Abstract
This work investigated the discharge properties of the Mg-6Al-xCd alloy as a potential anode material for the Mg–air battery. The influence of the Mg-6Al-xCd alloy with cadmium content ranging between 0.5% and 1% on the electrochemical and discharge performances was investigated by means of microstructure characterization, electrochemical performance measurement and discharge test of the alloys. The results show that Mg-6Al-1.0Cd has the highest anode efficiency and specific capacity of 41.38% and 993.54 mAh/g, respectively. This good discharge performance is attributed to the addition of cadmium, which can inhibit self-corrosion and reduce the galvanic corrosion of the magnesium anode. The Mg-6Al-1.0Cd alloy can be used as a potentially ideal material for the Mg–air battery anode.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
As the worldwide fossil fuel energy crisis and air pollution become increasingly serious, the storage and conversion of renewable and clean energy will be in urgent need of rechargeable batteries in the coming decades. The magnesium–air (Mg–air) battery has a high standard electrode potential (3.09 V versus SHE) and theoretical energy density (3910 Wh kg−1) [1–5]. The Mg–air battery is low cost and its discharge product is pollution free. The Mg–air battery consists of three parts: the magnesium (Mg) alloy anode, the air cathode and the saline electrolyte (commonly a 3.5 wt% NaCl solution) [1]. However, the Mg–air battery has not been commercialized so far, and the two main problems affecting its application are the polarization of the Mg alloy and the low coulombic efficiency [1, 4]. The reasons for these problems are as follows: (i) the Mg anode can react with water, resulting in self-corrosion; and (ii) the sluggish kinetics of the oxygen reduction reaction in the air cathode. The Mg anode is the only active substance transferred by the battery. It determines the capacity of the battery and its performance will affect the discharge performance of the battery. Alloying is a significantly important method to improve the properties of the Mg anode [6–9].
The Mg-Al-Zn (known as AZ) alloys are an important system of Mg alloys. Aluminium (Al) can form the β-Mg17Al12 phase in the Mg alloy, and this phase can form a grid structure at the grain boundary, hindering the corrosion process of the Mg-6Al alloy. The denser the grid, the stronger this blocking effect will be [10–16]. In addition, research results showed that the Mg alloy anode had good electrochemical performance when the Al content was 6 wt% [15].
In recent years, the influence of cadmium (Cd) on the corrosion of Mg alloys has attracted much attention. Cadmium has a hexagonal structure, and its lattice parameters are similar to those of Mg in terms of being infinitely soluble [17–19]. Magdalene et al studied the effect of Cd on the corrosion of the Mg-6Al alloy. The authors pointed out that Cd could inhibit the hydrogen evolution process of the Mg alloy and that the uniform distribution of Cd inhibited the micro-couple effect of the Mg alloy, thus improving the corrosion resistance of the alloy [20]. The improvement of the corrosion resistance may help inhibit the self-corrosion reaction of Mg anodes and improve their performance. However, there are few studies about the effect of Cd on the discharge performance of anode materials in Mg–air batteries. In this paper, a small amount of Cd was added to Mg-6Al as the substrate to explore its effect on the discharge performance of the Mg–air battery with the Mg-6Al anode material to provide a research basis for the search for a better performance of the Mg–air battery anode material.
2. Materials and methods
2.1. Materials preparation
Table 1 shows the chemical composition of the Mg alloys used in this study. The Mg-6Al-xCd (x = 0, 0.5, 1 wt%) trinary alloys were prepared via casting using pure Mg (99.9 wt%), pure Al (99.9 wt%) and pure Cd (99.99 wt%). The raw materials were melted in a graphite crucible and then put into a resistance furnace at 1003 K. Subsequently, the molten metals were poured into steel moulds and cooled in the air. The steel moulds were preheated to 573 K before casting. The dimensions of the casting ingots were 60 × 120 mm (diameter × height). Both melting and casting were protected by an argon (Ar) gas atmosphere.
Table 1. Chemical composition of the alloys (wt%).
| Alloys | Cd | Al | Mg |
|---|---|---|---|
| Mg-6Al | 0 | 6.13 | Bal. |
| Mg-6Al-0.5Cd | 0.508 | 6.08 | Bal. |
| Mg-6Al-1.0Cd | 1.34 | 6.13 | Bal. |
2.2. Microstructure characterization
The samples were ground with different grades of SiC paper and polished with an abrasive paste. The phase morphology and distribution were observed by scanning electron microscopy (SEM, Ultra Plus) combined with an energy dispersive spectrometer (EDS). An x-ray diffractometer (XRD, X'Pert Pro) was used to detect the phase composition (scan rate: 4° min−1 and scan range: 20–85°).
