Abstract
Highly efficient LPG sensor working at room temperature was developed using a simple and cost-effective route. For this purpose, ZnO/SnO2 heterostructure was synthesized using the hydrothermal route and thin films of the material were prepared. X-ray Diffraction revealed all the crystal parameters including grain size, texture coefficient, dislocation densities, surface area which are necessary for a sensor. Also, particle size, zeta potential, and conductivity were observed using nanozetasizer. Heterojunctions at the surface of the film were viewed by Scanning electron microscopy. An optical band-gap of ∼3.85 eV was measured using UV–vis absorption spectrum. Further, the film was used as room ambient sensor for different concentrations of LPG. Among them, the best sensor response and sensitivity of 276.51 and 3.78 respectively were obtained for 2.0 vol% of LPG whereas minimum response and recovery time of 10 s and 15 s were obtained for 0.5 vol% of LPG.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Liquefied petroleum gas (LPG) is a combustible gas, which threatens human beings as well as the environment. LPG is widely used in residents and industries. LPG consists of propane (5%–10%), butane (70%–80%) and propylene, butylene, ethylene and methane (1%–5%) [1]. Occupational Safety and Health Administration (OSHA) and National Institute for Occupation Safety and Health (NIOSH) are the agencies that calibrated the lower explosion limit (LEL) of propane as 2.2 vol% and butane as 1.8 vol% in the air [2, 3]. Therefore, any type of leakage of LPG may be highly hazardous which enforces several research groups all over the world to work on the development of an innovative LPG sensor for the entire range from LEL to upper explosive limit (UEL) at room temperature [2].
Nanoscale metal oxides are of remarkable current interest to scientists and engineers because it has the potential of their emerging applications from catalysts, sensors, and microelectronic devices to energy conversion devices including fuel and solar cells. The intrinsic characteristics of the metal oxide, in the form of nanoparticles, for example, large surface to volume ratio and Debye length comparable to their dimensions, enhances their usefulness in these emerging applications. Metal oxides e.g. ZrO2, MnO2, CoO, ZnO, TiO2, VO2, MgO, SnO2, CuO, Fe2O3 etc. based gas/humidity sensors play an important role due to their plentifulness, low price, and easy manufacture [4–7]. In these devices, the electrical resistance of the material is adjusted by phenomenon occurring at the surface of the material, the adsorbed air oxygen ions, and the target gases. These devices are sensitive to many toxic and flammable gases and are used extensively in climate protection, safety processes, monitoring, and medical equipment such as breath analyzer [8, 9]. Metal oxide semiconductor-based gas sensors attained restricted achievement because of their higher operating temperature and less selectivity. For the enhancement of the performance of gas sensor, in the past decade, researchers developed the heterostructured metal oxides-based sensor [10]. The heterostructured nanomaterials include electronic effects such as band bending due to Fermi level equilibration, charge carrier separation, depletion layer manipulation, chemical effects such as decrease in activation energy, targeted catalytic activity, synergistic surface reactions and geometrical effects such as grain refinement, surface area enhancement and increased gas accessibility [11].
Tin dioxide (SnO2) consisting of a tetragonal arrangement of the atoms which has a stable phase under normal condition namely rutile or mineral form viz., cassiterite and zinc oxide (ZnO) has wurtzite hexagonal structure. Both are the n-type transition metal oxide and have wide-bandgap. Both the ZnO and SnO2 have chemical stability, good optical transparency and electrical conductivity that make them a very attractive material for catalysis, optoelectronic devices, solar panels, and sensors.
Recently, S. Choudhary et al designed a LPG gas sensor based on hexagonal ZnO nanorods. In this work, the hollow ZnO nanorods exhibited the maximum sensor response of 49% with response and recovery time as 39 s and 46 s towards 100 ppm LPG at an operating temperature of 120 °C [12]. In another work, U.T. Nakate et al detected the LPG at room temperature by nanocrystalline CdO thin film for 1–10 vol% of LPG and it was found that the lowest response of 4.6% was found for 1 vol%, whereas 20% for 10 vol% of LPG [13]. Also, M. Singh et al fabricated perovskite barium titanate thin film LPG sensor for detecting 0.5–4.0 vol% of LPG [14]. The maximum sensitivity was found as 3.5 with sensor response 250 for 4.0 vol% of LPG. In another work, they fabricated nanostructured strontium ferrite thin film for detection of LPG below LEL [15]. In this work, the maximum sensitivity was found as 7 with sensor response 602 for 5.0 vol% LPG. The response and recovery time for 0.5 vol% LPG was found as 20 s and 40 s. Table 1 presents the recent literature review showing different types of metal oxide-based gas sensors. From the table it may be seen that the semiconductor metal oxide gas sensors have two major shortcomings; primarily, low sensitivity and another, high operating temperature [16–18]. As reported highly sensitive LPG sensor typically works at high operating temperature (200 °C–300 °C) and causes high power consumption to trace a small concentration of LPG, therefore, to enhance the sensitivity and other sensor attributes for low concentration of LPG, we need to employ nanocomposite materials for developing the LPG sensor.
