Abstract
Chromium (VI) electroplating plays a vital role in the surface engineering industry for metallic materials due to its high hardness and excellent chemical stability. However, the reduction of Cr (VI) is a series of complex reactions that exhibits low current efficiency. As such, this low current efficiency in the process poses a significant challenge in the adoption of CR (VI) electroplating in the surface engineering industry. Methane disulfonic acid sodium salt (MDAS) is an essential catalyst for Cr (VI) electroplating. In this work, the effects of MDAS on the current efficiency of Cr (VI) electroplating process is studied. It is discovered that the current efficiency increases to about 17% when 4–6 g l−1 MDAS is added in the bath, which is an improvement over the current efficiency of 12% for its counterpart without MDAS. Furthermore, rotating disk electrode method is employed to investigate its interaction mechanism with regards to the reactions which have occurred during this process. It indicates that MDAS demonstrates a remarkable catalytic ability towards the electrochemical reaction which occurs at the lower-potential peak. This lower potential peak corresponds to the electrochemical reaction of Cr (VI) to Cr (0) or Cr (III) to Cr (0).
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
Nomenclature
| Abbreviations | Explanation |
| Cr | chromium |
| MDAS | methane disulfonic acid sodium salt |
| SO4 2− | sulfate anion |
| H+ | hydrogen cation |
| H2 | hydrogen |
| F− | fluorine ion |
| SiF6 2− | silicon hexafluoride ion |
| BF4 − | boron tetrafluoride ion |
| CrO3 | chromium trioxide |
| H2SO4 | sulphuric acid |
| Cr3+ | trivalent chromium ion |
| GC | glassy carbon |
| Pt | platinum |
| LSV | linear sweep voltammetry |
| f | radians per second for the rotating disk electrode |
| IL | peak current density, A m−2 |
| ic | the current density due to the chemical reaction, A m−2 |
| id | Levich limiting diffusion current density, A m−2 |
| n | the number of electrons transfer |
| F | Faraday constant, 96500 |
| A | the area of electrode, m2 |
| D0 | the diffusion coefficient for the reactant ion, m2 s−1 |
| v | viscosity, Pa·s |
| C0 | the concentration of reactant, mol l−1 |
| MSE | mercury/mercurous sulfate electrode |
1. Introduction
As the third hardest element behind diamond and boron, chromium is an extremely hard metal with a Mohs hardness of 8.5. Furthermore, chromium metal is prone to passivation which forms a thin protective surface layer on the metal [1]. Thus, hard chromium electroplating is a widely used method to prevent the interaction of the mechanical parts with the harmful agents. In addition, it can also imbue special physical properties to the surface such as increased wear resistance, low frictional coefficient, and enhanced aesthetic appearance of the surface of the workpiece. Cr (VI) electroplating is widely used in some industries, such as hydraulic system, aviation industry, and nuclear industry [2–4]. However, Cr (VI) electroplating suffers from a low current efficiency and sophisticated construction technology. This is largely due to the series of complex reactions involved in the reduction of Cr (VI) to Cr (0), which requires the transfer of a total of 6 electrons in this process. Thus, the effective strategy to address this problem is to develop novel high-efficiency catalyst for the reduction of Cr (VI) to Cr (0) [5–9].
The deposition of chromium metal from aqueous hexavalent chromium electrolyte occurs only in the presence of a certain amount of SO4 2−. SO4 2− plays a critical role in the formation of the cathodic film, which is necessary for the reduction reaction [10]. However, Cr (VI) electroplating process still suffers from a low current efficiency of 12%–15%, with the evolution of gaseous hydrogen (2H+/H2) acting as a competing reaction. As such, the current efficiency of Cr (VI) electroplating process is far lower than the electroplating processes of other common metals such as copper and nickel. In the early stage of the study, some catalysts such as fluorine-containing anions (F−, SiF6 2−, and BF4−) have been developed to broaden the current for electroplating bright Cr layer and to simultaneously increase its current efficiency [11]. However, fluorine-containing compounds can increase the corrosion rate of the counter electrode and the difficulty in controlling the electroplating process. Till now, the most versatile catalyst used in Cr (VI) electroplating is saturated sulfonic acids such as methane disulfonic acid and ethane disulfonic acid [12]. The use of such catalyst can effectively increase the current efficiency and broaden the current range, which makes it easier for bright electroplating.
