Abstract
This research investigates the influences of different contents of Sc on the microstructure, mechanical, electrical conductivities and corrosion resistance properties of 7055 alloy. The effect of Sc can increase the non-uniform nucleation of the alloy and significantly refine the casting structure of the alloy. In addition, the addition of Sc not only inhibits grain coarsening, pins GBs and restrains recovery and recrystallisation, but also maintains the effect of fibre structure during the formation of high-density nano-Al3 (Sc, Zr) phase precipitation. Compared with pure 7055 alloys, the hardness, tensile strength, elongation, electrical conductivity and corrosion resistance of 7055 alloys with Sc are improved remarkably. After adding a little Sc, the grain size of the alloy is refined from 80um to 40um, and the properties of the alloy are improved. When Sc content is 0.25%, the tensile strength of the alloy increases from 623.1mpa to 685.9mpa, and the self-etching current density decreases from 1.56 × 10−5 to 8.74 × 10−7, and the corrosion resistance is also greatly improved.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Low density, high strength, good electrical conductivity and good corrosion resistance of metal materials have been the research focus of modern aerospace. Al-Zn-Mg-Cu (7xxx) alloys possess ultra-high strength and cracking toughness, which are widely used in aerospace, aircraft, rail transit, national defence and other domains [1–5]. With the rapid development of the aerospace industry, high requirements are introduced for the mechanical properties and density of structural materials. In addition, the 7xxx alloy must have outstanding conductivity to meet the demand for power transmission. Moreover, the 7xxx alloy is expected to have good power transmission performance and prolong the service life of the material, which requires materials with good corrosion resistance. Therefore, the performance of the Al-Zn-Mg-Cu alloy needs to be enhanced to achieve the requirements of high performance and low density in some key fields [4, 6–9].
In recent years, the research on the microstructure and mechanical properties of the 7xxx alloy containing Sc and Zr has attracted widespread attention. The engineering application of aluminium alloy is mostly concentrated on the ultra-high-strength 7-series alloy (Al-Zn-Mg-Cu). The effect of rare-earth elements on the microstructure and mechanical properties of the 7xxx alloy has been studied in considerable research [10, 11]. Adding microscale Sc can ameliorate the microstructure of Al alloy and improve its mechanical properties [12–14]. Several studies have investigated that Al3 (Sc, Zr) particles as a heterogeneous nucleation site of Al grains, for the as-cast alloys, form the primary Al3 (Sc, Zr) phases, which contribute to thin their microstructure and promotes their mechanical properties [15].
Although some encouraging results have been obtained in the application of Al-Sc alloys, the practical application and establishment of Al-Sc alloy series still need further study, and a systematic and in-depth study on alloying and application properties must be conducted. Most of the research focuses on improving the strength of the 7xxx alloy, which enhances the mechanical properties of the alloy. However, the research on the corrosion resistance of the 7xxx alloy is relatively lacking. With regard to application, in addition to the strength of the alloy, the service life of the alloy must also be extended. Consequently, the 7xxx alloy is required to have not only high strength, but also good corrosion resistance.
Therefore, this study explores the optimum mechanical properties, corrosion resistance and conductivity of the samples after adding the optimum Sc. In this study, after homogenisation treatment, rolling, solution treatment and aging treatment of 7055-xSc (x=0, 0.15, 0.25, 0.35, 0.45) alloys, the influences of different amounts of Sc on the microstructure, electrical conductivity, mechanical properties and corrosion property of 7055 alloys are studied.
2. Experimental
7055–xSc alloys with a rolled temperature of 420 °C were used as experimental samples. The composition of these samples is shown in table 1. These specimens initially experienced homogenisation treatment, solid solution treatment for 455 °C/2 h and then rapid water quenching. Finally, the samples were aged treatment at 120 °C/24 h.