Table 1. Recent literature review depicting metal oxides-based gas sensors.
| Detection range of LPG | LPG Conc. | Materials | Response time (s) | Recovery time (s) | Sensor response | Operating temp. (°C) | Authors | References. |
|---|---|---|---|---|---|---|---|---|
| 0.5–4.0 vol% | 4.0 vol% | BaTiO3 | 30 | 60 |
 = 250 = 250 | RT | Singh et al | [14] |
| 0.5–5.0 vol% | 5.0 vol% | SrFe12O19 | — | — |
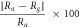 = 602 = 602 | RT | Singh et al | [15] |
| 100–800 ppm | 500 ppm | PANI-Nb2O5 | 30 | 50 |
 45.21 45.21 | RT | Kotresh et al | [19] |
| 400–1200 ppm | 1000 ppm | n-Bi2S3/p-PbS | 170 | 300 |
 71 71 | RT | Ladhe et al | [20] |
| 1300–5200 ppm | 2600 ppm | ZnO | 525 | 140 |
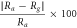 = 38 = 38 | 300 | Gaurav et al | [21] |
| 1000–500 ppm | 5000 ppm | ZnO | — | — |
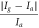 = 80 = 80 | 200 | Nksoi et al | [22] |
| 1.0–4.0 vol% | 4.0 vol% | NiFe2O4 | 220 | 250 |
 = 62.3 = 62.3 | RT | Srivastav et al | [23] |
| 100–1000 ppm | 1000 ppm | Au-activated ZnO | 18 | 36 |
 = 59 = 59 | 300 | Khojier et al | [24] |
| 50–500 ppm | 500 ppm | SnO2 | 9 | 15 |
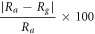 = 93.4 = 93.4 | 250 | Thomas et al | [25] |
The present article reports the synthesis of ZnO/SnO2 nanocomposite heterostructure by hydrothermal method and its LPG sensing. The synthesized nanocomposite was characterized using various tools like XRD, SEM, Zeta-Nanosizer and UV–vis spectrophotometer. Due to large active sites, ZnO/SnO2 has special magnetic resonance and absorbing properties that direct better sensing properties.
2. Experimental section
2.1. Preparation of ZnO/SnO2 heterostructured powder
Zinc acetate dihydrate and tin chloride dihydrate were used as the starting materials. 0.2M aqueous solution of Zn(CH3COO)2.2H2O and 0.1M aqueous solution of SnCl2.2H2O were mixed and stirred at room temperature for 30 min 0.05M NaOH solution was added dropwise to the mixture with continuous vigorous stirring till the pH of the solution became neutral. The solution was then transferred to a 100 ml Teflon-lined stainless-steel autoclave. Heat and pressure treatments were then given to the mixture by maintaining the temperature at 180 °C for 20 h. After cooling to room temperature, the obtained solution was centrifuged and washed with distilled water and ethanol, then dried at 80 °C for 5h in a hot air oven and calcined at 500 °C for 2h @ 10 °C min−1 in a programmable furnace. The final product obtained was ZnO/SnO2 heterostructure. The synthesis process of ZnO/SnO2 is depicted with the help of a flowchart shown in figure 1.
Figure 1. Synthesis flow chart to obtain ZnO/SnO2 heterostructures.
Download figure:
Standard image High-resolution image2.2. Preparation of thin film of ZnO/SnO2 heterostructure
The glass substrate of dimension 1 × 1 cm2 was taken and cleaned using distilled water and ethanol followed by acetone in an ultrasonic cleaner. Further, the substrates were dried at 80 °C on a hot plate for 15 min to remove VOCs. Then, the substrates were ready for film fabrication. A dilute solution of ZnO/SnO2 was made in isopropyl alcohol (IPA) while sonicating by ultrasonic waves for 4 h. The dilute solution was obtained which was then spun on the rinsed substrates using spin coater at a speed of 3500 rpm for 30 s and dried at 50 °C for 10 min on a hot plate. The film fabrication process of coating and drying were repeated three times for achieving the required thickness.