2.3. Electrochemical measurement
The size of the electrochemical samples taken from the ingot was 10 mm × 10 mm × 5 mm. A ChenHua CHI660E electrochemical workstation was used to test the electrochemical behaviours. Three alloys were used as working electrodes, with a platinum foil as the counter electrode and a saturated calomel solution (SCE) as the reference electrode. The anode material, with a test area of 100 mm2 (10 mm × 10 mm), was ground to 3000 particles with SiC paper and then immersed in an aqueous solution of 3.5 wt% NaCl at room temperature. The polarization curve was recorded at a scanning rate of 1 mV s−1. The electrochemical impedance spectroscopy (EIS) was measured, using a voltage amplitude of 5 mV and a frequency range from 100 kHz to 0.1 Hz. The EIS pattern was eventually implemented using ZSimpWin software. At least three replications were conducted to ensure repeatability.
2.4. Mg–air battery tests
NEWARE battery testing system combined with the assembled Mg–air battery was used to record the discharge curve of the three anodes during a 10 h discharge at room temperature. The electrolyte was a 3.5 wt% NaCl solution. The test current densities were 0.5, 1, 2, 5, 10, 15 and 20 mA cm−2. After the discharge tests, the anode was cleaned in a chromic acid solution containing 180 g l−1 CrO3 to remove the surface products. Subsequently, the discharge surface was observed by SEM. The discharge capacity and anode efficiency were calculated using the mass loss method. Repeatability tests were performed on at least three repetitions.
3. Results and discussion
3.1. Microstructures evolution
The α-Mg and β-Mg17Al12 phases are primarily indexed in the XRD patterns of the three alloys as shown in figure 1(a), which is consistent with the results of the phase diagram analysis [21]. Figures 1(b)–(e) show the SEM image of the investigated alloys. The addition of Cd changes the shape of the β-Mg17Al12 phase. The shape of the second phase changes from dotlike to chainlike. According to the element distribution scanned by the EDS maps, Cd does not form a second phase with Mg and Al and has an even distribution in the alloy matrix, which is consistent with XRD.
Figure 1. (a) XRD patterns of the investigated alloys; SEM images of as-cast (b) Mg-6Al alloy, (c) Mg-6Al-0.5Cd, (d) Mg-6Al-1.0Cd alloy and (e) EDS.
Download figure:
Standard image High-resolution image3.2. Electrochemical behaviours of the investigated alloys
The open circuit potential (OCP) can determine the corrosion probability of the alloy [22]. Figure 2(a) shows the OCP of three Mg alloys in 3.5 wt% NaCl solution. The average OCP of each alloy is denoted by Eocp as shown in table 2. The OCP curves are stabilized after an immersion time longer than 1500 s. The Mg-6Al anode has the most negative OCP value (−1.603 V versus SCE). The addition of Cd significantly positive the OCP value. The Mg-6Al-0.5Cd alloy has the most positive value.
Figure 2. Electrochemical results of the three investigated alloys: (a) open circuit potential; (b) polarization curves and (c) electrochemical impedance spectroscopy of the investigated alloys in 3.5 wt% NaCl solution.
Download figure:
Standard image High-resolution imageTable 2. Electrochemical parameters of the magnesium electrodes.
