Abstract
Green synthesis of zinc oxide nanomaterials has received recent attention due to the potential application of these nanomaterials as biocidal agents to combat antibiotic resistant organisms. In this paper, we provide a facile, one-step hydrothermal approach to prepare catechin-functionalized ZnO nanoclusters in aqueous solution. The obtained ZnO nanoclusters were characterized by UV spectroscopy (UV), Fourier transform infrared spectroscopy (FTIR), transmission electron microscopy (TEM), atomic force microscopy (AFM), x-ray diffraction (XRD) and x-ray photoelectron spectroscopy (XPS). The results of FTIR indicated that the catechin molecules were adsorbed on the surface of ZnO nanoclusters. TEM determination revealed that small ZnO nanoparticles tend to aggregate and form nanocluster structures. Antibacterial activity was tested by paper disk diffusion and the catechin-functionalized ZnO nanoclusters showed high biocidal activity against gram-positive bacteria, gram-negative bacteria and fungi.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Metal oxide nanomaterials such as titanium dioxide, copper oxides and zinc oxide have shown noticeable antibacterial activity in recent studies [1–4]. Some advantages of metal oxide nanomaterials as antibacterial agent are their specific targeting, minimum toxicity and inhibitory ability against antibiotic-resistant bacteria. The mechanism of bactericidal activity of metal oxide nanomaterials is still controversial. The possible mechanisms have been comprehensively reviewed in the past and include release of toxic ions as well as oxidative stress, cell lipid peroxidation, enzyme inhibition, and proteolysis [5, 6]. Among these metal oxide nanomaterials, zinc oxide has received considerable attention as an efficient bactericidal agent against multi-drug resistant organisms [7–10]. In addition to ZnO nanomaterials, zinc-based chelates such as Zineb (Zinc ethylenebisdithiocarbamate) and Mancozeb (Manganese Zinc ethylenebis(dithiocarbamate)) have been widely used in the agrochemical sector as fungicides [11, 12]. Mancozeb (Structural formula shown in figure 1(a)) has been used effectively upon over 70 crops for the control of plant pathogens.
Figure 1. Structural formula of mancozeb (a) and catechin (b).
Download figure:
Standard image High-resolution imageBeside the antibacterial effects, nano-sized ZnO is an promising material which can be used in photonics, electronics and sensing [13–15]. Syntheses of ZnO nanomaterials have been extensive explored in recent years. ZnO nanoparticles can be prepared by different physical and chemical approaches including vapor condensation, combustion sol-gel, microwave-assisted synthetic methods [16, 17]. However, these physico-chemical processes sometimes employ toxic reagents and solvents which lead to harmful post-process by-products [18, 19]. Green synthesis is regarded as an alternative route to avoid aforementioned disadvantages. Plants are considered as one of the richest sources for the green synthesis of the nanomaterials [20–23]. Green synthesized nanoparticles can be capped by one of many organic phytochemicals that facilitate ligand-based interaction with many potential receptors, including phospholipids, proteins and lipoteicholic acid, which enhances adhesion of gram positive bacteria to host or target organisms [13]. Ultimately, these interactions prevent biofilm formation and thus prevent the growth and spread of bacterial colonies upon host surfaces.
The phytochemicals often involved in green synthesis include polysaccharides, aldehyde, organic acids, polyphenol and flavones [24, 25]. Catechins (Structural formula shown in figure 1(b)), an efficient natural antioxidant of tea polyphenols, possess broad-spectrum pharmacological and therapeutic activities [26, 27]. The important role of catechin is being increasingly recognized with regard to human health. Catechins are also known as an antioxidant agents [28]. Recently, catechin-copper nanoparticles, synthesized through an ultrasonic crushing method, have been found to display excellent antimicrobial activity [29]. Based on their superior biological properties, catechins can be considered as potential coordination agents to be used for green synthesis of ZnO nanoparticles with high bactericidal activity yet having low adverse effects. Since the catechin is the main component of the green tea, the safety and the biocompatibility of the ZnO nanoparticles was considered to be improved after catechin combination. However, to our knowledge, the synthesis of catechin-functionalized ZnO nanomaterials has not been comprehensively explored.