2.3. Characterizations
XRD pattern of the prepared powder of ZnO/SnO2 heterostructure was recorded by glancing angle x-ray Diffractometer (Bruker D8 advanced Ecosystem), equipped with monochromatic Cu-Kα as radiation source (40 kV and 20 mA). Surface morphological study of the material was done by the Field emission scanning electron microscope (JEOL, JSM 7610F) equipped with W (Tungsten) hairpin filament & LaB6 gun operated at 20 kV. The particle size of the nanocomposite was analyzed by a Particle size analyzer (Nanozetasizer-ZS90). Optical characterization of the sample was performed by using UV–visible spectrometer (Evolution 201) to record the UV-Visible absorption spectra of ZnO/SnO2 heterostructures. Also, Fourier transform infrared spectrum was obtained using FTIR spectrophotometer (Thermoscietiic Nicole 6700) in the spatial frequency regime of 4000–400 cm−1.
2.4. Sensor fabrication
For LPG sensing, a gas chamber made of borosil glass along with the inlet and outlet knobs was designed. The inlet of the gas chamber was connected to the concentration measuring unit for the exact vol% of the gas inserted inside the chamber and an outlet knob for removal of LPG. A detailed description of the gas chamber was reported in our previous publication [14]. Silver contacts were made on the fabricated film which was then used as a LPG sensing element. For electrical measurements, Keithley electrometer (Keithley-6517B) was used.
3. Result and discussion
3.1. Crystal structure analysis
The x-ray diffraction pattern of ZnO/SnO2 heterostructure is depicted in figure 2(a). The diffraction peaks well match with the JCPDS card no. 79–0207 revealing ZnO wurtzite hexagonal structure (P63mnc space group) and with JCPDS card no. 21–1250 revealing SnO2 tetragonal structure (P42/mnm space group. The crystallite size (Dhkl) from every peak was calculated by using Scherrer's formula [26] given in equation (1).
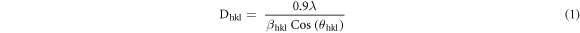
Here λ is the x-ray wavelength, θhkl is the Bragg diffraction angle and βhkl is the full width at half maximum (FWHM) of the main peaks in XRD pattern in radian. The average crystallite size of all peaks was obtained as 17.55 nm.
Figure 2. (a) XRD pattern and (b) W-H plot of ZnO/SnO2 heterostructure.
Download figure:
Standard image High-resolution imageThe Williamson and Hall (W-H) plot for relating the size and microstrain broadening was analyzed to estimate the average size and the strain in grains [27, 28]. The W-H plot shown in figure 2(b) has a slope value of 4.81 × 10–3, which reveals the compressive microstrain in ZnO/SnO2 heterostructure. The crystallite size estimated from the intercept value of W-H plot is 17.33 nm which is well matching with that calculated from Scherrer's formula (17.55 nm).
Also, the texture coefficient TC(hkl) for the domination of crystallite orientation in the grown sample has been calculated by using equation (2) from the XRD data. The TC (hkl) value greater than one reveals the preferred orientation, as well as the random orientation, are confirmed for the 1 value of TC. Among all texture coefficients, the value 1.59 associated with the plane (100) shows the most prominent growth of ZnO along (100) plane and in the case of SnO2 shows the value 1.26 associated with the (101) plane.

Here, I(hkl) is the measured relative intensity of the plane (hkl), Io(hkl) is the standard intensity of the plane (hkl), N is the reflection number and n is the number of diffraction peaks.
The quantitative phase analysis of ZnO (Hexagonal) and SnO2 (Tetragonal) structures was estimated approximately from the ratio of intensities of individual phase peaks to the intensities of all peaks in heterostructures using the relations given in equations (3) and (4):
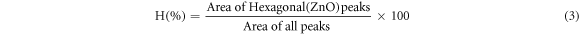
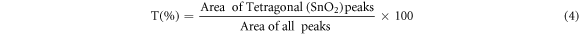
Here, H(%) denotes the percentage of hexagonal ZnO presence in ZnO/SnO2 and T(%) denotes the percentage of tetragonal SnO2 structure respectively. From the XRD data of ZnO/SnO2 heterostructure, it was found that tetragonal SnO2 has 53.18% whereas hexagonal ZnO has 46.91%.