| Alloys | Average open circuit potential V | Corrosion voltage V | Corrosion current μm/cm2 |
|---|---|---|---|
| Mg-6Al | −1.603 | −1.395 | 15.16 |
| Mg-6Al-0.5Cd | −1.595 | −1.277 | 1.645 |
| Mg-6Al-1.0Cd | −1.600 | −1.298 | 1.321 |
In terms of the polarization curves (figure 2(b)), the corrosion potential (Ecorr) also has a positive move due to the Cd addition. The Mg-6Al alloy has the most negative Ecorr, suggesting that the unmodified alloy has the largest corrosion driving force in a 3.5 wt% NaCl solution. The additional of Cd reduces this corrosion driving force. The corrosion current densities (Jcorr) were obtained by the Tafel extrapolation method according to the potentiodynamic polarization curve, as listed in table 2. The corrosion current density is proportional to the corrosion rate. The results of the polarization curves show that the addition of Cd decreases the Jcorr, suggesting that the modified Mg-6Al alloy has a relatively good corrosion resistance. In the anodic branch of polarization, the current of the modified anode increases with the positive potential shift faster than the current of the original anode, suggesting that the improved alloy has strong discharge activity during anodic polarization. The cathode branch of polarization curve is mainly related to the reaction of the hydrated proton on the anode surface of the Mg alloy [23]. At the same cathode potential, the cathode current density of the Mg-6Al-1.0Cd anode is lower than the current density of the Mg-6Al-0.5Cd anode, indicating that a high Cd content helps inhibit the hydrogen evolution reaction on the surface of the Mg anode during the cathodic polarization. According to the polarization curve, Cd makes the corrosion potential of the Mg anode shift positively but helps enhance the discharge activity during the anode polarization.
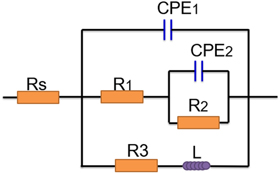
Figure 3. Equivalent circuit for the investigated anodes based on the EIS.
Download figure:
Standard image High-resolution imageFigure 2(c) shows the EIS at the OCP. The EIS of the three investigated alloys consists of two capacitive loops at high and intermediate frequency ranges, with one inductive loop at the low frequency region. The equivalent circuits simulating the two processes are shown in figure 3. The fitting values of the electrochemical impedance parameters tested using ZSimpWin software are shown in table 3. A constant phase element (CPE) is used to show the double layer capacitance [24]. Rs represents the solution resistance, which is related to the distance between the Luggin capillary and the working electrode. CPE1 is a double layer capacitance. Y0 and n are the two parameters of CPE1 describing the properties of the capacitor. The range of n is from 0 to 1, representing the variation tendency between the pure resistance and the capacitance of condenser. R1 is described as the charge transfer resistance of the three investigated alloys. R2 and CPE2 represent the Mg(OH)2 film resistance and the Mg(OH)2 film capacitor, respectively. L and R3 are the inductance and the resistance related to the desorption ability of the partial protective film on the surface of the Mg anodes [25–27]. Compared with the original alloy, the modified alloy has smaller transfer charge and thin film resistances. For the improved Mg alloys, the addition of Cd increases the desorption capacity of the intermediate products, which is conducive to the shedding of the Mg(OH)2 film [26]. According to the EIS results (figure 2(c)), the higher inductance value suggests a larger anode surface area involved in the discharge process and shows a stronger desorption ability for the discharge product. The L value is higher for the Mg-6Al-1.0Cd alloy than for Mg-6Al-0.5Cd, indicating that Mg-6Al-1.0Cd has a better desorption ability.
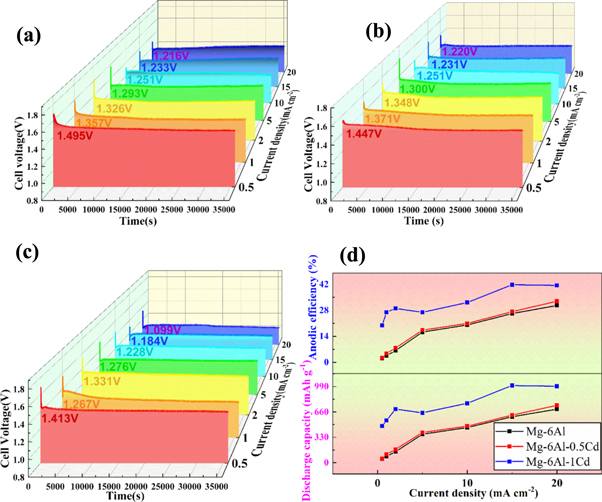
Figure 4. Discharge curves of the investigated anodes (a) Mg-6Al, (b) Mg-6Al-0.5Cd and (c) Mg-6Al-1.0Cd in 3.5 wt% NaCl solution at different current densities for 10 h. (d) Anodic efficiency and discharge capacity of the investigated anodes.
