Abstract
Ternary Ag-In-S and quaternary Ag-In-Zn-S nanoparticles with different ratio of Ag/In/Zn/S are synthesized. The incorporation of Zn into Ag-In-S nanoparticles leads to the increase in the optical bandgap and the blue shift of photoluminescence (PL). The optical properties of these nanoparticles are significantly dependent on the chemical composition of nanoparticles. Time-resolved PL spectroscopy in nanosecond time regime is used to study the recombination processes of carriers, which involve the surface states and intrinsic crystallographic defects. These measurements support the donor-acceptor model, in which the PL is achieved by radiative recombination of the localized electron and hole.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Due to the unique optical and electronic properties, the colloidal nanoparticles have attracted much attention in the field of optoelectronics such as photovoltaic devices, light emitting diodes, photodetectors and bioimaging applications [1–8]. Although binary chalcogenide nanoparticles such as CdSe exhibit considerable optical properties [9, 10], their toxic heavy-metal elements limit their further applications. Toxic element-free nanoparticles have been an alternative such as ternary I-III-VI metal chalcogenide nanoparticles. Among them, ternary AgInS2 (AIS) and its analogues are of particular interest owing to the tunable photoluminescence (PL) wavelengths and high fluorescence quantum yield [11–27].
Since 1980s, the donor-acceptor pair transitions in bulk AIS have been investigated by the time-resolved spectra method [28]. The defect states such as vacancies and interstitial atoms have been found in bulk and film AIS [29–31]. Recently, most studies are focused on AIS nanoparticles, which exhibit broad PL spectra, relatively large Stokes shifts and long emission lifetimes. Their PL emissions are attributed to the recombination of carriers trapped in donor and acceptor levels, which are formed due to different kinds of defects in AIS nanoparticles such as sulfur and silver vacancies and interstitial atoms [32–37]. In addition, due to the high surface-to-volume ratio in these nanoparticles, other defects such as surface states cannot be ignored since surface trap states provide local sites for relaxation of photoexcited electrons, resulting in short PL lifetimes compared with deep trap states [12]. The PL of the nanoparticles can be tuned by controlling the diffusion of Zn into AIS to form quaternary Ag-In-Zn-S (AIZS). In 2007, composition tuning of AIS-ZnS solid solution and observation of PL emission from the near infrared to the green region were first reported by T. Torimoto et al [38]. Another effective way to tune the PL emission is to incorporate an alloying compound with wider bandgap such as ZnS to form core–shell structures [14, 39–42]. Moreover, other methods by doping Zn have been applied, including cation exchange of In or Ag with Zn [15, 43] and preparation of solid solution of (AgIn)xZn2(1−x)S2 [44–46]. Early studies have shown that photoluminescence quantum yield (PLQY) of (AgIn)xZn2(1−x)S2 nanoparticles initially increases and then decreases with further incorporating Zn. The explanation is that the addition of Zn eliminates non-radiative surface states at the first stage and increases the density of radiative channels within nanoparticles [46]. The decrease in PLQY with further addition of Zn is due to the enhanced non-radiative relaxations, which is related to the intrinsic defects. The change of the mean nanoparticle size is also accompanied by the variance of the structural imperfection and disorder [14], which is also one of causes responsible for the change in PLQY. Despite the extensive studies of these nanoparticles, their PL properties and related carrier dynamics have not been fully understood yet. We would like to see how the recombination mechanism changes when incorporating Zn ions into the lattice.
In this work, we focus on the effect of Zn amount on the PL properties and the related carrier dynamics in AIS nanoparticles. The PL emission band varies with the incorporation of Zn. The time-resolved PL measurements indicate that the radiative recombination involves the electrons trapped by donor and the holes trapped by acceptor. It enables the utilization of such ternary and quaternary semiconductor nanoparticles in photovoltaic cells and white light-emitting diodes [47].