In this work, it is shown that sulfonic acids have significant effects on the electrochemical reactions that occur during the Cr (VI) electroplating process. The impacts of sulfonic acids on Cr (VI) electrodeposition have also been studied by researchers. For instance, Efimov investigated the effects of methanesulfonic acid on the electrodeposition of chromium. It was reported that methanesulfonic acid can accelerate the deposition of chromium and increase the current efficiency by increasing the overpotential of the hydrogen evolution reaction on the surface of the workpiece [10]. Protsenko also investigated the kinetics and mechanism of the Cr (III) reduction, which subsequently discovered that it has an analogous mechanism for the bath with sulfate[13]. However, Distelrath argued that Cr (VI) electrodeposition proceeds via a different mechanism from that found with sulfuric acid, as the action of these two acids work in opposition to one another [3, 8]. Thus, it remains to be a debatable point with regards to the catalytic mechanism of sulfuric acid in the Cr electroplating reaction. In this present work, a rotating disk electrode setup is employed to investigate the effect of MDAS on the Cr (VI) electrodeposition reactions in the Cr (VI) bath. The effects of the addition of MDAS on the ion diffusion and electrochemical reaction are elucidated. The result shows that MDAS is able to demonstrate a remarkable catalytic ability in the electrochemical reaction of Cr (III) to Cr (0).
2. Experimental
2.1. Synthesis
The current efficiency of Cr (VI) electroplating process is measured using a home-made laboratory electrolytic cell, with a stainless-steel plate (dimension: 3 cm × 3 cm) as the cathode and a Pb-Sn alloy plate as the anode. As stainless-steel is the common workpiece for Cr coating and Pb-Sn alloy was the major anode for Cr electroplating industry. The distance between the anode and cathode is fixed at 50 cm. The hexavalent Cr bath is prepared with 20 g l−1 CrO3, 2.5 g l−1 H2SO4, 2.5 g l−1 Cr3+, and a certain concentration of MDAS as additives. In this test, a current density of 0.4 A cm−2 is used for the stainless-steel cathode, which is a specified value used in crack-free Cr electroplating industry. Also, an electronic coulometer is connected to a circuit in series to measure the total electricity consumed. Furthermore, the quality of the as-obtained electroplated layer is inspected using a dye penetrant testing according to the reports obtained from relevant researches [14, 15]. DPT-5 penetrant was used in this test and the piece was cleaned using a dye remover before evaluating the quality of Cr layer.
2.2. Characterization
For the electrochemical characterization of Cr (VI) reduction, the following stock solutions are used in this work; 20 g l−1 CrO3, 0.2 g l−1 H2SO4 and varied concentrations of MDAS (0, 0.2 g l−1, 0.4 g l−1, 0.6 g l−1, and 0.8 g l−1) as the catalyst. The solutions are firstly purged with N2 (with flow rate of 100 ml min−1) before use. The reduction reaction of Cr (VI) is performed on a glassy carbon (GC) surface in the above-mentioned solutions. The GC rotating disk electrode possesses a geometric area of 0.196 cm2 (diameter of 5 mm). The surface of the GC electrode is treated prior to use, with accordance to the previous report 14. linear sweep voltammetry (LSV) is carried out in a three-electrode cell using an electrochemical workstation (Princeton Applied Research Model VersaSTAT 3). A Pt electrode with a dimension of 2 cm × 2 cm is used as the counter electrode, while a mercury/mercurous sulphate electrode (MSE) is used as the reference electrode. All potentials mentioned in this work are with respect to the MSE reference electrode. LSV is scanned from the open circuit potential to −1.6 V with a scan rate of 20 mV S−1. The rotation speed of RDE is varied from 100 to 3600 rpm. A fresh GC electrode is used for each measurement to preclude artifacts from possible inactivation of the GC surface after being in contact with strongly oxidizing Cr (VI). To this end, a relatively diluted Cr (VI) bath is used in this study. All chemicals are obtained from commercial sources and are of the highest purity available. They are used as received unless it is otherwise stated. Double distilled water is used in the preparation of all solutions.