In this study, the synthesis of catechin-functionalized ZnO nanoclusters by a hydrothermal method were explored and antibacterial activity of the obtained product was also investigated. We provide a one-step hydrothermal method to synthesize large-scale hydrophilic catecin-functionalized ZnO nanoparticles. This method is facile, cost effective, safe and environmentally friendly. In the synthesis process, only two reactants were needed, which include ZnCl2 and catechin (plant metabolites). Furthermore, the test results indicated that the catechin-functionalized ZnO nanoparticles have high antibacterial activity against both bacterial and fungus.
2. Materials and methods
2.1. Materials
(+)-Catechin (>98%) was purchased from Sigma-Aldrich Co., Ltd (Shanghai, China). ZnCl2 (>98%) was the product of Tianjin Tianli Chemical Reagent Co., Ltd, and used without further purification. Ammonia (AR) was provided by Tianjin Fuyu Chemical Co., Ltd Gram-positive S. aureus (ATCC 6538), Gram-negative E. coli (ATCC 8739) and fungi C. albicans (ATCC16404) were obtained from the institute of applied microbiology, Heilongjiang academy of science (China). The water used in this study was obtained by a Millipore Milli-Q system.
2.2. Synthesis of ZnO nanoclusters
In a typical experiment, 40 ml of 0.32 mmol l−1 catechin solution was prepared by dissolving of catechin powders with pure water. 0.4 ml of 0.3668 mol l−1 ZnCl2 aqueous solution was added into the as-prepared catechin solution. A small amount of concentrated ammonia was added into the solution to adjust the pH to 8.0. 40 ml of the mixed solution was then taken out and put into a Teflon-lined autoclave. It was incubated at 140 °C for 4 h. Finally, a brown colloidal solution was obtained. The product was purified by a succession of washing steps involving centrifugation, decantation of supernatant and resuspension of the pellet in Millipore water. Finally the pellet was crushed and dried to give a brown powder.
2.3. Characterization of ZnO nanoclusters
2.3.1. UV–vis absorption spectra
UV-5500 spectrophotometer (Metash, China) was used for the measurement. The brown ZnO colloidal solution was diluted fourfold before measurement.
2.3.2. Fourier transform infrared spectroscopy
Fourier transform infrared spectrometer (IR Affinity-1, Shimadzu, Japan) was utilized for the measurement by using KBr pellet method. The ZnO nanoclusters sample were collected from the centrifugation of the catechin-functionalized ZnO colloidal solution.
2.3.3. Transmission electron microscopy
The ZnO nanomaterials were observed by JEM-2100 (JEOL Ltd) at 200 kV. It is equipped with energy dispersive spectrometer (EDS). 5 μl of the as-prepared ZnO colloid was dried on a carbon coated copper grid and stored in a desiccator prior for TEM observation.
2.3.4. Atomic force microscopy (AFM)
A PicoPlus II system from Molecular Imaging Inc. was used for the AFM imaging. 50 μl of the as-prepared ZnO colloid were dropped onto a freshly cleaved mica surface and then rinsed by a copious of pure water. The mica surface were then dried under a flow of pure Ar gas and stored in a desiccator before AFM imaging.
2.3.5. X-ray diffraction
A Rigaku D/MAX 2200 x-ray diffractometer (Tokyo, Japan) using CuK radiation (40 kV, 300 mA) of wavelength 0.154 nm was used for the measurement.
2.3.6. X-ray photoelectron spectroscopy
XSAM HS surface analysis spectrometer (Kratos instrument) with Mg-Kα as the x-ray source (1253.6 eV) was used for XPS analysis. The XPS spectra were measured at 10 mA with 14 kV of energy.