Table 1. Chemical composition of the 7055–xSc alloys.
| Wt% | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Si | Fe | Cu | Mn | Mg | Cr | Zn | Ti | Zr | Sc | |
| 0.10 | 0.15 | 2.0–2.6 | 0.05 | 1.8–2.3 | 0.04 | 7.6–8.4 | 0.06 | 0.08–0.25 | ||
| 7055–0Sc | 0.0296 | 0.0672 | 2.3774 | 0.0046 | 1.8761 | 0.0018 | 7.9948 | 0.0342 | 0.0945 | 0 |
| 7055-0.15Sc | 0.0289 | 0.0612 | 2.2508 | 0.0020 | 2.0331 | 0.0012 | 7.9402 | 0.0448 | 0.0939 | 0.15 |
| 7055-0.25Sc | 0.0271 | 0.0620 | 2.2541 | 0.0018 | 2.0479 | 0.0013 | 7.9789 | 0.0430 | 0.1034 | 0.25 |
| 7055-0.35Sc | 0.0257 | 0.0622 | 2.3038 | 0.0022 | 2.1163 | 0.0015 | 7.9247 | 0.0418 | 0.0912 | 0.35 |
| 7055-0.45Sc | 0.0244 | 0.0588 | 2.1910 | 0.0022 | 2.0507 | 0.0013 | 7.7523 | 0.0354 | 0.1023 | 0.45 |
The sample size of 8 mm × 8 mm × 8 mm was derived from the as-cast ingots and rolled sheets. The microstructures were observed by optical microscopy (OM), scanning electron microscopy (SEM) and energy-dispersive spectroscopy (EDS) after being derived by the Keller reagent for 20 s. Thin sections were cut from the studied alloy for transmission electron microscopic (TEM) observation. The films were prepared by twin-jet electropolishing at 20 V in a solution of 25% nitric acid and 75% methanol solution cooled to 30 °C and observed with accelerating voltage of 200 kV by a TECNAIG2 20 electron microscope. Phases were determined by X-ray spectrometer (XRD), X-ray diffraction analysiswas carried out using CuKα , and 2θ from 10° to 90°. Hardness was measured using Vickers hardness equipment. The tensile test of sheet specimens was performed at room temperature and at test speed of 0.5 mm/ min. The polarisation curves of the 7055-xSc alloy were measured by a PS-268A electrochemical measuring instrument. The experimental environment was a solution with 3.5% NaCl concentration. The reference electrode was a saturated calomel electrode, and the auxiliary electrode was a platinum plate.
3. Results and discussion
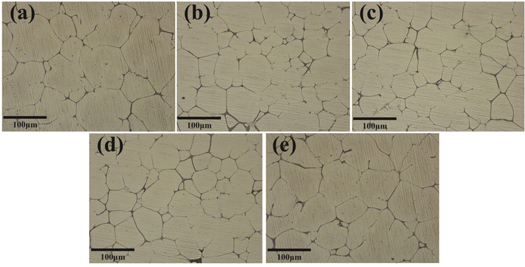
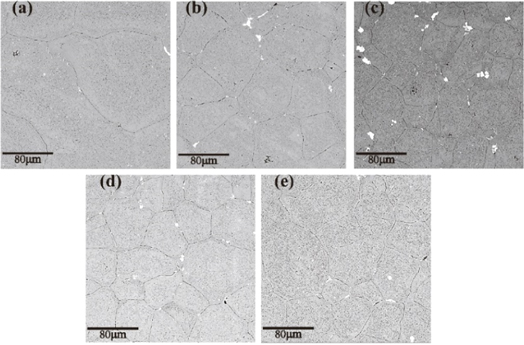
The SEM and OM images of the studied alloy were shown in figure 1, 2 and 3. OM images of 7055-xSc alloys with different Sc contents are shown in figure 1. It can be found from the figure, the as-cast organization of dentrite shaped ɑ-Al matrix, and at the same time can be clearly observed serious segregation in the as-cast samples, as-cast organization boundary exists for the coarse second phase, at the same time there are many other residual phase, at the grain boundary can still be observed in the insoluble phase containing Fe, and rod-shaped insoluble phase. The coarse grain size of sample7055-0Sc can be obviously observed, and the grain size was between 50–80um. In contrast, the grain size of sample added with rare earth Sc has been refined to varying degrees. When the Sc content was 0.25%, it has a strong effect on refining the grain size of the ingot, and the grain size is relatively uniform, and the grain size was between 30–40um [16]. As shown in figure 2, after homogenisation heat treatment, a great mass of the residual phases was dissolved in the α-Al matrix, which made the element distribution in grain and grain boundary (GB) uniform, and the GBs became thin and clear after homogenizing. The insoluble phases containing Fe element could still be viewed in the grain and GBs; simultaneously, the continuous residual phases along the GBs were transformed into discontinuous residual phases [14, 17]. The as-cast structure of the 7055-xSc alloy changed from dendrite to equiaxed grain, and the size of the grain was refined with the increase of Sc addition. After the Sc content reached 0.25%, the effect of grain refinement was remarkable. In the grain centre of the 7055-xSc alloys, block particles were formed, which were in the primary Al3 (Sc, Zr) phase. In the alloy melt, including Sc and Zr atoms, the metastable L12 Al3Zr phase was firstly generated during solidification [18]. When the temperature increased the line at 659 °C, Sc atoms diffused into the Al3Zr phase and replaced some of the Zr atoms to form the L12 Al3 (Sc, Zr) phase. For the Al3 (Sc, Zr) phase, the lattice parameters were similar to those of the Al matrix; the initial Al3 (Sc, Zr) phase during solidification promoted the liquid heterogeneous nucleation of the 7055 alloy, which further refined the as-cast alloy grain significantly and transformed dendrite into an equiaxial crystal [11, 15, 19].
