Abstract
Zn-Ni ferrite nanoparticles were successfully synthesized by coprecipitation method. The samples were annealed at various temperatures, i.e., 200 °C, 400 °C, 600 °C, 800 °C, and 1000 °C. The nanoparticles have the mixed spinel phase structure as confirmed by the X-ray diffraction patterns. The crystallite size was 15.1 nm and increased to 25.1 nm after annealing at 1000 °C. Transmission electron microscope images showed that the annealed sample exhibited better dispersion and grain boundaries compared to the as-prepared sample. Fourier transform infra-red spectra showed the existence of vibrations at 378 cm−1 and 555 cm−1, confirming bonding for mixed spinel ferrites. The hysteresis measurement by using vibrating sample magnetometer confirmed that the sample possessed soft magnetic properties with a coercivity of 45 Oe and increased after annealing. The saturation magnetization of the as-prepared sample was 11 emu g−1, and increased to 58 emu g−1 after annealing at a temperature of 800 and 1000 °C. The specific absorption rate (SAR) with an alternating current magnetic field (50 Hz and 100 Oe) of Zn-Ni ferrite before and after annealing (at 800 °C) was 63.7 and 92.4 mW g−1, respectively. The results showed that annealing temperature has a significant role in determining the microstructural, the magnetic properties and the SAR of the nanoparticles.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
In recent years, ferrite magnetic nanoparticles (MNPs) with the formula MFe2O4 (M: divalent metals) are one of the most promising nanoparticles as they have wide application opportunities, such as biomedical system, magnetic storage, microwave devices, ferrofluid technology, gas sensors, and photocatalysis [1–3]. In the biomedical applications, MNPs have been proposed as magnetic guidance in drug delivery system and as heat magnetic mediators in hyperthermia treatment [4–7]. Hyperthermia treatment has an obstacle that is the difficulty in the local heating of the tumor region up to the desired temperature with no damage to the adjacent healthy tissue [8, 9]. To overcome this limitation, MNPs are good candidates for hyperthermia treatment [10–13]. The great characteristic of MNPs is their ability to be targeted to a specific area, interaction with an external magnetic field and generating heat under exposure to an alternating current (AC) magnetic field [14, 15]. High-frequency magnetic field may cause heating in MNPs on basis of the relaxation mechanisms of Brownian and Néel as well as hysteresis loss, which subsequently enables the tissue-specific hyperthermia [16–19]. Moreover, MNPs have the potential to be applied in hyperthermia because they have high specific absorption rate (SAR) values at low amplitudes and frequencies. The SAR is a concept used to calculate the efficiency of magnetic material that is affected by an external AC magnetic field [20].
In the last recent years, there were many kinds of magnetic materials have been extensively studied for any applications, for instant Y3−xSrxFe5−xZrxO12 [21], c-CuFe2O4 [22], La1−xSrxFe0.5Co0.5O3 [23], MF12O19 with M = Ba, Pb, and Sr [24], Bi0.95Sr0.05Fe1−xMnxO3 [25], and Zn-subtituted ferrite such as Zn-CoFe2O4 [26–28], Zn-MnFe2O4 [29], Zn-CuFe2O4 [30], and Zn-NiFe2O4 [31–35]. Among those materials, the Zn-subtituted ferrite nanoparticles has found to have the largest magnetization and the smallest coercivity [35]. The Zn-subtituted ferrite is interesting to be studied further for tuning their magnetic properties as well as their SAR properties for magnetic hyperthermia application.
Nevertheless, there are few papers which have studied the SAR for Zn-substituted ferrite [36, 37]. The reason is their complex synthesis and investigation methods, reduced biocompatibility, and chemical instability [38–40]. Recently, the theoretical investigation of the SAR of the Zn-substitutedMNPs has been reported [41]. In our previous research, Zn-substituredFe2O4 nanoparticles synthesized by coprecipitation method have a low crystallinity [42, 43]. An effort is needed to improve the microstructural and magnetic properties of the nanoparticles. Controlling these two parameters is necessary to improve the properties of the nanoparticles. The performance of the microstructural and magnetic properties of Zn-substituted Fe2O4 had been improved using annealing treatment [44–47]. Hence, in connection with the purpose of improving the microstructural, magnetic properties, and SAR of Zn-substituted ferrite nanoparticles, application of an annealing treatment to the MNPs is interesting to be investigated. There have been recently reported the effect of the method of preparation, sintering temperature, sintering time, and sintering atmosphere on the physical properties of ferrites [48–56].