Download figure:
Standard image High-resolution imageTable 3. Electrochemical parameters of the equivalent circuits of the investigated anodes .
| Alloy | Rs Ω·cm−2 | CPE1 | R1Ω·cm−2 | CPE2 | R2 Ω·cm−2 | L | R3 Ω·cm−2 | |
|---|---|---|---|---|---|---|---|---|
| Y0 × 10−5Ω−1cm2Sn | n | |||||||
| Mg-6Al | 4.851 | 0.00001201 | 0.916 | 52.37 | 0.001194 | 15.44 | 25.01 | 159.9 |
| Mg-6Al-0.5Cd | 4.095 | 0.0000118 | 0.9427 | 28.75 | 0.001764 | 3.203 | 8.519 | 58.46 |
| Mg-6Al-1.0Cd | 4.579 | 0.000008815 | 0.9498 | 12.24 | 0.00000376 | 3.73 | 14.31 | 68.96 |
3.3. Discharge performance of the Mg–air batteries with Mg-6Al-xCd anodes
A constant current density is applied to the Mg anode during the discharge process so that the Mg anode can be electrochemically dissolved to output electrons. The average battery voltage, specific capacity and anode efficiency are important criteria to evaluate the discharge performance of the Mg anode. The average battery voltage can be obtained directly from the discharge curve. The discharge capacity is calculated as follows [28–30]:
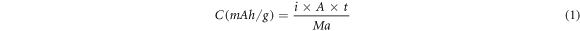
where C is the discharge capacity, i is the given current density (0.5, 1, 2, 5, 10, 15 and 20 mA cm−2), A is the surface area (cm2), t is the discharge time (10 h) and Ma is the theoretical mass loss (g). The utilization efficiency (η) of the anode is obtained by:
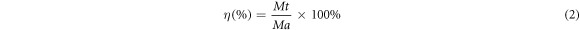
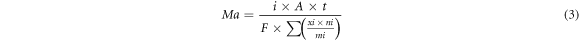
where Mt is the actual mass loss (g), F is the Faraday constant (96485 c mol−1), xi is Mg and the mass fraction of each element, ni is the number of electrons exchanged between Mg and each element and mi is Mg and the molar mass fraction of each element (g/mol).
Figures 4(a)–(c) present the discharge curves of the assembled Mg–air batteries with Mg-6Al-xCd (x = 0, 0.5, 1) anodes for 10 h. Figure 5(d) summarizes the average discharge cell voltages, the discharge capacity and the utilization efficiency of the three anodes [31]. The Mg–air battery with the modified Mg-6Al anodes has a higher cell voltage at small current densities (for instance, 0.5 mA cm−2). In addition, Mg-6Al-0.5Cd has the highest cell voltage at 20 mA cm−2 (1.22 V). The three anodes have a stable plateau of cell voltage, even at high current densities. The average cell voltage of the Mg–air battery is generally affected by its internal resistance, electrode polarization and discharge products. The high current density can accelerate the generation rate of discharge products on the anode surface, which is filled with Mg(OH)2, and promotes the decline rate of the average cell voltage of the Mg–air battery. According to the electrochemical results (table 3), the transfer charge resistance can be seen as a part of the internal resistance of the battery [29]. After adding Cd, the value of the transfer resistance decreases, indicating that the internal resistance of the Mg–air battery is small (table 3). Under high current densities, a small internal resistance can improve the discharge capacity and result in a more sufficient electric energy release.
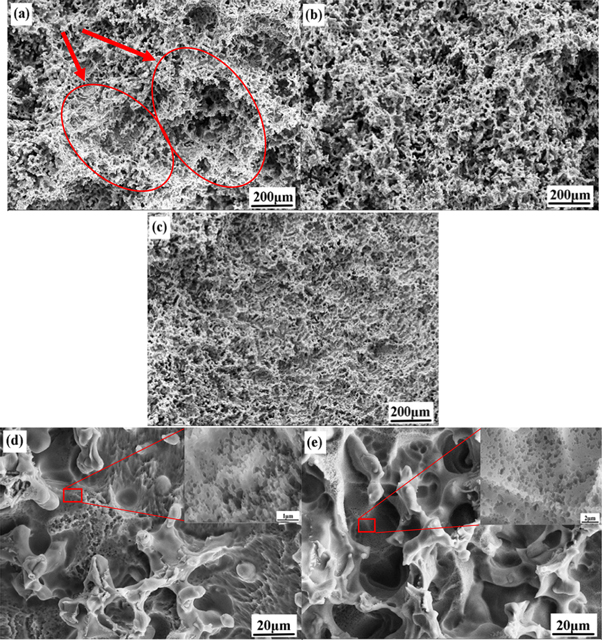
Figure 5. The surface morphologies of (a) Mg-6Al, (b) (d) Mg-6Al-0.5Cd and (c) (e) Mg-6Al-1.0Cd anodes after 10 h discharge testing in a 3.5 wt% NaCl electrolyte at a 0.5 mA cm−2 current density with the discharge products removed.