The particle size of the ZnO/SnO2 heterostructure was analyzed by using Nanozetasizer in which the average particle size was measured by dynamic light scattering (DLS) method provided the nanomaterials are dispersed in solution. This method assumes the spherical particle with an aspect ratio of 1[29].
The graph obtained from Nanozetasizer for particle size analysis is shown in figure 3(a). From figure 3(a), it can be observed that the hydrodynamic diameter lies in the range 50–80 nm and the average particle size was found 64.23 nm.
Figure 3. (a) Particle size distribution and (b) Zeta potential distribution for ZnO/SnO2 nanoparticles dispersed in isopropyl alcohol at 25 °C.
Download figure:
Standard image High-resolution imageAlso, Zeta potential was measured using Nanozetasizer which defines the voltage at the edge of the shipping (shear) plane with respect to the bulk dispersion medium. Zeta potential predicts the long-term stability of nanoparticles. The measurement was done at 25 °C with iso-propyl alcohol solvent containing 1.0 mmol l−1 solvent. From figure 3(b), the value of zeta potential and conductivity was found −10.3 mV and 0.0116 (mS cm−1) respectively for ZnO/SnO2 heterostructure in isopropyl alcohol at 25 °C.
3.2. UV-Visible spectroscopy analysis
The UV-Vis (250–900 nm) absorbance spectrum for ZnO/SnO2 heterostructure is shown in figure 4(a). The absorption was observed in the UV region (∼352 nm). The slow increase in absorbance and broadening of absorbance peak may be attributed to defects and particle size distribution. The absorption coefficient (α) can be calculated by Beer–Lambert's relation shown in equations (5) and (6):
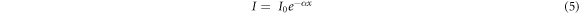
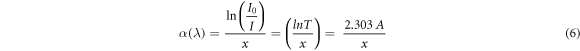
Where I0 is the initial photon intensity, I is instantaneous photon intensity and x is the thickness of the cuvette. The variation of α(λ) of ZnO/SnO2 heterostructure with wavelength is depicted in figure 4(b) and it was seen that as wavelength was increased, the absorption coefficient was decreased. The reason for this decrease may be due to internal electric field or distortion of lattice due to strain of charge carrier's inelastic scattering by phonons. The higher value of α in UV-region (330–400 nm) for the heterostructure is due to transition among extended states in valence and conduction bands.
Figure 4. UV–visible spectrum of ZnO/SnO2 heterostructure (a) Absorbance spectrum with inset of Tauc's plot and (b) Variation of absorption coefficient with wavelength.
Download figure:
Standard image High-resolution imageThe optical energy band gap value for ZnO/SnO2 heterostructure was investigated using 'Tauc's plot' (inset in figure 4(a)) [9]. The Tauc's relation given in equation (7) was used for obtaining the optical band-gap (Eg) [30].

Here hν is photon energy, α is absorbance coefficient, αo is a characteristic parameter, h is Planck's constant, and n is power factor. The value of n may vary depending upon the type of transition taking place. Here, the transition is a direct allowed type, therefore 'n' is taken as ½. The estimated value of Eg for ZnO/SnO2 heterostructure is ∼3.85 eV.
3.3. FTIR analysis
FTIR spectroscopy operated at 4000–400 cm−1 was used for the identification of the functional group in ZnO/SnO2 heterostructure. The corresponding spectrum is shown in figure 5. The data reveals that the absorption peak at 485.26 cm−1 is assigned to the Zn-O bonding, 534.18 cm−1 for Zn-O-Sn bonding and 601.68 cm−1 corresponds to O-Sn-O antisymmetric stretching. The broad absorption peaks observed at 3423.02 cm−1 reveals the O–H stretching due to the absorption of water molecules. Some additional peaks at 1635.34 cm−1, 1392.35 cm−1, and 1116.58 cm−1 are assigned to N–O stretching (nitro compound), O–H bending (due to alcohol), and C–O stretching (due to secondary alcohol). These peaks are occurring because in the synthesis process ammonia solution was used for precipitation and alcohol was used for washing.
Figure 5. FTIR spectra of ZnO/SnO2 heterostructure.