Zn-NiFe2O4 is one of the most interested ferrites. Divalent metal ions of Ni and Zn are known to have strong preferences for the tetrahedral A and octahedral B sites. The bulk material of Ni ferrite has an inverse spinel structure, in which Ni2+ cations mainly occupy octahedral sites, while Zn ferrite has a normal spinel structure with a preference for Zn2+ cations mainly on its tetrahedral sites [48]. The occupancy of these divalent metal ions on these lattice sites can influence the properties of nanoparticles [46], such as the microstructural and the magnetic properties. Magnetic properties of Zn-NiFe2O4 nanoparticles depend on several factors, including the distribution of nanoparticle constituents, grain shape, microstructure, lattice stress, and defects that occur between the crystal surface and their internal structure [46, 48]. Microstructural and magnetic properties of Zn-NiFe2O4 nanoparticles can be also controlled by modifying the chemical composition and varying the preparation method [35, 48, 57]. Coprecipitation is a convenient method for preparing nanoparticles due to the relatively small size of the particles that can be obtained and being a simple procedure without the need of a high synthesis temperature [44, 48]. Unfortunately, the nanoparticles commonly exhibit agglomeration with a broad size distribution [46].
There have been much research into Zn-NiFe2O4 nanoparticles. Most research are demonstrating the relationship between synthesis methods, microstructural and magnetic properties of Zn-NiFe2O4 nanoparticles. For example, Shahane et al [48], Velmurugan et al [57], Ghasemi et al and Hwang et al only studied the microstructural and magnetic properties of Zn-NiFe2O4 in varying concentrations using coprecipitation and sol-gel methods, respectively [35, 58], while Singh et al [59] reported the cation distribution of annealed Zn-NiFe2O4 nanoparticles. Ojha et al also reported an annealing study of Zn-Ni ferrite [60]. However, the effect of annealing on SAR of Zn-NiFe2O4 has not been studied yet. Therefore, it is important to investigate the relationship among microstructural, magnetic properties, and SAR to obtain more comprehensive information through annealing treatment. The objective of this research is to determine the microstructural and magnetic properties, and SAR of Zn-NiFe2O4 nanoparticles through annealing treatment at various temperatures and synthesized by the coprecipitation method. The relationship between the annealing temperature and the microstructural, magnetic properties, and SAR will be studied in detail.
2. Materials and methods
Ni-ZnFe2O4 nanoparticles were synthesized by using the coprecipitation method. The starting materials were 2.16 g ferrite chloride hexahydrates (FeCl3.6H2O), 0.48 g nickel chloride hexahydrates (NiCl2.6H2O), 0.58 g zinc sulfate heptahydrates (ZnSO4.7H2O), and 3 g sodium hydroxide (NaOH) as the mineralizer. All pure analytical materials were purchased from Merck (Darmstadt, GFR) and used without further purification. FeCl3.6H2O and the combination of ZnSO4.7H2O and NiCl2.H2O were used as precursors.
The precursors were dissolved in distilled water to make solution with mol comparison of 1 : 1 : 4 for NiCl2.H2O, ZnSO4.7H2O, and FeCl3.6H2O, consecutively. Subsequently HCl solution was added into it. After 5 min stirred, the mixed solution was added dropwise into 12 M NaOH solution at a temperature of 60 °C for 2 h. The slurry was filtered and then washed for 7 times with distilled water to remove the incomplete chemical precursors during synthesis. The slurry was then dried at 90 °C in a furnace for 4 h to relieve water content and obtain powder. The powder was annealed at five different temperatures, i.e., 200 °C (T1), 400 °C (T2), 600 °C (T3), 800 °C (T4), and 1000 °C (T5) for 120 min in the vacuum [61]. As-prepared (T0) nanoparticles were used for comparative data against the annealed nanoparticles.