2.4. Antibacterial tests
The antibacterial activity of the as-prepared ZnO nanomaterials has been tested against gram-positive bacteria S. aureus and gram negative bacteria E. coli, the fungus C. albicans using the disk diffusion approach. E. coli and S. aureus were incubated in nutrient broth at 37 °C, and C. albicans in yeast peptone dextrose (YPD) broth at 28 °C. E. coli, S. aureus and C. albicans (108 colony units (CFU)/ml) were inoculated on the agar plates under sterile condition. Antibiotic discs were prepared with the filter papers impregnated with 15 μl 0.3 mg ml−1 of catechin-functionalized ZnO nanomaterials or control sample solution. They were gently placed onto the top of agar. The samples after incubation for 24 h were taken out and photographed with a digital camera. Minimal inhibitory concentration (MIC) and minimal bactericidal concentration (MBC) of the as-synthesized ZnO nanomaterials were also measured. The samples of catechin-functionalized ZnO nanomaterials were prepared by two-fold (1:2) dilution method and then pipetted into 96-well microtiter plates. The microbial suspensions were then transferred into each well. The microtiter plate was incubated for 24 h. The lowest ZnO colloid concentration that induced no microbial growth was regarded as MIC. The lowest concentration of ZnO colloid that resulted in the complete killing of the inoculated bacterial strains was determined as MBC.
The primary toxicity experiments of the catechin-functionalized ZnO nanoclusters at the MBC concentration were also tested. Six healthy zebrafish were put into the as-prepared solution at MBC. After one week, the survival of these zebrafish was investigated and photographed.
3. Results and discussion
3.1. Spectra characterization
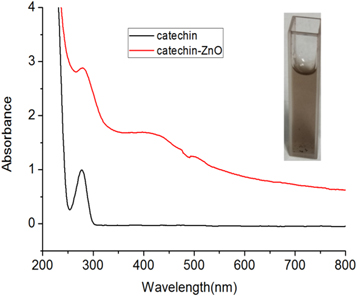
Figure 2 shows the UV absorption spectrum of the obtained catechin-functionalized ZnO colloidal solution in a typical experiment. The inset shows the photograph of the prepared solution. The broad absorption band ranging from 330 nm to 430 nm in figure 2 can be attributed to the absorption band of the ZnO colloid according to the previous report [30]. As reported by Zheng et al., the edge of the absorption band of the ZnO semiconductor is located at around 375 nm [30]. Furthermore, the absorption peak at 400 nm can also be ascribed to the polymerization of catechin under the basic condition. Catechin molecules can be dimerized by autoxidation under hydrothermal conditions [31].
Figure 2. UV absorption spectrum of the catechin -functionalized ZnO colloid.
Download figure:
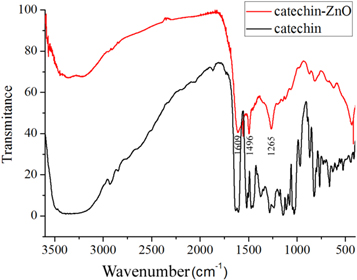
Standard image High-resolution imageThe FTIR spectrum of the catechin-functionalized ZnO nanomaterials and pure catechin is illustrated in figure 3. In the spectrum of catechin, the absorption band ranging from 3500 to 3200 cm−1 was attributed to the stretching vibration of O-H bond of catechin. The absorption peak at 1634−1 and 1604 cm−1 were assigned to the C=C group of the benzene ring in catechin. The absorption peaks at 1282−1 and 1144 cm−1 were derived from C–O stretching vibration of catechin [31] . The characteristic absorption peaks were dramatically reduced as for the FTIR spectrum of catechin-functionalized ZnO nanomaterials in comparison to the pure catechin. There is only one sharp peak around 1600 cm−1 (belong to C=C groups) instead of two peaks in the pure catechin sample. Furthermore, there is also one peak at 1265 cm−1 (C–O stretching vibration) in catechin-functionalized ZnO sample whereas there are three strong peaks in the pure catechin sample. This diminishing phenomena of the absorption band are quite similar with FTIR spectra of catechin contacted with Al2O3 [31]. It is indicated that ten peaks were less for the sample contacted with alumina [31]. Thus, the present RTIR results can be attributed to the adsorption of catechin on the surface of ZnO nanomaterials.