2. Sample preparation
The synthesis process of AIS and AIZS nanoparticles can be found in the previous work [47], which are briefly described below. The chemicals used for synthesis included oleic acid (OA, 90%, from Aladdin reagent), oleylamine (OAm, 80%–90%, from Aladdin reagent), toluene (anhydrous, 99.5%), silver nitrate (AgNO3, 99.8% from Aldrich reagent), indium acetate (In(Ac)3, 99.99% from Aldrich reagent), zinc stearate (Zn(St)2, 90% from Aldrich reagent), sulfur (S, 99.99%, from Aldrich reagent), n-dodecylthiol (DDT, 99.8%, from Aladdin reagent) and 1-octadecene (ODE, 90%, from Aldrich reagent). The nanoparticles are obtained from a mixture of AgNO3 (0.1 mmol), In(Ac)3 (0.4 mmol), oleic acid (0.2 ml), n-dodecylthiol (0.75 ml) and 1-octadecene (4 ml) in a three-neck flask. The mixture was heated to 60 °C under flowing nitrogen with magnetic stirring and kept for 30 min. Then it was heated to 90 °C and kept for 10 min. Subsequently, sulfur (0.5 mmol) dissolved in oleylamine (0.5 ml) and 1-octadecene (0.5 ml) was added. To prepare AIS nanoparticles, the reaction solution was heated to 110 °C and kept for 30 min. To synthesize the AIZS nanoparticles, Zn(St)2 (0.05, 0.1, 0.2 mmol) dissolved in oleylamine (0.5 ml) and 1-octadecene (0.5 ml) was slowly injected immediately after the reaction solution was heated to 110 °C. The mixture was further kept for 30 min to form AIZS nanoparticles with different Ag/In/Zn/S ratio. For purification, a batch of toluene (10 ml) was added to the crude solution. The mixture solution was centrifuged at 10000 rpm at 5 min and the supernatant was decanted. Finally, the nanoparticles were re-dissolved in toluene for storage and study.
3. Characterization
UV–vis absorption spectra were measured by a spectrophotometer (UV-2100, SHIMADZU, Japan). The x-ray diffraction (XRD) patterns were examined by Cu Kα radiation (XRD-6100, SHIMADZU, Japan). TEM was recorded by an electron microscope (Libra 200 FE, Zeiss, Germany). The x-ray photoelectron spectroscopy (XPS) spectra were measured using an ESCA Lab220I-XL. The PLQY was obtained from a spectrofluorometer (Edinburgh Instruments Ltd, FLSP920, United Kingdom). The photoluminescence spectroscopy was implemented by a fluorescence spectrophotometer (PL: Agilent Cary Eclipse, Australia). The time-resolved PL spectra were obtained by a home-made streak camera equipped with a spectrometer. The excitation source was a 266 nm pulsed laser with a duration of ∼10 ps. All the experiments were performed at room temperature.
4. Experimental results and discussion
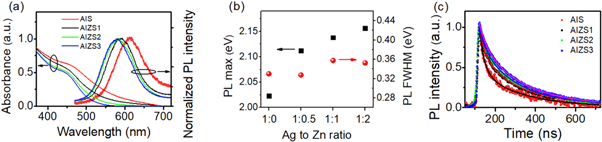
The cation precursor ratios of Ag/In/Zn/S are 1:4:0:0.5, 1:4:0.5:0.5, 1:4:1:0.5 and 1:4:2:0.5, which we refer to as AIS, AIZS1, AIZS2 and AIZS3, respectively. These nanoparticles are stable in air for many months. The XRD patterns of shown in figure 1(a) indicates that all nanoparticles exhibit a cubic spinel AgInS phase with diffraction peaks located at 27.7°, 45.8° and 53.9°. And with an increase of ZnS content, the diffraction peaks are slightly shifted to a higher angle, approaching to those of (111), (220) and (311) planes of the sphalerite ZnS phase (PDF#05-0566). It suggests the formation of AIZS nanoparticles. The nanoparticles exhibit torispherical shape due to the well dispersity, which can be seen from the TEM image (figure 1(b)). The size is found to be 4.2 ± 0.8 nm for AIZS2 nanoparticles. The high-resolution TEM image in the inset demonstrates good crystallinity with clear lattice fringes. The elemental composition of nanoparticles are also measured with EDS, which evidences that Zn ions are incorporated into nanoparticles (table S1 (available online at stacks.iop.org/MRX/8/085002/mmedia)). XPS was further carried out to determine the valence states of the ions in nanoparticles. The Ag 3d exhibits two peaks located at 367.1 eV and 373.2 eV, corresponding to Ag 3d5/2 and Ag 3d3/2. The In 3d also shows two peaks centered at 444.2 and 451.5 eV, indexing to In 3d5/2 and In 3d3/2. While the Zn 2p is located at 1021.0 and 1044.6 eV, corresponding to Zn 2p3/2 and Zn 2p1/2. The S 2p shows a peak at 160.8 eV. These results reveal that the valence states of the ions are Ag+, In3+, Zn2+ and S2− in the AIZS2 nanoparticles, which are in agreement with the reported data in the literature [48]. The UV–vis absorption spectra and PL spectra of nanoparticles are shown in figure 2(a). The absorption spectra exhibit a broad absorption band from ∼350 nm to 700 nm and an increase in absorption with increasing the photon energy. Similar to the previous studies, no distinct excitonic peaks can be clearly seen and are hidden in tail absorption due to the intraband gap transitions arising from the defect states. The absorption edge is blue-shifted and the absorption coefficient decreases in the near infrared and visible spectrum range. It is attributed to the Zn incorporation into the lattice of nanoparticles. The nanoparticles exhibit typical broad PL emissions in the visible spectral range. The large Stokes shifts with respect to the Zn incorporating is observed (figure 2(b)). The FWHM of PL emission is around 330 meV. The large stoke shift indicates that the band-edge emission is almost absent and defect-induced emissions are dominant in these alloyed nanoparticles [46].