The magnetic properties and crystal structure of nanoparticles were characterized using a vibrating sample magnetometer (VSM) Riken Denshi Co., Ltd. of Nagoya University and X-ray diffraction (XRD), respectively. Vibrational spectra of nanoparticles were studied by Fourier transform infra-red (FTIR). Meanwhile, their size, morphology, and diffraction of nanoparticles were studied by transmission electron microscopy (TEM) and selective are electron diffraction (SAED).
The efficiency of heat dissipation of nanoparticles can be experimentally measured in terms of SAR using calorimetric heating measurements with an alternating current magnetic field (50 Hz and 100 Oe), which is the energy dissipated per unit of mass of nanoparticles (Wg−1), and is given by [62]:
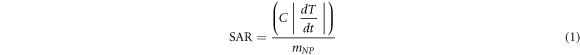
where C is the specific heat of the medium, T = temperature, t = time, mNP = mass of nanoparticles. The temperature increases of the nanoparticle sample (ΔTNP) and the associated water sample (ΔTw) (at the same frequency) were obtained by subtracting the initial temperatures from their measured temperatures at each time, t, ΔTNP/w(t) = TNP/w(t) −TNP/w(0). Subsequently, the temperature increase of the water blank was subtracted from that of the nanoparticle sample, resulting in the net temperature increase, ΔTnet(t) = ΔTNP(t)−ΔTw(t). The SAR was estimated from the slope of this net temperature increase [63].
3. Results and discussion
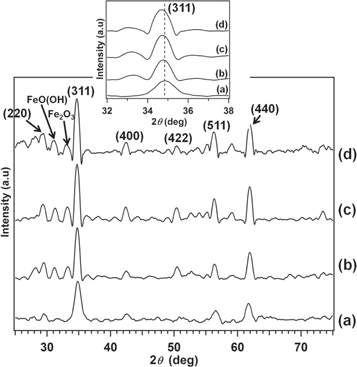
The XRD pattern of the as-prepared and annealed Zn-NiFe2O4 nanoparticles is shown in figure 1. The diffraction peaks correspond to (220), (311), (400), (422), (511), and (440) planes belonging to the cubic close-packed spinel structure (JCPDS, Card No. 080234). The peak intensity of the sample increased with increasing annealing temperature. The (311) plane showed the highest peak intensity, indicating that the grains of nanoparticles were dominant in the direction of the diffraction plane (311) [45, 64]. The appearance of α-Fe2O3 with the reflection plane of (104) (JCPDS, Card No. 330664) and FeO(OH) with the reflection plane of (221) (JCPDS, Card No. 220353) are considered to have been caused by thermal decomposition and oxidation during annealing. Others peaks such noisy background are not belonging of the Zn-NiFe2O4 nanoparticles. Those noises might come from the XRD instrument. The crystallite size of the sample was calculated using Debye–Scherrer's formula from XRD patterns for the reflection plane of (311) [2].
Figure 1. XRD pattern of Ni-ZnFe2O4 nanoparticles for various temperatures: (a) as-prepared sample (T0); (b) 600 °C (T3); (c) 800 °C (T4); and (d) 1000 °C (T5). Inset shows a shift of peaks (311) in the XRD pattern of the annealed Ni-ZnFe2O4 nanoparticles.
Download figure:
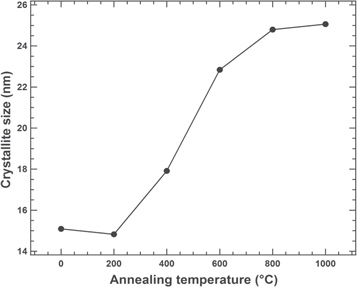
Standard image High-resolution imageThe annealing temperatures' dependence on the crystallite size is shown in figure 2. The crystallite size of the as-prepared sample was 15.1 nm and nearly the same after annealing at a temperature of 200 °C. The crystallite size significantly increased with increasing temperature (≥ 400 °C), and the size became 25.1 nm at a temperature of 1000 °C. The crystallite size increased with increasing annealing temperature due to a re-crystallization of the nanoparticles and decreased their lattice strain broadening [53]. In fact, the grain of the nanoparticles experience a growth and merging between small grains via coalescence and coarsening processes. Thus, it appears that the crystallite size may be controlled by varying the annealing temperature of the sample.