Figure 1. OM images of as cast 7055-xSc alloys (a) 0%Sc, (b) 0.15%Sc, (c) 0.25%Sc, (d) 0.35%Sc,. (e) 0.45%Sc
Download figure:
Standard image High-resolution imageFigure 2. SEM images of 7055-xSc alloys after homogenizing (a) 0%Sc, (b) 0.15%Sc, (c) 0.25%Sc, (d) 0.35%Sc,. (e) 0.45%Sc
Download figure:
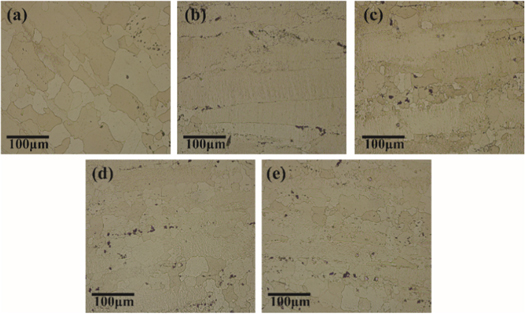
Standard image High-resolution imageFigure 3. OM micrographs of 7055-xSc alloys after aging (a) 0%Sc, (b) 0.15%Sc,. (c) 0.25%Sc, (d) 0.35%Sc, (e) 0.45%Sc
Download figure:
Standard image High-resolution imageFigure 3 has presented OM images of 7055-xSc alloys after aging. For the 7055 alloy, the artificial aging process eliminated several dislocations and vacancies. Therefore, the GBs provided precipitates favourable nucleation sites. Firstly, precipitates were formed in the GBs, then kept to germinate and transformed into a stable phase by absorbing the solute atoms around the particles; then, the nucleation of precipitates near GBs was limited to form the precipitate-free zone (PFZ) [4, 10]. Moreover, abundant Mg and Zn were used in the Al-lattice. The production of η phase and the growth of η'-precipitated phase were inhibited in the 7055 alloy added with Sc because of the high efficiency of precipitation nucleation. Therefore, compared with the 7055 alloy, the 7055-0.25Sc alloy had lower η phase density and finer η' phase size under the same heat treatment condition [19, 20].