Figure 1. (a) XRD patterns of AIS and AIZS nanoparticles. The bottom lines are the standard diffraction lines of cubic AIS and cubic ZnS. (b) Size distribution and TEM image of AIZS2 nanoparticles.
Download figure:
Standard image High-resolution imageFigure 2. (a) Absorbance and PL spectra of nanoparticles dispersed in toluene. (b) PL max and FWHM. (c) Time-resolved PL decay curves of nanoparticles.
Download figure:
Standard image High-resolution imageFigure 2(c) shows the time-resolved decay curves of integrated PL for AIS and AIZS nanoparticles. The long-time decay of the PL emission indicates the existence of multiple emissive states with multi-exponential decay feature in these nanoparticles. The PL decay traces can be well fitted by a bi-exponential function with a fast component and a slow component as follows
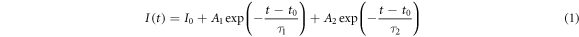
where  (
( ) is the fast (slow) decay time constant, and
) is the fast (slow) decay time constant, and  (
( ) is the corresponding intensity weight factor. Fitting results show that the fast time constant is in the region of 25–40 ns and the slow time constant is in the region of 180–220 ns. The intensity weight factor of fast decay component decreases after the incorporation of Zn. The average lifetimes
) is the corresponding intensity weight factor. Fitting results show that the fast time constant is in the region of 25–40 ns and the slow time constant is in the region of 180–220 ns. The intensity weight factor of fast decay component decreases after the incorporation of Zn. The average lifetimes  can be given as intensity weighted times
can be given as intensity weighted times  It is found that the average times extend into hundreds of nanoseconds and increase with increasing the Zn content. The average PL lifetimes of AIZS nanoparticles are comparable to previously reported AIZS systems [12], but shorter than (AgIn)xZn2(1−x)S2 nanocrystals [46] and other AIZS nanocrystals [14, 27, 35, 43].
It is found that the average times extend into hundreds of nanoseconds and increase with increasing the Zn content. The average PL lifetimes of AIZS nanoparticles are comparable to previously reported AIZS systems [12], but shorter than (AgIn)xZn2(1−x)S2 nanocrystals [46] and other AIZS nanocrystals [14, 27, 35, 43].
Further analyses of the PL decay dynamics at different wavelengths provide more insight into the optical properties and the effect of Zn doping on defect states of the nanoparticles. Figures 3(a)–(d) show the PL emission decay spectra recorded by a streak camera coupled with a spectrometer for the four samples. Examples of PL emissions at different delay times are fitted with a Gaussian function as shown in figures 3(e)–(h). With increasing the delay time, the significant red shift of PL emission peak can be observed for all four samples, which indicates that PL decay rate is dependent on emission wavelength. The long-wavelength emissions decay slower than the short-wavelength emissions. Compared with AIS nanoparticles, the PL red shift of AIZS nanoparticles is less pronounced with increasing the delay time. It results most probably from the incorporation of Zn ions into the nanoparticle leading to the partial elimination of the lattice defects.