Figure 2. Trends in annealing temperatures versus the crystallite size of the Ni-ZnFe2O4 nanoparticles.
Download figure:
Standard image High-resolution imageThe lattice parameter was calculated from x-ray diffraction data using the lattice spacing (d) value and the Miller indices (h, k, l) of the plane, especially for (311). There was no significant change in the lattice parameter by annealing, as shown in table 1. However, if we compare the lattice parameter of the as-prepared and the as-annealed samples, there is a slight difference. The lattice parameters of the as-prepared and the as-annealed samples were 8.52 Å and 8.54–8.56 Å, respectively. Variation in the lattice parameter with increasing the calcination temperature suggests that a change in the cations distribution between tetrahedral A and octahedral B sites due to thermal fluctuation [47]. A change in the cations distribution causes a change in the mean ionic radii of A-site (rA) and B-site (rB) [65].
Table 1. Lattice parameter of Zn-NiFe2O4 nanoparticles at various annealing temperatures.
| Annealing Temperature ( °C) | Lattice parameter (Å) | Standard deviation |
|---|---|---|
| As prepared | 8.52 | 0.01 |
| 200 | 8.52 | 0.02 |
| 400 | 8.54 | 0.06 |
| 600 | 8.55 | 0.04 |
| 800 | 8.55 | 0.04 |
| 1000 | 8.56 | 0.05 |
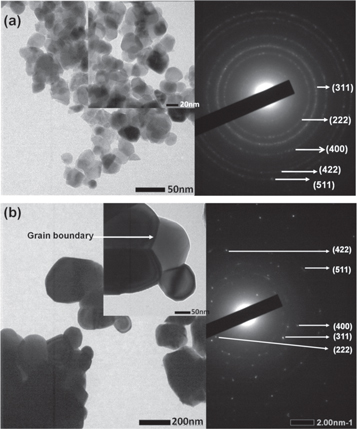
TEM image was used to characterize the structural morphology of the Ni-ZnFe2O4 nanoparticles, as shown in figure 3. The TEM micrograph showed that the morphology of as-prepared nanoparticles mainly tended to agglomerate with a spherical shape. However, the TEM micrograph showed that the annealed sample exhibited better dispersion and grain boundaries was well-developed compared with the as-prepared sample, as shown in figure 4. Ni-Zn ferrite nanoparticles with high dispersibility have the potential for any applications. Figure 4(a) shows the TEM micrographs of the annealed Ni-ZnFe2O4 nanoparticles at 600 °C with 50 nm in scale while figure 4(b) is at 1000 °C with 200 nm in scale. It is evident that the particle size increased with increasing annealing temperature as a consequence of the surface energy that was reduced, and small particles merged to form larger particles [47]. These results were in agreement with those of the XRD analysis.
Figure 3. TEM and SAED images of the as-prepared Ni-ZnFe2O4 nanoparticles.
Download figure:
Standard image High-resolution imageFigure 4. TEM and SAED images of annealed Ni-ZnFe2O4 nanoparticles: (a) 600 °C and (b) 1000 °C.
Download figure:
Standard image High-resolution imageThe SAED patterns (inset images are shown on the right-hand sides of figure 4) recorded on a nanoparticle assembly showed spotty diffraction rings. The SAED image for both samples confirmed the formation of polycrystalline nanoparticles. The diffraction rings were successfully identified from these SAED images and in agreement with the reflection peaks given by the XRD pattern as shown in figure 1.
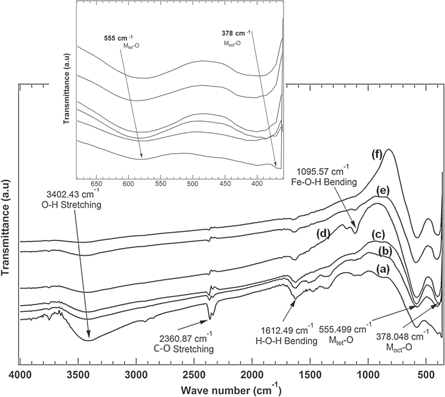
Atomic vibrational study using FTIR spectroscopy yielded information about the local chemical bonds among the atoms of the nanoparticles, as shown in figure 5. FTIR absorption spectra were obtained using the KBr pellet technique at room temperature. The two principal absorption bands of the spinel ferrite structure were found in the wave number range of 600–350 cm−1. The bands at around 378 cm−1 and 555 cm−1 were due to the vibration of the chemical bonds Moct-O at the location of the octahedral and Mtet-O at the location of the tetrahedral sublattice, respectively.