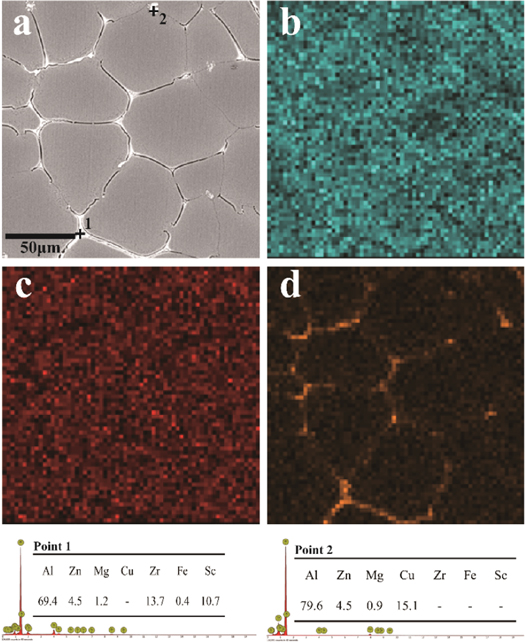
Figure 4 shows the SEM image and EDS analysis of the studied as-cast alloys. The EDS maps of Mg, Zn and Cu in the alloy were also shown. The concentration of Mg, Zn and Cu was directly proportional to the brightness of the EDS map (figure 4). The distribution of EDS showed the relative concentration of Mg, Zn and Cu, indicating that the segregation degree of Mg, Zn and Cu was different at GBs [21]. By EDS analysis, at point 1 of figure 4(a), the content ratio of Zr and Sc is about 1:1, so it contains some Al3 (Sc, Zr) phases formed during the solidification process of the alloy, which have not yet dissolved into the inner crystal cell. Point 2 of figure 4(a) was sheet eutectic, and it was the T phase (Al2Mg3Zn3) with a small amount of Cu dissolved [14, 22].
Figure 4. SEM images and corresponding EDS of the as-cast alloys: (a) SEM images, (b) Zn and (c) Mg (d) Cu.
Download figure:
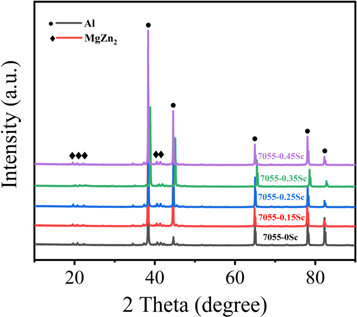
Standard image High-resolution imageFigure 5 has showed the XRD diffraction pattern of 7055-xSc alloys after aging treatment. As can be seen from figure 5 the main phases of the 7055-xSc alloy are ɑ-Al and η (MgZn2) phases. However, the diffraction peak of η phase is shifted from the standard diffraction peak of MgZn2, which is mainly due to the solid solution of Cu and Al in the phase. After solution treatment, solute atoms dissolve into the matrix, resulting in lattice distortion and lattice parameters increased. With the increase of lattice parameters, more residual phases dissolve in ɑ-A1 matrix. Therefore, the residual phase is completely dissolved in ɑ-A1 matrix. At the same time, the larger the lattice parameters, the greater the lattice distortion, which can improve the aging strengthening effect. The results are in good agreement with the test results of mechanical properties.
Figure 5. X-Ray diffraction patterns of the 7055-xSc alloys after aging treated.
Download figure:
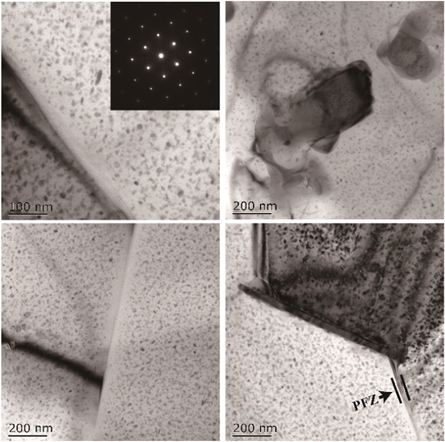
Standard image High-resolution imageFigure 6 shows the TEM micrographs of the 7055-0.25Sc alloy after aging. In addition, several nano-sized high-density precipitates could be observed in the grain after aging of the 7055-0.25Sc alloy. The significant strengthening mechanism of the 7xxx aluminium alloy after aging treatment was precipitation strengthening. The precipitation sequence of the 7xxx alloy was described as follows: supersaturate solid solution → Guinier–Preston (GP) zones → metastable η' phase → stable η phase. The η' phase played the primary role in the strengthening stage [4, 23].
Figure 6. TEM images of 7055-0.25Sc alloy.
Download figure:
Standard image High-resolution imageHigh-density rod-like nano-phase appeared in the 7055-0.25Sc alloy. The precipitated phase in the 7055 alloys significantly reinforced aluminium matrix η' or η. The results showed that the Al3 (Sc, Zr) phase with a face-centred cubic structure was formed in the aged 7055-0.25sc alloy, and PFZ was observed in the sample. After adding Sc to the 7055 alloy, the nanometre Al3 (Sc, Zr) phase was retained after solution aging treatment, and part of the phases was pinned at the GBs [3, 24]. Given the strong pinning effect of nanometre Al3 (Sc, Zr) relative to GBs, the recrystallisation of the 7055-xSc alloy during solution aging was effectively inhibited, and the fine grain structure was retained.