Figure 3. PL decay spectra for (a) AIS, (b) AIZS1, (c) AIZS2, and (d) AIZS3. (e)–(h) The corresponding time-resolved PL spectra at delay times of 122, 172, 272 and 372 ns, fitted with a single Gaussian function. PL intensity decay curves monitored at the wavelengths of (A) 555 ± 5 nm, (B) 580 ± 5 nm, (C) 605 ± 5 nm, and (D) 630 ± 5 nm for (i) AIS, (j) AIZS1, (k) AIZS2, and (l) AIZS3. The PL decay behavior is fitted with a bi-exponential function.
Download figure:
Standard image High-resolution imageSuch behavior can be primarily described with a donor-acceptor model [13, 34, 36, 42, 49–53], which is a commonly accepted emission mechanism in I−III−VI2 QDs. In this model, the observed PL emission originates from the radiative recombination involving the electrons trapped by donor and the holes trapped by acceptor. The PL photon energy  can be expressed as [13, 34, 36, 52]
can be expressed as [13, 34, 36, 52]
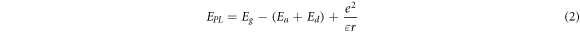
where  is the optical bandgap (or exciton energy),
is the optical bandgap (or exciton energy),  and
and  are the binding energies of the acceptor and donor that trap a hole and an electron, respectively.
are the binding energies of the acceptor and donor that trap a hole and an electron, respectively.  is the Coulombic electron-hole interaction, which is a function of the distance
is the Coulombic electron-hole interaction, which is a function of the distance  between the trapped charge carriers.
between the trapped charge carriers.  is the electron charge, and
is the electron charge, and  is the dielectric constant of the material.
is the dielectric constant of the material.
Equation (2) shows that the energies of the emitted photons can be affected by at least three factors, which include: (1) a size distribution of nanoparticles, (2) a distribution of binding energy of the acceptor and donor, and (3) a distance distribution of trapped electron and hole in nanoparticles. A size distribution of the nanoparticles in a colloidal ensemble induces a distribution of  since the energy of interband transition is a function of nanoparticle size in particular for the nanoparticles with sizes smaller than the exciton diameter. The contribution of this factor to the energy distribution can be investigated by using the size-selected nanoparticles. A recent study shows that PL spectral width remains almost unchanged for nanoparticles in the size-selected fractions [42], which confirms that the variation in the distribution of nanoparticle size does not significantly affect the PL spectral width of nanoparticles. The acceptor and donor defects responsible for emissions in such nanoparticles result from the various nanoparticle lattice defects and other possible origins of electron and hole traps (e.g., surface defects). Trapping the photo-generated electrons and holes in these defects leads to the distribution of the binding energy
since the energy of interband transition is a function of nanoparticle size in particular for the nanoparticles with sizes smaller than the exciton diameter. The contribution of this factor to the energy distribution can be investigated by using the size-selected nanoparticles. A recent study shows that PL spectral width remains almost unchanged for nanoparticles in the size-selected fractions [42], which confirms that the variation in the distribution of nanoparticle size does not significantly affect the PL spectral width of nanoparticles. The acceptor and donor defects responsible for emissions in such nanoparticles result from the various nanoparticle lattice defects and other possible origins of electron and hole traps (e.g., surface defects). Trapping the photo-generated electrons and holes in these defects leads to the distribution of the binding energy  and
and  The contribution of this factor to the PL width can be studied by a modification of the defect states either by decreasing the temperature and reducing the possibility of the thermal activation [53] or by passivating the nanoparticle surface with a shell [14, 39–42]. The Coulombic electron-hole interaction depending on the distance between the trapped electron and hole is the third contribution to the PL energy distribution. The radiative recombination of the trapped electrons and holes located closer to each other leads to higher energy photons, while the lower energy photons are generated from the trapped carriers situated further apart [27]. It is challenging to clearly evaluate the contribution of this factor to the PL energy broadening, in which the size variation of the nanoparticles, the temperature and excitation energy should be taken into account. It is commonly accepted that these three factors contribute to the distribution profile of PL energy.