Figure 5. Vibrational spectra of Ni-ZnFe2O4 nanoparticles at various temperatures: (a) as-prepared sample (T0); (b) 200 °C (T1); (c) 400 °C (T2); (d) 600 °C (T3); (e) 800 °C (T4); and (f) 1000 °C (T5). Inset shows a shift of wave number in the FTIR spectra of the Ni-ZnFe2O4 nanoparticles at low wave numbers (350–700 cm−1).
Download figure:
Standard image High-resolution imageThe presence of asymmetric stretching vibration of CO2 at wave number 2360 cm−1 confirmed the reaction between carbon dioxide and hydrogen in the atmosphere. The bending modes in the as-prepared sample (T0) at around 3402 cm−1, 1612 cm−1, and 1095 cm−1 were associated with O–H stretching, H–O–H bending, and Fe–O–H bending vibration, respectively. The increase in annealing temperature caused the absence of O-H absorption peaks. Further, at a temperature of 1000 °C, only the absorption peaks at around 378 cm−1 and 555 cm−1 were observed, which were assigned to the Ni–O, Zn–O, and Fe–O stretching bands. Fe–O–H bending was clearly observable for the sample annealed at 600 °C. This might have appeared from FeO (OH) and hydration of Fe2O3 as shown in the XRD patterns.
The hysteresis loops of the sample were measured at room temperature using VSM, as shown in figure 6. A detailed magnetic parameter is presented in table 2. The saturation magnetization (σs) and coercivity (Hc) of the as-prepared sample were 11 emu/g and 45 Oe, respectively. Shahane et al. reported that the saturation magnetization and coercivity of the as-prepared Ni-Zn ferrite nanoparticles were 37 emu g−1 and 200 Oe, respectively [48]. The difference in magnetic properties may have been due to the differences in the microstructures.
Figure 6. Hysteresis loop of Ni-ZnFe2O4 nanoparticles at various annealing temperatures: (a) as-prepared sample (T0); (b) 200 °C (T1); (c) 400 °C (T2); (d) 600 °C (T3); (e) 800 °C (T4); and (f) 1000 °C (T5). Inset shows enlarged hysteresis loop of as prepared and annealed (800 °C) samples.
Download figure:
Standard image High-resolution imageTable 2. Crystallite size with standard deviation, saturation magnetization (σs) and coercivity (Hc) of Ni-ZnFe2O4 nanoparticles at various annealing temperatures.
| Parameters | ||||
|---|---|---|---|---|
| Annealing Temperature (°C) | Crystallite size (nm) | Standard deviation | σs (emu/g) | Hc (Oe) |
| As prepared | 15.1 | 0.1 | 11 | 45 |
| 200 | 14.8 | 0.3 | 29 | 49 |
| 400 | 17.9 | 0.2 | 41 | 50 |
| 600 | 22.8 | 0.2 | 50 | 94 |
| 800 | 24.8 | 0.2 | 58 | 94 |
| 1000 | 25.1 | 0.3 | 58 | 94 |
It was found that the increase in σs and Hc were inconsistent with the increase in crystallite size as a result reported by Shamgani & Gholizadeh [65]. The hysteresis loops confirmed that nanoparticles possess soft magnetic properties with Hc in the range of 45 to 94 Oe. An increase in Hc due to an increase in the crystallite size with increasing annealing temperature and a change in the particles' shape. The physics behind of this phenomenon can be explained as follows. The Hc depends on the anisotropy energy of the nanoparticles. Higher anisotropy energy will cause higher coercivity. This energy is influenced by some parameters, for instant shape anisotropy, surface energy, magneto-elastic energy, and magneto-crystalline anisotropy energy [66]. In these results, the shape of the nanoparticles changes with the calcination temperature. So that, the shape anisotropy energy gives much effect on Hc changes. Furthermore, as the crystallite size change with temperature so the surface energy, the magneto-elastic energy, and magneto-crystalline anisotropy energy will change. It causes a change on Hc [65, 66].