Figure 7 shows the conductivity of the 7055-xSc alloy, and the conductivity of the 7055-0Sc alloy was 32.8% IACS. By adding 0.15% contents of Sc to the 7055-0Sc alloy, the conductivity of the 7055-0.15Sc alloy decreased because the addition of alloy elements would affect the purity of the matrix, which was not conducive to conductivity. However, when the Sc content reached 0.25%, the conductivity of the 7055-0.25Sc alloy reached 32.9%. If the Sc content continued to increase, then the conductivity of the alloy would drop sharply. The results showed that proper Sc could improve the conductivity of the 7055 alloy, and trace amounts of Sc could form precipitated intermetallic compound with impurity elements Fe and Si, which reduced the influence of impurity elements on conductivity. In this case, Sc is a good rare-earth additive for grain refinement and improvement of conductivity and resistivity reduction. Electron scattering caused by lattice distortion determined the electrical conductivity. Defects, impurities and solid solute atoms were the main causes of lattice distortion [25, 26]. On the one hand, the decrease in resistivity caused by solute atoms was due to the release of Sc and Zr atoms, which alleviated lattice distortion. On the other hand, during subsequent aging, most dislocations would be renovated and form tiny recrystallised grains, which weakened the effect of dislocations on conductivity. The increase in conductivity after aging was due to the continuous precipitation of Sc and Zr atoms. As aging progressed, the supersaturation of solute atoms decreased, and the concentration of the solute in α-Al decreased rapidly at the beginning of aging, and then the precipitation rate gradually slowed down. Precipitation continued to form in the late aging period, resulting in a continuous decline in solute content, thereby providing an additional increase in electrical conductivity [11, 26, 27].
Figure 7. Conductivity of 7055-xSc alloy with different Sc content.
Download figure:
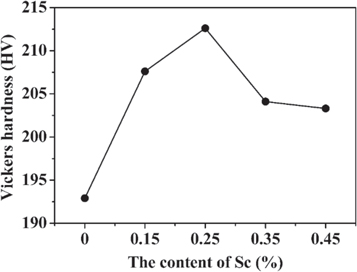
Standard image High-resolution imageFigure 8 shows the Vickers hardness curve of the 7055-xSc alloy with different Sc contents after aging. The hardness of the 7055-0Sc alloy was 192.9 HV. After scandium was added, the hardness of the 7055-xSc alloy was significantly improved, and the maximum hardness value reached 212.6 HV (7055-0.25Sc). Compared with the 7055-0Sc alloy, the hardness of the 7055-0.25Sc alloy increased by 10.2% and decreased rapidly after reaching the peak value. The hardness of the 7055-0.45Sc alloy decreased to 203.3 HV. The results indicated that the hardness of the 7055 alloy could be remarkably improved by doping appropriate Sc under the same heat treatment condition. Significant increase in hardness was observed in the 7055-0.25Sc alloy as compared with the 7055-0Sc alloy.
Figure 8. Vickers hardness of 7055-xSc alloys with different Sc content.
Download figure:
Standard image High-resolution imageThe hardness of 7055-xSc increased with the addition of Sc, and the hardness value was the highest when Sc content reached 0.25% because the grain size incessantly reduced and reinforced coherent Al3 (Sc, Zr) particles [28–30]. The 7055 alloy was a representative age-hardening alloy, whose hardness could be increased after aging derived from the formation of GP zones and metastable η' phase in the matrix. During deformation, the GP zones and η' phase would be sheared by dislocation motion. Increasing the size and volume score of the GP zones and η' phase resulted in good hardening effect, thereby increasing hardness [30].
