from DC polarization technique [7, 29, 32]. Especially,polarization of charge carriers [29]occur in a controlled way at electrode-electrolyte interface when an external fixed voltage is applied for an enough long time to the system, placing in between two blocking electrodes. As soon as the process of polarization of charge carriers is over, reversal of the polarity of the external voltage takes place [7, 29, 32]. This may lead to move the cloud of polarized charge carriers towards the opposite end of the glassy sample under study. The mobility of charge carriers can be displayed as [7, 29, 32]:
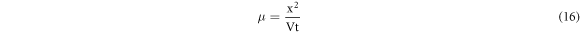
where, x is the sample thickness, V is the voltage applied and t is the time taken by the charge carriers to cross the thickness x of the sample.The expression of the carrier density 'N' of the glassy sample may be projected as [7, 29, 32]:
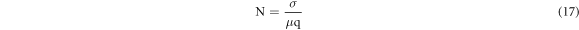
where σ is the conductivity, μ is the mobility of charge carriers and q is the charge of an electron/ polaron.figure 12(a) shows the mobility versus reciprocal of temperature plots for the present system of all compositions.It is found to increase with the rise in temperature of the system. The mobility is found to increase first and then decrease with the increase of composition. In figure 12(b), carrier density (N) is projected with reciprocal of temperature. It is noteworthy from figure 12(b) that N does not show any changes with temperature for all compositions. This result directly indicate that N does not play any role in the conduction process. So, this discussion and equation (17) should suggest that mobility of charge carriers (μ) is mostly pronounced in the conduction process.
Figure 12. (a) Mobility of charge carriers (μ) versus 1000/T; (b) carrier density (N) versus 1000/T for x = 0.05, 0.1 and 0.2.
Download figure:
Standard image High-resolution image4. Conclusion
Thermally activated nature of AC conductivity and dielectric properties of the present chalcogenide glassy system have been established in a wide range of frequency and temperature. AC conductivity is found to increase linearly with frequency at the high frequency region. Modified correlated barrier hopping (CBH) model has been found suitable to explore the conduction mechanism. Average crystallite size, estimated from XRD study decreases with compositions. Incorporation of more Ag2S content in the present system suggests to enhance the dislocation and to decrease the crystallite sizes. Higher order defects may insist polaron conduction in a higher order via hopping of polarons up to a limit with x = 0.1. The gradual fall in the values of lattice strain (ε) up to x = 0.1 suggests to more stable network structure. FT-IR spectra have been used to explore the presence of O-H group and other functional groups. Optical phonon frequency (ν0) and Debye temperature (θD) are found to be composition dependent. Almond West Formalism reveals percolation type motion of polarons in the present system. The activation energy related to hopping frequency (EH) is found to decrease first and then increase with Ag2S content. Activation energy associated with the relaxation process (Eτ ) is found to increase first and then decrease with compositions. The temperature dependent relaxation process in the present system can be confirmed from the study of electric modulus formalism. Carrier mobility and carrier density in the present system have been estimated and correlated with conduction process. Carrier density is found to be maximum for x = 0.1 in the composition. The temperature and composition scaling of AC conductivity as well as M// spectra indicate that the electrical relaxation process is independent on temperature and depends on composition of the present system.
Acknowledgments
The financial assistance for the work by the DST-CRG (Department of Science and Technology, Govt. of India) via Sanction No. CRG/ 2018/ 000464 is thankfully acknowledged. A Chamuah is thankful to Department of Science and Technology, Govt. of India for providing junior research fellowship. Dr S Bhattacharya (PI) also acknowledges 'Sophisticated Analytical Instrument Facility (SAIF)'of Gauhati University, Guwahati 781014 for providing XRD facility. Prof. Dipankar Chattopadhyay, Department of Polymer Science and Technology, University of Calcutta, West Bengal, India is also thankfully acknowledged for providing FT-IR facility.
Data availability statement
The data generated and/or analysed during the current study are not publicly available for legal/ethical reasons but are available from the corresponding author on reasonable request.