Saturation magnetization tends to increase from 11 to 58 emu g−1 with increasing annealing temperature. Lin Leng et al. reported that the saturation magnetization increased from 24 to 38 emu g−1 with increasing annealing temperature [46]. Physically, the magnetic saturation is influenced by three parameters, such as magnetic diameter, cation distribution, and Yafet-Kittel angle between moments on B-site [65]. An increase of the annealing temperature causes a change in the cations distribution. According to the Neel's theory, the total magnetization of the nanoparticles is contributed by the net magnetization between the cations in B-site and A-site [65, 66]. A change in the cation distribution leads to a change in the inversion parameter and the resultant exhange interaction between magnetic moment of cations in both sites. The interaction attributes a change in the magnetic saturation of the nanoparticles [51]. In other hand, increases in annealing temperature cause the formation of larger crystallite sizes, so nanoparticles may contain multiple domain walls. In this work, we found that the nanoparticles have a range of the crystallite size of 15.1 nm—25.1 nm. For the crystallite size larger than the critical size (≈ 20 nm) so the formation of others extra domain walls is probably occur due to thermal activation [51, 53]. Having numerous domain walls will increase the total number of magnetic moments so that a large magnetization will be obtained [47].
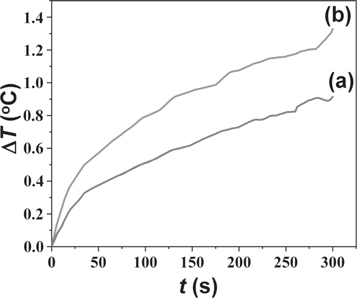
The temperature change in nanoparticles measured by calorimetric method is shown in figure 7. Zn-NiFe2O4 nanoparticles were exposed to an alternating magnetic field of 100 Oe with frequency of 50 Hz. As characterized by the SAR equation, the SAR value of Zn-NiFe2O4 nanoparticles was calculated to be 63.7 mW gram−1. The SAR of the annealed Zn-NiFe2O4 nanoparticles (at 800 °C) was 92.4 mW gram−1. The increase of SAR is due to the increase of the saturation magnetization of Zn-NiFe2O4 nanoparticles after annealing at 800 °C, as shown in table 2. The nanoparticles after annealing have better dispersion and grain boundaries compared to the nanoparticles before annealing, as shown in figure 3 and figure 4. Such condition may also cause the increase of the SAR value. Of course, for the magnetic hyperthermia application, the SAR value is very low. Because the frequency of an alternating magnetic field used in this experiment was very low. The SAR value can increase by the increase of the frequency. However, the focus of this research is to study the modification of the SAR value of nanoparticles by the annealing treatment, which is due to the change of the microstructural and the magnetic properties of nanoparticles.
Figure 7. Temperature changes in Zn-NiFe2O4 nanoparticles under an AC magnetic field.
Download figure:
Standard image High-resolution image4. Conclusions
Ni-ZnFe2O4 nanoparticles with a crystallite size in the range of 15.1 nm to 25.1 nm were successfully synthesized by a coprecipitation method and annealed at five different temperatures, 200 °C, 400 °C, 600 °C, 800 °C, and 1000 °C. The average crystallite size showed an increasing trend after annealing as confirmed by the XRD analyses. TEM images showed that particle size increased for annealed nanoparticles, the grain boundary was well-developed, and the grain exhibited better dispersion. FTIR analysis established the presence of pure Ni-ZnFe2O4 nanoparticles and the complete removal of organic substances at the higher-temperature annealed nanoparticles. Saturation magnetization and coercivity of the samples increased due to the increasing annealing temperature. The specific absorption rate (SAR) of Zn-Ni ferrite was 63.7 and increased to 92.4 mW/g after annealing. The results showed that the annealing temperature has a significant role in determining the microstructural, the magnetic properties, and the SAR of nanoparticles for specific applications such as the magnetic hyperthermia.
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).
Fundings
This work was partly supported by the grant of PTUPT 2020-2022, the Ministry of Research & Technology, Republic of Indonesia and Nanofabrication Platform Consortium Project of Nagoya University, Minister of Culture, Sports, Science and Technology (MEXT) Nano-Project Platform, Japan.