Figure 9 shows the tensile properties of the 7055-xSc alloy with different Sc contents. The results of the mechanical property test were shown in table 2. Under the same heat treatment condition, the properties of ultimate tensile strength and elongation of the samples containing Sc were better than that of samples without Sc content. The ultimate tensile strength of the 7055-0Sc alloy was 623.1 MPa, and the elongation was 10.0%. Compared with the 7055-0Sc alloy, the ultimate tensile strength of the 7055-0.25Sc alloy was 685.9 MPa, and the elongation was 18.3%. The mechanical property was remarkably improved. However, the ultimate tensile strength of the 7055-0.35Sc and 7055-0.45Sc alloys was 653.1 MPa and 632.8 MPa, respectively, and their elongation was 16.7% and 16.2%, respectively. Furthermore, the mechanical property was reduced.
Figure 9. Stress-strain curves of 7055-xSc alloys.
Download figure:
Standard image High-resolution imageTable 2. Tensile properties of 7055-xSc alloys with different content of Sc.
| UTS (MPa) | EL (%) | |
|---|---|---|
| 7055-0Sc | 623.1 | 10.0 |
| 7055-0.15Sc | 676.1 | 12.7 |
| 7055-0.25Sc | 685.9 | 18.3 |
| 7055-0.35Sc | 653.1 | 16.7 |
| 7055-0.45Sc | 632.8 | 16.2 |
The high GBs and precipitation strengthening of the 7055-0.25Sc alloy provided higher tensile strength than that of the 7055-0Sc alloy. The high thermal stability of the 7055-0.25Sc alloy was due to the coarsening of the metastable phase and precipitation inhibition of the transition stable phase caused by the adjunction of Sc [18, 24]. The 7055-0.25Sc sample exhibited higher hardness, UTS and EL compared with the 7055-0Sc samples. The 7055-xSc alloys contained several Zr elements, which served as a grain-refining agent. Compared with adding Sc elements alone, adding Sc and Zr simultaneously could generate Al3 (Sc, Zr) dispersion particles. Primary Al3 (Sc, Zr) could be used as Al grain during solidification of all other nuclear sites, which was conducive to refine the grain size. The nano-precipitates led to the accumulation of dislocations in the aluminium alloy during stretching, thereby increasing the ultimate tensile strength and improving the elongation of the 7055 alloys. In the current study, the 7055-0.25Sc sample showed a better work hardening rate than the 7055-0Sc sample because a finer precipitate was produced. Therefore, the 7055-0.25Sc alloy obtained high ultimate tensile strength and elongation [31].
The excellent elongation of the 7055-0.25Sc alloy was due to the following reasons. Firstly, the addition of trace Sc could produce Al3 (Sc, Zr) particles. The Al3 (Sc, Zr) particles were fixed with the GBs of the alloy during deformation and subsequent solution treatment. Therefore, the grain size of the 7055-0.25Sc alloy was finer than that of the 7055-0Sc alloy. Tiny grain structure improved extensibility because the redistribution of stress during stretching prevented strain localisation. Therefore, a considerable elongation was achieved before failure [32, 33]. Secondly, Al3 (Sc, Zr) particles conduced to pin GBs and dislocations, and during the tensile test, the accumulation of dislocations was delayed, thereby suppressing the crack propagation of the 7055-0.25Sc alloy [20, 34].
As shown in table 3 and figure 10, the corrosion resistance of the 7055-xSc alloy with Sc addition was better than that of the sample without Sc. Ecorr was a thermodynamic parameter that reflected the corrosion trend. As a dynamic factor, Icorr could directly reflect the corrosion rate [16, 35]. Compared with the 7055-0Sc alloy, the corrosion current density of 7055 alloys with Sc addition tended to decrease. From the perspective of corrosion kinetics, the corrosion current density of the 7055-0.45Sc alloy (8.74 × 10−7) was two orders of magnitude lower than that of the 7055-0Sc alloy (1.56 × 10−5). The corrosion rate of the alloy was inversely proportional to the current density; thus, the corrosion rate of the 7055-0.45Sc sample was slow (figure 7 and table 3). The corrosion resistance of the alloy was improved by adding Sc, which was related to the size and distribution of precipitates. In the sample with refined grains after Sc insertion, abundant GBs might reduce the corrosion rate by the following three aspects: (1) the passivation kinetics was accelerated, and then, the intensity of the galvanic couple between the grain interior and GBs was reduced; (2) the fine grain structure contributed to the formation of thicker and denser passivation films; and (3) grain refinement also affected precipitation/grain size and distribution [16, 22]. It can be seen from figure 11, 12 and table 4 that with the increase of Sc content, the AC impedance value of 7055 alloy gradually increases, indicating that the corrosion resistance of the alloy is getting better and better. Comparing the Nyquist diagrams of the five alloys, it can be seen that the arc of 7055 alloy is the largest and the corrosion resistance is the best when the Sc content is 0.45%, which is consistent with the polarization curve analysis results.