Figure 3. The FTIR spectrum of the catechin and the catechin-functionalized ZnO nanoclusters.
Download figure:
Standard image High-resolution image3.2. Topography and elemental composition characterization
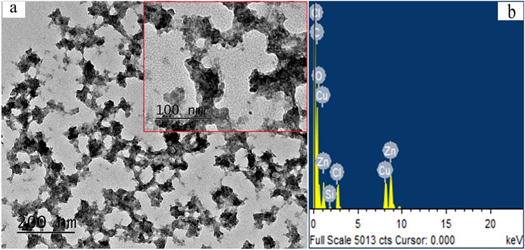
The typical TEM image of the synthesized ZnO nanomaterials is showed in figure 4(a). The results indicate that the most of the obtained products were the nanocluster composed of many small nanoparticles. Furthermore, these nanoclusters were connected with each other and transformed a network structure as shown in the inset in figure 4(a). The size of these ZnO nanocluster ranges from 10 nm to 30 nm. The average diameter is 24.1 ± 2.5 nm (n = 100). It has been revealed that the size and morphology of the ZnO nanomaterials may significantly influence on their activity [32]. Recently, Das and Pramanik utilizing catechin as the additive (crystal habit modifier) synthesized the large ZnO microspheres. The role of catechin in their study is quite different with that in the present research [33]. Energy dispersive x-ray spectroscopy (EDS) of the obtained ZnO nanomaterials by using electron microscope JEM-2100 was showed in figure 4(b). As expected, the elements including C, O and Zn have been detected, respectively. The strong C and O in EDS spectra can be attributed to the catechin components capped on ZnO nanomaterials whereas Zn peak can be attributed to the ZnO nanomaterials [34, 35]. In addition, the elements Cu and Cl have been detected as indicated in figure 4(b). The presence of element Cu can be ascribed to the copper grid used for TEM and EDS measurement. The presence of a small amount of Cl in the sample indicated that the Cl ions also exist on the ZnO nanoparticle surface, which originated from ZnCl2 in the reaction process. Chlorine-doped ZnO nanomaterials have been comprehensively investigated in the recent studies. It is revealed that the increase of Cl concentration mixed in the ZnO crystal structure was kinetically driven [36, 37]. The chlorine ions preferentially adsorbed on the polar {0001} facet [37]. It was revealed that Cl significantly influenced on morphological, structural and electrochemical properties of the synthesized ZnO nanomaterials.
Figure 4. (a) The TEM images of the obtained ZnO nanoclusters (b) The EDS spectrum of the obtained ZnO nanoclusters.
Download figure:
Standard image High-resolution imageIn addition to the TEM determination, the obtained ZnO nanomaterials were further observed by AFM. Figure 5 shows the AFM image of the catechin-functionalized ZnO nanoparticles adsorbed on the mica surface. The nanoparticles observed in figure 5 are considered to be the ZnO nanomaterials. This was verified by the line section analysis as indicated in figure 5. The heights of these observed ZnO nanomaterials are around of 30 nm, which are close to the size results measured by TEM.
Figure 5. The AFM image of the catechin-functionalized ZnO nanomarerials adsorbed on the mica surface.