Download figure:
Standard image High-resolution image3.4. Raman spectra analysis
Raman spectroscopy is one of the effective tools to analyze the vibrational modes, sensitive defects, size, and structural phase purity of the nanomaterials. Figure 6 shows the Raman spectrum of ZnO/SnO2 heterostructure at room temperature. Zinc oxide has a hexagonal crystal structure with a point group  and space group (P63mc) and tin oxide has a tetragonal crystal structure with point group
and space group (P63mc) and tin oxide has a tetragonal crystal structure with point group  and space group P42/mnm. From figure 6 the Raman peaks observed at 334, 439, and 582 cm−1 [31] which attributed to the E2H-E2L, E2H, and E1L vibrational modes of hexagonal ZnO respectively, whereas the peaks at 381, 480 and 763 cm−1 can be described to the Eu, Eg and B2g vibrational mode of tetragonal SnO2, respectively [32]. The vibrational modes of ZnO as E2H-E2L, E2H, and E1L are associated with the vibrational of heavy Zn sublattice and an oxygen atom. Another mode such as Eu, Eg and B2g represent IR active mode, doubly degenerates mode, and B2g non-degenerate mode respectively. In non-degenerate Raman active modes (B2g) vibrations in the plane perpendicular to the c-axis while the doubly degenerates Raman Eg mode is along to the direction of the c-axis. The Sn-atom is in rest position, whereas the O-atom vibrates. The same Raman active modes were observed in earlier literature which is in good agreement with standard hexagonal ZnO and tetragonal SnO2 structure [33, 34].
and space group P42/mnm. From figure 6 the Raman peaks observed at 334, 439, and 582 cm−1 [31] which attributed to the E2H-E2L, E2H, and E1L vibrational modes of hexagonal ZnO respectively, whereas the peaks at 381, 480 and 763 cm−1 can be described to the Eu, Eg and B2g vibrational mode of tetragonal SnO2, respectively [32]. The vibrational modes of ZnO as E2H-E2L, E2H, and E1L are associated with the vibrational of heavy Zn sublattice and an oxygen atom. Another mode such as Eu, Eg and B2g represent IR active mode, doubly degenerates mode, and B2g non-degenerate mode respectively. In non-degenerate Raman active modes (B2g) vibrations in the plane perpendicular to the c-axis while the doubly degenerates Raman Eg mode is along to the direction of the c-axis. The Sn-atom is in rest position, whereas the O-atom vibrates. The same Raman active modes were observed in earlier literature which is in good agreement with standard hexagonal ZnO and tetragonal SnO2 structure [33, 34].
Figure 6. Raman spectrum of ZnO/SnO2 heterostructure at room temperature.
Download figure:
Standard image High-resolution image3.5. Morphological analysis
The morphological analysis of ZnO/SnO2 heterostructure film was done at 1 μm and 100 nm scales using a FESEM and shown in figure 7(a) and (b). It can be observed from figures that the particles have formed spherical clusters leaving the voids on the film. These clusters together with voids serve as active centres for the adsorption/desorption of gas molecules. Also, the heterostructured material due to the composite formation provides enhanced sensing activity by changing the material physics at the electronic level [15]. Also, the composition of the sensing film was analyzed by Energy-dispersive x-ray spectroscopy. The obtained spectrum is shown in figure 8 which clearly showed the existence of Zn, Sn, and O elements. The % composition of elements is shown in the inset of figure 8.
Figure 7. FESEM micrograph of ZnO/SnO2 heterostructure film at (a) 1 μm and (b) 100 nm scales.
Download figure:
Standard image High-resolution imageFigure 8. EDX spectrum of ZnO/SnO2 heterostructure film showing the presence of key elements.
Download figure:
Standard image High-resolution image3.6. Gas sensing measurement
The prepared film of ZnO/SnO2 heterostructure was exposed to LPG and the variation in electrical resistance with time for different concentrations of LPG was recorded using an electrometer at room temperature. The sensing behaviour was studied by focusing on its sensing parameters with the exposure of LPG. For this, sensor response, response time, recovery time, sensitivity, and selectivity were studied.
In the case of metal oxide, the gas sensors work on the principle of adsorption and desorption of air and target gas. Generally, sensitivity and % sensor response of the gas sensor for n-type material are defined by the relations given in equations (8) and (9) respectively [24]. Also, sensitivity is the change in the resistance of the film corresponding to the change in time and % sensor response is the percentage ratio of variation in resistance of the film after interaction with analyte gas to the resistance of the film in the air.