3. Results and discussion
The effects of MDAS on the current efficiency of hexavalent Cr plating is firstly studied using the electroplating test. In this work, a square stainless-steel electrode with a dimension of 4 cm × 4 cm, is masked with adhesive tape. The photographic image of the as-obtained Cr coating layer is shown in figure 1. As shown in figure 1, the Cr coating layer on the stainless-steel is relatively smooth with a metallic lustre. This coating layer can be considered to be of high quality as no apparent crack can be observed, which is an important aspect towards increasing its corrosion resistance and durability in industrial applications.
Figure 1. Typical Cr coating on stainless-steel substrate by electroplating process.
Download figure:
Standard image High-resolution imageBy changing the amounts of MDAS in the Cr (VI) bath, Cr coating layers with different amount of catalyst are prepared. The mass increase in the test electrode is recorded by weighing the mass of the stainless-steel electrode before and after electroplating. The electricity consumed during the electroplating process is measured with an electronic coulometer. The current efficiency is calculated based on the mass of the deposited Cr coating layer and the corresponding electricity consumption, with accordance to the references [16, 17]. In this work, the background solution comprises of 250 g l−1 CrO3, 2.5 g l−1 H2SO4, and 2.5 g l−1 Cr3+. Furthermore, various electroplating baths with different concentrations of MDAS are prepared based on the above-mentioned solution for the Cr (VI) electroplating test. The effects of the amount of MDAS on the current efficiency of hexavalent Cr electroplating is shown in table 1. As shown in table 1, the current efficiency for the bath without any MDAS added is about 12%. However, when the concentration of MDAS added into the bath reaches 4–6 g l−1, the current efficiency increases to about 17%. The results show that the addition of MDAS into a hexavalent chromium electroplating bath could increase the current efficiency. Also, the dye penetrant test results for the Cr electroplated layer without MDAS and that with 6 g l−1 MDAS are shown in figure 2. As shown in figure 2, there are no observable cracks on the surface of the Cr electroplated layer that is obtained from the bath with MDAS.
Table 1. Effects of the amount of MDAS on the current efficiency of hexavalent chromium electroplating process.
| MDAS addition (g) | 0 | 2 | 4 | 6 |
|---|---|---|---|---|
| Current efficiency (%) | 12.1 | 15.0 | 16.6 | 17.4 |
Figure 2. Dye penetrant test results for the Cr electroplating layer without MDAS (a) and with 6 g l−1 MDAS (b).
Download figure:
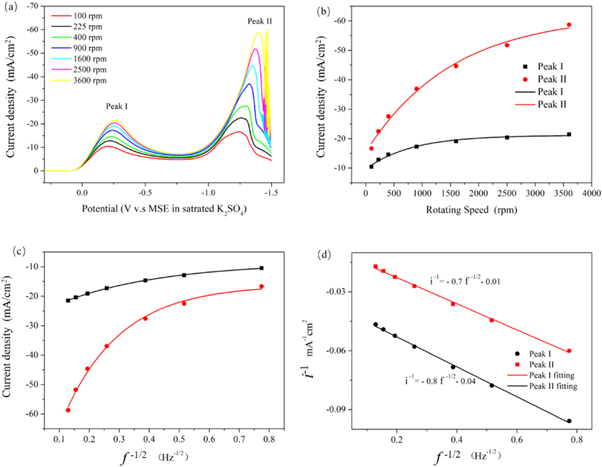
Standard image High-resolution imageRotating disk electrode voltammetry has proven to be an important method for the kinetic analysis of the electrochemical reaction. Figure 3(a) shows the typical voltammogram obtained in Cr (VI) bath using the rotating disk electrode setup. It is performed in a series of Cr (VI) baths using a rotating disk electrode with a fixed rotating speed of 900 rpm. As shown in figure 3(a), the reduction peak located at about −0.3 V corresponds to Cr (VI)/Cr (III) reaction (Peak I) [10]. In the voltage range between −0.4 V to −1.0 V, the electrode surface is partially blocked by a complex solid electrolyte film which can inhibit the reactions on the electrode, which is according to the report [12]. The reduction peak located at about −1.3 V corresponds to the reactions of Cr (VI)/Cr (0), Cr (III)/Cr (0), and H+/H2 (Peak II) [18, 19]. As shown in figure 3(a), the current density for both peak I and peak II can be enhanced by increasing the electrode rotating speed. The influence of electrode rotating speed on peak current density is shown in figure 3(b).