Download figure:
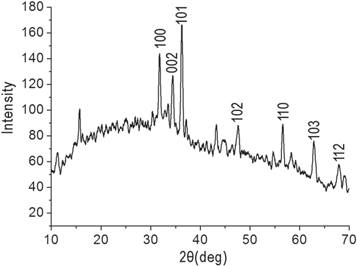
Standard image High-resolution imageFigure 6 shows XRD pattern of the obtained product. The diffraction peaks in figure 6 at 31.8°, 34.4°, 36.2°, 47.5°, 56.7°, 62.9°and 67.8°can be ascribed to (100),(002), (101), (102), (110), (103) and (112) planes of hexagonal ZnO [5] (JCPDS No. 74-0534). This result confirmed that the nanoclusters in this study are the ZnO nanomaterials. The width of the (101) peak was employed to calculate the average crystallite size using the Scherrer equation. The calculated crystal grain size was 25.5 nm, which is close to that of TEM measurement.
Figure 6. XRD patterns of the obtained ZnO nanoclusters.
Download figure:
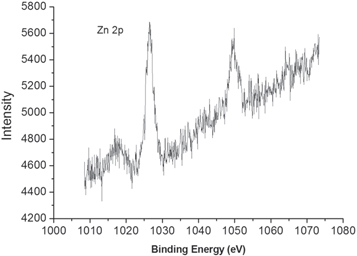
Standard image High-resolution imageThe representative XPS spectra of the synthesized ZnO nanomaterials are showed in figure 7. The two peaks at 1026.5 and 1049.5 eV are corresponding to Zn 2p3/2 and Zn 2p1/2, respectively. The spin–orbit splitting of 23.0 eV is evidently in good accordance with the previous reported values [18, 38] .
Figure 7. The representative XPS spectra of the obtained ZnO nanoclusters.
Download figure:
Standard image High-resolution imageHowever, the binding energy of Zn2p3/2 in this study is much higher than that of some previous reports [38, 39]. The reason for high binding energy might be derived from the coordination of the Zn2+ ion with catechin. Lupan et al attributed the two peaks at high binding energies (~1049 eV and 1026 eV) in their recent study to a Zn-containing compound [39]. Similar phenomena have been observed in previous report. For example, the measured binding energy of Zn within zinc acetate dihydrate is 1026.3 eV [35]. It is possible that catechin molecules binding on the surface of ZnO with their oxygen coordination with Zn2+ ion.
3.3. Antibacterial activities of catechin-functionalized ZnO nanoclusters
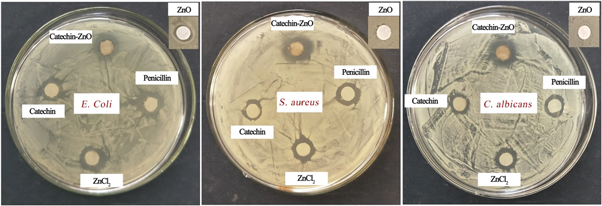
Antibacterial activities of the as-synthesized catechin-functionalized ZnO nanoclusters have been investigated. Figure 8 presents the zone of inhibition (ZOI) results of catechin-functionalized ZnO nanoclusters. Control samples including penicillin, catechin solution, ZnCl2 solution and ZnO in absence of catechin were also investigated against E. coli, S. aureus and C. albicans for comparison. It can be seen that the ZnO nanoclusters can inhibit the growth of both bacteria and fungus. Catechin-functionalized ZnO nanoclusters are more efficient to inhibit the growth of C. albicans and S. aureus in comparison to the positive control penicillin. It has been noticed that catechin and ZnCl2 solution also have bactericidal effect whereas ZnO in absence of catechin have no obvious antibacterial activity as shown in figure 8.
Figure 8. Zone of inhibition of catechin-functionalized ZnO nanoclusters, catechin solution, ZnCl2, penicillin and ZnO in absence of catechin.