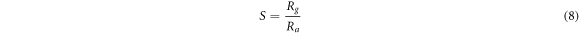
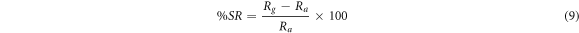
Here, Ra is stabilized resistance in air and Rg is sensor resistance after LPG injection in the gas chamber. Also, when the sensor reaches 90% of the maximum resistance of the film during adsorption of LPG, the time taken is called response time. Similarly, recovery time is the time required to come back 90% of its initial value during the desorption [14].
To study of the gas sensing properties of ZnO/SnO2, the LPG concentration (in vol%) was varied, and correspondingly different sensing curves were plotted and shown in figure 10. The curves represent the sensing characteristics for 0.5, 1.0, 1.5, and 2.0 vol% of LPG. Figure 9(a) shows the change in resistance of the film with the exposure time for different concentrations of LPG. From figure 9(b), it can be observed that % sensor response increases almost linearly with the increasing concentration of LPG which is good for an efficient gas sensor, and the maximum value was obtained as 276.51 for 2.0 vol% LPG. Figure 9(c) exhibits the sensitivity for different concentrations of LPG and the maximum sensitivity was found as 3.78 correspondings to 2.0 vol% LPG. The minimum response time was found for 0.5 vol% of LPG and the values of which are 10 s and 15 s respectively. The sensing characteristics of the film were repeated after two months of fabrication, a minute change (± 4%) was observed indicating the stability and reliability of the sensor.
Figure 9. Curves showing (a) LPG sensing characteristic (b) sensor response (c) sensitivity and (d) response/recovery time (e) repeatability and (f) selectivity.
Download figure:
Standard image High-resolution imageThe repeatability curve for ZnO/SnO2 film was observed by alternatively exposing 0.5 vol% LPG and air for three consecutive cycles at room temperature is shown in figure 9(e). The sensing response curve shows an almost similar response (Ra to Rg and again Rg to Ra) repeated to three consecutive cycles which display good repeatability. The repeatability of the reported LPG sensor after 3 weeks showed 97.59% repeatably. Selectivity is one of the most imperative and challenging parameters for gas sensors and sensor response towards a specific gas needs to be noticeably higher than those of other gases for selective gas detection. To study the selective behaviour of the ZnO/SnO2 heterostructure operating at room temperature, the gas sensor response towards LPG, ethanol and acetone with concentration of 0.5 vol% each were measured. The corresponding results are shown in figure 9(f). The ZnO/SnO2 heterostructure exhibits a higher % sensor response to LPG (∼138.51), whereas it shows a considerably lower sensor response to CO2 (∼79.17), acetone (∼56.18), and ethanol (∼28.56). In order to quantify the selectivity to LPG, the selectivity coefficient (K) of LPG to another gas is defined by equation (10).
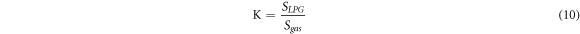
Here SLPG and Sgas are the sensor response of LPG and another gas respectively. The selective coefficient for the ZnO/SnO2 heterostructure was 3.54 towards CO2, 8.56 towards acetone and 16.18 towards ethanol. Higher K values imply more selective detection of LPG in presence of other gases, e.g. K value of 16.18 for ethanol indicates that the sensor responses 16.18 times higher for LPG than that for ethanol. Thus the experimental results indicate that the ZnO/SnO2 heterostructure-based sensor has good selectivity towards LPG. The required sensor parameters for different concentration of LPG has been tabulated and shown in table 2.
Table 2. Sensor parameters for ZnO/SnO2 heterostructure film towards various concentrations of LPG.