Figure 3. Rotating disk electrode voltammograms Cr (VI) bath (a) and the corresponding Peak current-rotating speed plots (b), with a rotating speed from bottom to top: 100, 225, 400, 900,1600, 2500, and 3600 rpm. Levich plots for the reduction of Cr (VI) with electrode rotating speed of 100, 225, 400, 900, 1600, 2500, and 3600 rpm, (c) and that with electrode rotating speed of 100, 225, 400 and 900 rpm (d).
Download figure:
Standard image High-resolution imageAs shown in figure 3(b), the current density of the two peaks increases rapidly at the low rotating speed range (100–900 rpm). On the other hand, current density remains relatively stable at high rotating speed range (1600–3600), especially for the current density of peak I. The plot of the current limiting IL versus the square root of the electrode rotating speed f1/2 (where f is expressed in radians per second) is the first step in the diagnosis of the rate-limiting step. The relationship between them can be described according to the following equation [20–22].
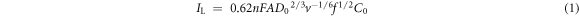
In equation (1), IL, n, F, A, D0, v, f, and C0 are the peak current density, the number of electrons transfer, Faraday constant, the diffusion coefficient for the reactant ion, viscosity, radians per second for the rotating disk electrode, and concentration of the reactant in the bath, respectively. Linear plot of IL versus f1/2 is diagnostic of the fact that the substrate transport in this solution is rate-controlling. On the other hand, a curvature in the IL versus f1/2 is diagnostic of the fact that substrate transport in this solution is an electrochemical-controlled process. Figures 3(c) and (d) show the Levich plots constructed based on the data presented in figures 3(a) and (b). As shown in figures 3(c) and (d), linear relationship is not observed for high rotating speed (when 0.4 < f1/2, 1600–3600 rpm). This result indicates that the reduction of Cr (VI) on the glass carbon electrode is a typical rate-controlling process at low rotating speed range. The linear behaviour with similar slope indicates that the reaction of Cr (VI) in this bath is a first order reaction with respect to the electroactive species and that the process is irreversible. The behaviour obeys the following equation [23–25].
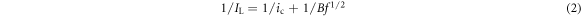
where IL is the total current density, ic is the current density due to the chemical reaction, and id = B f1/2 is the Levich limiting diffusion current density. The dependence of IL on the rotation frequency of the disk and of 1/IL on 1/f1/2 for the different electrode rotating speed for peak I and II are shown in figure 3(d). As shown in figure 3(d), 1/IL and f1/2 exhibit a relatively linear relationship for peak I and peak II, which agree with the law described in equation (2). Also, the corresponding linear fitting equations are presented in figure 3(d). This result indicates that the reduction of Cr (VI) is a mixture-controlled process which involves both diffusion and electrochemical processes.
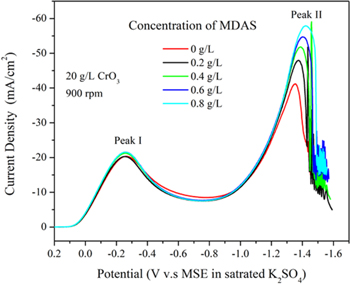
Furthermore, the influence of MDAS concentration on its voltammogram of Cr (VI) reduction is also studied. The voltammograms of Cr (VI) reduction for different MDAS concentrations in the solution are shown as figure 4. As the concentration of MDAS added increases, the current density of peak II increases uniformly while peak I shows negligible changes. This result indicates that MDAS could catalyse the reduction of Cr (VI) through enhancing the electrochemical reaction that occurs at peak II. Also, the addition of MDAS shows negligible impact on the middle voltage range, which indicates that there is no evidence that MDAS can influence the formation of complex cathodic film, which is reported as an interface that partially blocks the electrode surface during electroplating.
Figure 4. Typical voltammograms of Cr (VI) reduction in a 20 g l−1 CrO3 + 0.2 g l−1 H2SO4 solutions.