Download figure:
Standard image High-resolution imageTable 1 presents the quantitative MIC and MBC of catechin-functionalized ZnO nanoclusters. The MIC and MBC of catechin and penicillin were also simultaneous measured and shown in table 1. A lower MIC corresponds to higher antibacterial effectiveness. The MIC and MBC of catechin against for bacteria and fungi are relatively high, which revealed it only has weak antibacterial ability. The MIC and MBC of catechin-functionalized ZnO nanoclusters for E. coli, S. aureus and C. albicans are 29.8 μg/ml and 59.5 μg/ml, respectively. In comparison to the results in recent literature, the MIC of catechin-functionalized ZnO nanoclusters in this study is relatively lower. For example, the reported MIC of ZnO nanoparticles towards E. coli. and S. aureus is 80 μg/ml [6, 7]. In comparison to the results of catechin-Cu nanoparticles in recent study, The MIC and MBC of catechin-functionalized ZnO nanoclusters for E. coli and S. aureus in this study is slightly higher [29]. Nevertheless, the relative low MIC and MBC values confirmed that catechin-functionalized ZnO nanoclusters have strong antibacterial activity. Furthermore, its bactericidal efficiency is nearly equal against gram-positive bacteria S. aureus, gram negative bacteria E. coli and the fungus C. albicans. Thus, it can be confirmed that the catechin-functionalized ZnO nanoclusters have broad -spectrum bactericidal activities.
Table 1. The MIC and MBC (in μg/ml) of the catechin-functionalized ZnO nanocluster, catechin solution and penicillin against S. aureus, E. coli and C. albicans.
| E. coli | S. aureus | C. albicans | ||||
|---|---|---|---|---|---|---|
| μg/ml | MIC | MBC | MIC | MBC | MIC | MBC |
| catechin-ZnO | 29.8 | 59.5 | 29.8 | 59.5 | 29.8 | 59.5 |
| catechin | 919 | 1838 | 460 | 919 | 919 | 1838 |
| Penicillin | 12.5 | 25.0 | 12.5 | 25.0 | 25.0 | 50.0 |
The inhibition ratio of the catechin-functionalized ZnO nanoclusters and the control samples at 2MIC concentration were further measured as shown in figure 9. The results demonstrated that catechin-functionalized ZnO nanoclusters have high inhibition ratio for S. aureus, E. coli and C. albicans at 2MIC. Especially for S. aureus and C. albicans, the inhibition ratio is even higher than the positive control penicillin. This result further confirmed that the catechin-functionalized ZnO nanoclusters have high antibacterial efficiency.
Figure 9. Inhibition ratio of catechin-functionalized ZnO nanocluster at 2MIC against S. aureus, E. coli and C. albicans. Catechin, penicillin and ZnCl2 solution were investigated for comparison.
Download figure:
Standard image High-resolution imageThe action mechanism of ZnO nanomaterials against bacterial is still under debate [5, 40]. One important mechanism is concerned with oxidative stress of Reactive Oxygen Species (ROS) generated by ZnO nanomaterials [5]. ZnO nanomaterials could produce ROS such as superoxide ion, hydroxyl ion OH−, O2− and peroxide H2O2 under the different light conditions. These ROS especially peroxide ion can easily penetrate the membrane of bacterial and induce cell death by interfering DNA replication , protein synthesis and lipid assembly.
Another important mechanism is related to cell membrane disruption induced by ZnO nanomaterials. ZnO nanomaterials can cause bacteria cell membrane distortion and leakage after their attachment. Internalization of ZnO nanomaterials could lead to disruption of phospholipid bilayer and outflow of intracellular components such as ATP and lipopolysaccharide out of the cell finally inducing cell death. Furthermore, ZnO nanomaterials may interact with membrane proteins on the cell wall of bacteria and inactivate them. ZnO nanomaterials interact with the thiol group of membrane proteins to form a stable S-metal group, unfold protein and denature them thus inducing cell death.