Download figure:
Standard image High-resolution imageThe results show that the modified anodes have higher discharge capacity and utilization efficiency than the original Mg-6Al anode. In addition, the values of these two parameters increase as Cd is added to the Mg-6Al anode. For Mg–air batteries, the discharge reaction is as follows [2]:
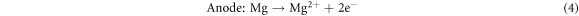
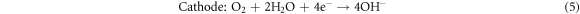
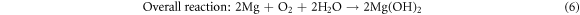
Magnesium reacts with water and oxygen in the air releasing energy. But in the discharge reaction process, there is another reaction that cannot be ignored. Magnesium anodes can also react with an aqueous electrolyte to release hydrogen gas:
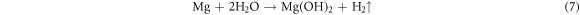
The hydrogen evolution reaction (HER) of the Mg anode is an important factor affecting the utilization efficiency of the anode. According to the results of the polarization curves, adding Cd reduces the self-corrosion current density of the Mg-6Al alloy. A small self-corrosion current density indicates a small self-corrosion rate. It means that the modified Mg alloys have good corrosion resistance, showing that the addition of Cd can inhibit the reaction between the Mg alloy and the electrolyte. This reduces the generation of hydrogen, making the Mg alloy more involved in the discharge process, and thus improving the discharge performance of the modified anodes, which is associated with the battery results. In addition, the self-corrosion potential of the β-phase (−1.30 V) in NaCl solution is 0.3 V higher than for the α-phase (−1.60 V) in the matrix [32]. As a consequence, the α-phase of the matrix corrodes due to its extremely negative free corrosion potential, and the corrosion rate is accelerated by the micro-electric coupling between the α-phase and the β-phase. The presence of Cd reduces the electro-couple corrosion of the different phases, which increases the effective reactants in the battery reaction, thus improving the anode efficiency and the discharge capacity [19]. Therefore, the discharge capacity of the Mg–air battery increases with the addition of Cd and with the increase of the current density.
3.4. Surface morphologies
Figure 5 shows the surface morphologies of the Mg-6Al-xCd (x = 0, 0.5, 1) anodes after 10 h discharge testing in a 3.5 wt% NaCl electrolyte at a 0.5 mA cm−2 current density with the discharge products removed. There are obvious coral-like structures in the original Mg-6Al anode. The structure can be used as a porous container for the discharge products, which prevents the contact between the Mg substrate and the salt solution, increasing the difficulty of the discharge products falling off the anode surface. Large and deep pits appeared on the surface of the Mg-6Al anode after discharge. Compared with the Mg-6Al anode, the pits on the surface of the modified anode are shallower and smaller. The smooth surface helps the discharge products fall off the electrode surface. A more effective area can improve anode efficiency and specific capacity, in accordance with the results of the battery experiments. In addition, some small pores are observed in the modified anodes. These are hydrogen pores related to the hydrogen evolution reaction (equation (7)) of the Mg alloy. According to the results of the polarization curves, the improved anode has a better corrosion resistance rate with the increase of Cd content, and the corrosion resistance of Mg-6Al-1.0Cd is better than Mg-6Al-0.5Cd, reducing the generation of hydrogen.
4. Conclusion
Three alloys with different compositions were studied in this work. All three alloys contain only α-Mg and β-Mg17Al12 phases, and Cd is evenly distributed in the Mg matrix. Cadmium can inhibit the self-corrosion effect of the Mg anode and reduce the generation of hydrogen. The specific capacity of the Mg anode and the anodic utilization of the sample anodes is increased with the addition of Cd. The Mg-6Al-1.0Cd anode has the highest specific capacity and anodic efficiency (1004.02 mAh g−1 and 41.75%, respectively) at the current density of 15 mA cm−2. Therefore, the Mg-6Al-1.0Cd alloy can be used as an optional anode material for Mg–air batteries.
Data availability statement
The data that support the findings of this study are available upon reasonable request from the authors.