The contribution of this factor to the PL width can be studied by a modification of the defect states either by decreasing the temperature and reducing the possibility of the thermal activation [53] or by passivating the nanoparticle surface with a shell [14, 39–42]. The Coulombic electron-hole interaction depending on the distance between the trapped electron and hole is the third contribution to the PL energy distribution. The radiative recombination of the trapped electrons and holes located closer to each other leads to higher energy photons, while the lower energy photons are generated from the trapped carriers situated further apart [27]. It is challenging to clearly evaluate the contribution of this factor to the PL energy broadening, in which the size variation of the nanoparticles, the temperature and excitation energy should be taken into account. It is commonly accepted that these three factors contribute to the distribution profile of PL energy.
Figures 3(i)–(l) illustrate extracted PL decay curves of nanoparticles at different emission bands. These curves typically show longer lifetimes for emissions at longer wavelengths. Emission bands at shorter wavelengths (555 ± 5 nm) have fast decays of 12.1, 19.4, 24.7, and 25.2 ns for AIS, AIZS1, AIZS2 and AIZS3 nanoparticles, respectively (table S2). The time constants of slow decays vary from around 100 ns for emission band with shorter wavelengths (555 ± 5 nm) to over 250 ns at longer wavelengths (630 ± 5 nm). The ratio of the fast decay component decreases with increasing wavelength from 550–635 nm. The fastest radiative processes are generally attributed to band-edge emission, which can be confirmed when monitoring near the bandgap. The PL lifetime increases with an increase in emission wavelength, which strongly indicates that the dominant radiative mechanism at longer wavelengths in these AIZS nanoparticles is the radiative donor-acceptor recombination. PL decay lifetimes intermediary between the band-edge emission and the donor-acceptor recombination are free-to-bound transitions, which evolve an electron in the conduction band recombining with a hole in an acceptor defect level [27]. Note that  monitored at the PL emission maximum has a value close to the average lifetime of all emissions.
monitored at the PL emission maximum has a value close to the average lifetime of all emissions.
Generally, the defects in these nanoparticles can be categorized into two types: intrinsic crystallographic defects and surface defects [27, 43]. Surface defects provide local sites for relaxation of photoexcited electrons and holes, leading to the faster recombination. Intrinsic crystallographic defects are deep trap states, which provide deep donor-acceptor recombination pathway responsible for slower decays. The average time increases with increasing Zn content, which indicates that the addition of Zn eliminates the lattice defects on the nanoparticle surface and the lattice cation vacancies, thereby reducing non-radiative decay pathways. The passivation of surface states is similar to that of ZnS coating, and the elimination of cation vacancies changes the local environments of the deep traps from AIS to AIZS [53]. The size of these nanoparticles also changes with incorporating the Zn. The PL lifetime is found to be dependent on the bandgap of nanoparticles. The smaller the size is, the shorter the lifetime is. As the smaller nanoparticles exhibit the higher surface to volume ratio, the lifetime can be significantly affected by radiative/non-radiative surface-related transitions. Due to larger overlap of carrier wave function, the probabilities for donor-acceptor transition and recombination for smaller particles is higher than those for larger ones.
To further examine the influence of the Zn inclusion on the radiative and non-radiative recombinations, we calculate the radiative and non-radiative decay rates ( and
and  ) by taking into account the absolute photoluminescence quantum yield (
) by taking into account the absolute photoluminescence quantum yield ( ) and PL lifetimes
) and PL lifetimes  for all samples [42]. The radiative decay rate can be expressed as
for all samples [42]. The radiative decay rate can be expressed as  From the total decay rate
From the total decay rate  on can obtain non-radiative decay rate
on can obtain non-radiative decay rate  The calculated values of
The calculated values of  and
and  are given in table 1. As shown in figure 4,
are given in table 1. As shown in figure 4,  increases from 53.1% for AIS to 60.3% for AIZS2, which indicates that the radiative recombination pathway increases with increasing Zn concentration.
increases from 53.1% for AIS to 60.3% for AIZS2, which indicates that the radiative recombination pathway increases with increasing Zn concentration.  decreases to 57.5% for AIZS3, which means that the non-radiative relaxations are enhanced due to the further addition of Zn, resulting probably in an increase in strain of the lattice.
decreases to 57.5% for AIZS3, which means that the non-radiative relaxations are enhanced due to the further addition of Zn, resulting probably in an increase in strain of the lattice.