Zinc oxide nanomaterials tend to disassemble in the aqueous medium and dissolve out Zn2+ ion under neutral and acidic condition. We have monitored the release of Zn2+ of catechin-ZnO nanoclusters. The catechin-ZnO nanoclusters (0.3 g l−1) were collected by centrifugation and re-dispersed in PBS solution. The concentration of Zn2+ was 0.45 mg l−1 after 3 days release. Zn2+ has also shown anti-bacteria activity as revealed in this study. Zinc ion can cause a conformational change of the enzyme or induce transformation of the active site leading to the competitive or non-competitive reversible inhibition. ZnO nanoclusters indicated better antimicrobial action in compared to zinc chloride in this study. This result can be ascribed to their active targeting ability, disruption of cell membrane integrity ability and ROS generation capacity. In addition, it is also possible that the Zn-catechin complexes formed and released under the strong acidic condition [41]. However, under neutral or weak basic conditions, the generation ratio of Zn-catechin complexes was estimated to be low.
In this study, the high antibacterial activity of the ZnO nanoclusters can also be ascribed to the catechin molecules capping on its surface. This facilitated the ZnO nanoparticles adsorbed onto the bacterial surface. The large surface area to volume ratio further enhances them to combine onto multiple-ligand binding sites on the bacterial cell surface.
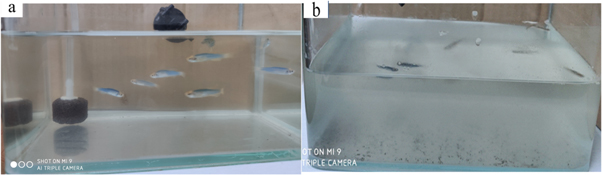
The nanoclusters synthesized by the green synthesis route in present study have great bactericidal activity than those prepared by chemical or physical methods since they are coated with plant active molecule on its surface, which facilitates phytochemicals-based nanoparticles to bind to the receptor on the bacterial membrane. The complexation of the nanoparticles with bacteria prevents the formation and growth of biofilms. The primary toxicity experiments of the catechin-functionalized ZnO nanoclusters at the concentration of MBC were also tested. The use of zebrafish has been proved as a promising biological platform for toxicity testing of pesticides such as fungicides, herbicides and insecticides [42]. Six healthy zebrafish were put into the as-prepared solution at MBC as shown in figure 10 (a). After one week, no one Zebrafish was died as shown in figure 10 (b). This result confirmed that the catechin-functionalized ZnO nanoclusters at the MBC concentration were nontoxicity. Catechin and catechin complextions are increasingly recognized with regard to human health. Zinc oxide is one of the most investigated inorganic compound and is rapidly attracting interest in the food and biomedical applications. Taken together, the catechin-ZnO nanoclusters with high antibacterial activity is expect to be applicable in package materials, cosmetics and biomedical applications such as using for wounds dressing and implant material coatings.
Figure 10. The photograph of six zebrafish in the catechin-functionalized ZnO nanoclusters solution at the MBC concentration at (a) day one, (b) day seven.
Download figure:
Standard image High-resolution image4. Conclusion
In present study, catechin-functionalized ZnO nanoclusters were synthesized via a hydrothermal method. TEM determination revealed that small ZnO nanoparticles tend to interact with each other and form nanocluster structures. FTIR results indicated that the catechin molecules were adsorbed on the surface of ZnO nanoclusters. Antibacterial tests revealed that catechin-functionalized ZnO nanoclusters have high antibacterial activity toward bacterial and fungus. The catechin-functionalized ZnO nanoclusters in this study have the potential to be developed as antibacterial agents used in multiple related fields.
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).
Competing interests
The authors declare that they have no competing interests.
Funding
The authors gratefully acknowledge the financial support by the Fundamental Research Funds for the Central Universities (2572020DR07), Natural Science Fund of Heilongjiang Province (LH2019B001), the 111 Project (B20088) and Heilongjiang Touyan Innovation Team Program (Tree Genetics and Breeding Innovation Team).
Authors' Contributions
B Z, S D, J L and C S, prepared and characterized the ZnO nanomaterials. Y F and Z L designed the synthesis method and wrote the manuscript. All authors revised and approved the final manuscript.