Download figure:
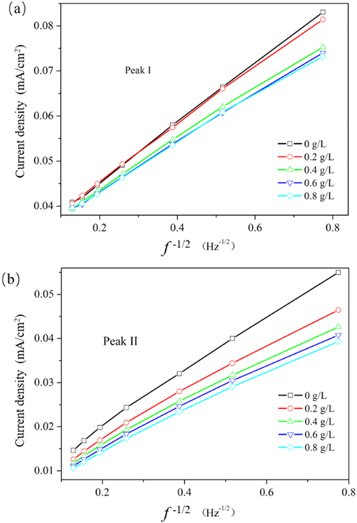
Standard image High-resolution imageThe dependence of IL on the rotation frequency of the disk and of 1/IL on 1/f1/2 for the different MDAS concentrations for peak I and II are shown in figures 5(a) and (b). As shown in figures 5(a) and (b), varying the concentration of MDAS added results in a linear relationship between 1/IL and 1/f1/2 for both peak I and peak II. This result can be well fitted to the law described in equation (2).
Figure 5. IL of peak I versus f1/2 plots (a) and IL of peak II versus f1/2 plots (b) for various concentrations of MDAS in 20 g l−1 CrO3 and 0.2 g l−1 H2SO4.
Download figure:
Standard image High-resolution imageConsidering that ic is the current density due to the chemical reaction and id = f1/2 is the Levich limiting diffusion current density, equation (1) can be presented as equation (3) [23–25].
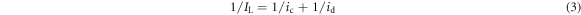
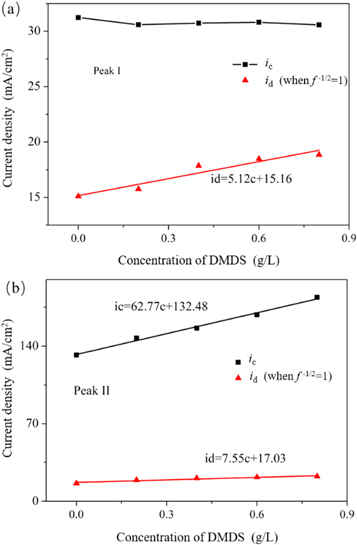
Thus, ic can be attributed to the contribution from the electrochemical-controlled process, while id can be attributed to the contribution from the diffusion-controlled process. Figures 6(a) and (b) present the dependence of ic and id on the MDAS concentration for peak I and peak II, respectively, which are constructed based on the results shown in figures 5(a) and (b). As shown in figures 6(a) and (b), the relationship between ic or id versus MDAS concentration can be fitted linearly, and the fitting results are inserted. It is noticed that ic for peak I is not affected by the addition of MDAS. However, ic for peak II is dramatically enhanced as the concentration of MDAS increases. This result indicates that MDAS is able to demonstrate a remarkable catalytic ability in the electrochemical reaction that occurs at peak II. According to the previous report, this electrochemical reaction may be attributed to the reaction of Cr (VI) to Cr (0) or Cr (III) to Cr (0). Furthermore, id also maintained a linear relationship with MDAS concentration for both peak I and peak II, while the slopes for their fitting lines are remarkably less than those of ic versus MDAS concentration for peak II. This result indicates that the introduction of MDAS could partly enhance the diffusion of reactant in the reduction of Cr (VI).
Figure 6. Dependence of ic and id on MDAS concentration for peak I (a) and peak II (b).
Download figure:
Standard image High-resolution image4. Conclusion
In this work, the influence and interaction mechanism of MDAS on the chromium electroplating process from Cr (VI) bath are studied. The electroplating experiment using Cr (VI) bath with MDAS is performed. The addition of MDAS (4–6 g l−1) into the bath can increase the efficiency of Cr (VI) electroplating from about 12% to 17%, and concurrently eliminates the cracks on the as-obtained Cr layer. Rotating disk electrode voltammetry analysis is performed to investigate the nature and the interaction mechanism of MDAS in Cr (VI) electroplating process. The result indicates that the reduction of Cr (VI) is a mixture-controlled process which contains both diffusion and electrochemical-controlled processes. By comparing the effects of the concentration of MDAS on the electrochemical-controlled process and that on the diffusion-controlled process, it is discovered that MDAS demonstrates a remarkable catalytic ability in the electrochemical reaction for Cr (0) generation.
Acknowledgments
This research is supported by the National Natural Science Foundation of China (No. 51702027) and the project of Sichuan Chengdu Science and Technology Bureau (No. 2019-YF05–02170-SN).
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).