Table 1. Summary of PL parameters for different compositions of nanoparticles.
| Sample | Ag/In/Zn | PLQY (%) | τlifetime (ns) | krad (s−1) | knr (s−1) |
|---|---|---|---|---|---|
| AIS | 1:4:0 | 53.1 | 173.5 | 3.06 × 106 | 2.70 × 106 |
| AIZS1 | 1:4:0.5 | 58.3 | 180.9 | 3.22 × 106 | 2.31 × 106 |
| AIZS2 | 1:4:1 | 60.3 | 184.1 | 3.28 × 106 | 2.16 × 106 |
| AIZS3 | 1:4:2 | 57.5 | 190.5 | 3.02 × 106 | 2.23 × 106 |
Figure 4. PLQY of nanoparticles.
Download figure:
Standard image High-resolution imagePrevious studies show that the recombination evolving surface defects is mainly non-radiative, via the free-to-bound transition and donor-acceptor transitions [27, 51]. The surface states arising from dangling bonds could be significantly suppressed by forming a shell of ZnS as a capping material for the AIS nanoparticles [40]. The dominant radiative emissions have been attributed to the donor-acceptor recombination resulting from intrinsic crystallographic defects which include vacancies, interstitial atoms and antisite defects in nanoparticles by many theoretical and experimental studies [12, 27, 51–55]. However, the specified intrinsic defects is difficult to be addressed since the nanoparticle composition is complicated. Based on the previous studies and the experimental results in present study, we speculate that silver vacancies VAg, zinc atom on silver site ZnAg+ and zinc atom on indium site ZnIn- are main intrinsic defects responsible for donor-acceptor radiative emission. More studies on the further determination of main intrinsic defects and their energy level are required.
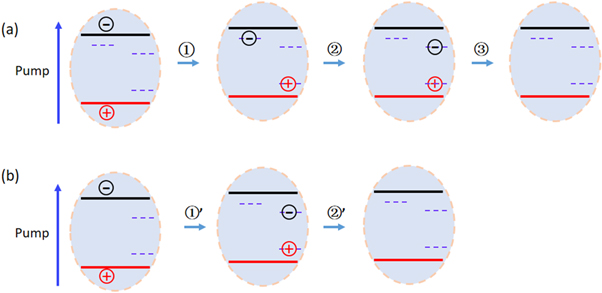
Based on these observations, we build a sample model to describe the charge carrier dynamics and radiative/nonradiative recombination processes in these nanoparticles as illustrated in figure 5. Upon photoexcitation, the electrons are excited into conduction band. And the holes residing in the valence band can be trapped by the acceptor on the subpicosecond time scales [26, 27]. In AIS nanoparticles (figure 5(a)), the electron moves first into the defect state (process ①) and subsequently into the donor state (process ②) [26, 27]. The trapped electron in donor state recombines radiatively with the hole localized in the acceptor state (process ③), resulting in nanosecond decay dynamics of the excited-state absorption. It is consistent with the long-time PL decay observation. In most of AIZS nanoparticles (figure 5(b)), the electron moves directly into the donor state (process ①') and then recombines radiatively with the hole localized in the acceptor state (process ②') on the nanosecond time scales.
Figure 5. Schematic illustration of the charge carrier dynamics and radiative/nonradiative recombination processes for (a) AIS and (b) AIZS nanoparticles.
Download figure:
Standard image High-resolution image5. Conclusion
We have presented the characterization and photophysical properties of the ternary AIS and quaternary AIZS nanoparticles with varying Ag/In/Zn/S ratios. The nanoparticles exhibit typical broad emissions in the visible spectral range. The incorporation of Zn results in the widening of the optical bandgap and the blue shift of PL emission maximum. The PL decay behaviors of these nanoparticles indicate that the surface trapping states are responsible for fast non-radiative recombination and the intrinsic crystallographic defects are responsible for long-time radiative recombination. Time-resolved PL spectroscopy shows that the addition of Zn into AIS nanoparticles leads to the increase in the PL decay times. The electron decay pathways play a key role in determining the process of electron-hole recombination. More attention will be paid to the elimination of electron and hole traps in order to develop high-quality AIZS nanoparticles.
Acknowledgments
This work was supported by the Defense Industrial Technology Development Program (Grant No. JCKY2017110C065) and the Presidential Foundation of China Academy of Engineering Physics (Grant No. YZJJLX2017010). The authors would like to thank Dr Zhigang Zang for his help in sample preparation and collecting linear absorption spectra.
Data availability statement
The data that support the findings of this study are available upon reasonable request from the authors.