| LPG vol.% | Response time (s) | Recovery time (s) | Sensor Response | Sensitivity |
|---|---|---|---|---|
| 0.5 | 10 | 15 | 138.51 | 2.29 |
| 1.0 | 14 | 19 | 166.64 | 2.65 |
| 1.5 | 18 | 24 | 240.36 | 3.11 |
| 2.0 | 22 | 29 | 276.51 | 3.78 |
3.7. Gas sensing mechanism
The schematic of LPG sensing using ZnO/SnO2 heterostructure film is shown in figure 10. LPG is a reducing gas that donates an electron to n-type metal oxide semiconductor. Here ZnO is n-type semiconductor and also SnO2 n-type semiconductor with a wide optical bandgap. ZnO is coupled with SnO2 grains to form a n-n heterojunction. In this junction, electron transfer occurs from a semiconductor with low work-function (SnO2) to the other with high work-function (ZnO) until Fermi level equalizes. This creates an electron-depleted layer at the interface of ZnO and SnO2 which bends the energy band. The enhanced sensing performance of ZnO/SnO2 heterostructure is attributed to the combined effect of the formation of a depleted layer at the surface of individual ZnO and SnO2 as well as at the formation of hetero-junction between ZnO and SnO2. The electron density in the conduction channel of SnO2 is more in comparison to that of ZnO. After contact with each other, the electrons from the conduction channel of SnO2 move towards the conduction channel of ZnO forming the depletion region. This depleted region works as a barrier and is called the potential barrier. The energy band diagram is shown in figure 10. The LPG sensing using ZnO/SnO2 heterostructure thin film takes place in two stages i.e. oxidation and reduction [24, 25]. In the first stage, air oxygen oxidizes the ZnO/SnO2 heterostructure sensor surface by capturing conduction electrons. This gives rise to depletion region formation beneath the sensor surface and corresponding conduction channel narrows down. This depletion width depends on the number of air oxygen molecule adsorption and the number of conduction electrons available in the ZnO/SnO2 heterostructure at that temperature. This depleted ZnO/SnO2 heterostructure film offers additional resistance besides grain boundary resistance due to the increase in electric Schottky barrier potential (qVb) in the presence of air. This adsorption of oxygen continues until the equilibrium is reached between the interfaces due to the interactions of oxygen molecules with the chemisorption sites at that particular temperature. This progress of adsorbed oxygen species can be described by equations (11)–(13) as follows:

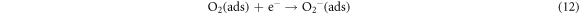
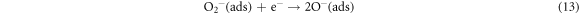
Figure 10. Schematic diagram showing band bending and gas sensing mechanism for heterostructured ZnO/SnO2 film as LPG sensor.
Download figure:
Standard image High-resolution imageThe negatively charged species present on the surface of the material play a crucial role in detecting the LPG. However, the reaction mechanism of LPG is complex and still not fully explained. The main component in the LPG is propane (C3H8) and butane (C4H10). There is an exchange of electrons between the LPG molecule and oxygen species adsorbed on chemisorption centres as shown by the reaction given in equations (14)–(15).


Here, CnH2n+2 represents the various hydrocarbon [14]. In the second stage, LPG is exposed to ZnO/SnO2 heterostructure. The LPG donates the electron after interaction with oxygen species and these ejected electrons recombine to form the electron-hole pair. In the case of n-type semiconductor, the resistance drastically increases in the beginning due to the rapid adsorption of hydrocarbon, afterwards, it increases slowly and finally gets saturated. When the flow of the LPG is stopped for the recovery characteristics, the oxygen molecules in the air will be adsorbed on the surface of the film. The depletion width of ZnO/SnO2 heterostructure sensor decreases by gaining electrons from oxygen ion species. Hence the corresponding channel width increases. Consequently, the resistance of the sensor decreases.
4. Conclusion
ZnO/SnO2 heterostructure has been successfully synthesized by the hydrothermal route. Thin-film of the material has been prepared by the spin coating technique. Various crystal parameters including grain size, texture coefficient, dislocation densities, surface area have been calculated using x-ray Diffraction. Particle size, zeta potential, and conductivity of the sample were observed using Nanozetasizer. A morphological image of the film was obtained using FESEM. EDX revealed the elemental information of the synthesized heterostructure. The optical band-gap was measured using UV-Vis absorption spectrum. FTIR spectrum confirmed the bond formation. Further, the film was investigated as LPG sensor at room ambient. Best sensor response and sensitivity were obtained as 276.51 and 3.78 respectively for 2.0 vol% of LPG whereas the least response and recovery time were observed as 10 s and 15 s respectively for 0.5 vol% of LPG.
Acknowledgments
The authors acknowledge the Department of Science and Technology (DST), Govt. of India for support under FIST program (ps-i-79-2019). Mr Ajeet Singh is grateful to Joint CSIR-UGC, Government of India for financial support in the form of Junior Research Fellowship (F.No16-9(June2017)/2018 NET/CSIR).
Data availability statement
The data generated and/or analyzed during the current study are not publicly available for legal/ethical reasons but are available from the corresponding author on reasonable request.
Conflicts of interest
The authors declare that there are no conflicts of interest.