Table 3. Electrochemical character of 7055-xSc alloy.
| Ecorr(V) | Icorr(A/cm2) | |
|---|---|---|
| 7055-0Sc | −0.690 | 1.56 × 10-5 |
| 7055-0.15Sc | −0.705 | 1.46 × 10-6 |
| 7055-0.25Sc | −0.695 | 1.58 × 10-6 |
| 7055-0.35Sc | −0.683 | 1.56 × 10-6 |
| 7055-0.45Sc | −0.703 | 8.74 × 10−7 |
Figure 10. Polarization Curves of 7055-xSc alloys.
Download figure:
Standard image High-resolution imageFigure 11. Nyquist diagram of 7055-xSc alloys.
Download figure:
Standard image High-resolution imageFigure 12. Bode diagram of 7055-xSc alloys.
Download figure:
Standard image High-resolution imageTable 4. Electrochemical fitting impedance of 7055-xSc alloys.
| C1(Ω−1·cm−2) | Rpo (Ω·cm2) | C2(Ω−1·cm−2) | Rct | |||
|---|---|---|---|---|---|---|
| Y1 | n1 | Y2 | n2 | (Ω·cm2) | ||
| 7055-0Sc | 4.22 × 10-3 | 0.718 | 16.72 | 1.38 × 10-4 | 0.731 | 52.82 |
| 7055-0.15Sc | 8.59 × 10-5 | 0.727 | 19.80 | 1.88 × 10-3 | 0.415 | 71.74 |
| 7055-0.25Sc | 9.13 × 10-5 | 0.752 | 23.99 | 1.46 × 10-3 | 0.467 | 72.7 |
| 7055-0.35Sc | 1.14 × 10-3 | 0.741 | 34.62 | 1.14 × 10-5 | 0.839 | 77.78 |
| 7055-0.45Sc | 7.88 × 10-4 | 0.784 | 28.93 | 2.49 × 10-5 | 1.000 | 87.08 |
For the 7055 alloy, the θ-CuAl2 and PFZ phases on the GBs might be used as the anode phase compared with the Al matrix in the corrosion environment, and both phases could be dissolved preferentially, but the tendency of the θ-CuAl2 phase as the anode was more serious, which caused the corrosion to expand along the GBs [16, 17, 22]. Therefore, the corrosion performance of the alloy was primarily determined by the size and distribution of the precipitates at the GBs. Al3(Sc, Zr) particles were precipitated in 7055 alloys with Sc content, which was close to the crystal structure and lattice constant of the Al matrix. Consequently, the particles had an excellent grain refining effect, making the alloy structure more uniform and fine. Furthermore, the 7055 alloy could prevent the subcrystal structure and movement of dislocation and inhibit the precipitation and aggregation of the θ-CuAl2 phase at the GBs, thereby reducing the corrosion sensitivity of the alloy [16, 35, 36].
4. Conclusions
The effects of proper Sc on the properties of the 7055 aluminium alloy were researched. The following conclusions were drawn:
- (1)After the addition of Sc in the 7055 alloy, the initial Al3 (Sc, Zr) phase generated during casting promotes the heterogeneous nucleation and refines the grain.
- (2)The appropriate amount of Sc not only promotes the η' stage of uniform precipitation, but also inhibits the η' stage of coarsening and prevents its transformation into η phase 7055 alloy.
- (3)The 0.25% amount of Sc improves the mechanical properties of the 7055 alloy, such as tensile strength, hardness and elongation, and it also improves the corrosion resistance of the alloy, extends the service life of the alloy and increases the strength. Moreover, the conductivity of the alloy is improved.
Acknowledgments
This work was supported by The National Natural Science Foundation of China (U20A20276) and the Science and Technology Major Project of Guangxi Province (AA17204021-3).
Conflicts of interest
The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this paper.