Abstract
CsxWO3-ZnO-SiO2 smart coatings were proposed to achieve multiple functions, such as thermal insulation, anti-dust, anti-fogging and blocking harmful ultraviolet light, etc. The transparent hydrophobic hybrid resins were prepared from methyltriethoxysilane (MTES) and SiO2 sol, which was used as the substrate of the composite coating. The CsxWO3 is served as the near-infrared (NIR) absorber, and the ZnO is functioned as the ultraviolet (UV) blocking agent. Coatings with different ratios of CsxWO3 and ZnO were successfully prepared by co-blending with nano-SiO2 resin. The morphology, microstructure, surface composition, hydrophobicity, thermal stability, anti-dust and optical property of the composite coatings were investigated comprehensively. The transmittance of C10Z10 (CsxWO3 10%, ZnO 10%, nano-SiO2 resin 80%) coating at 370 nm is 2.3%, and the value of Solar Energy Transmittance Selectivity (SETS) is 0.665, which exhibits excellent NIR shielding ability. Compared with nano-SiO2 resin coating, the thermal insulation temperature difference can reach 7.0 °C. The C10Z10 coating showed good durability in the twenty times anti-dust repeated tests and the efficiency could maintain 70.3% after the repeating tests. The coating showed excellent sustainability after a 45-day outdoor exposure experiment and a 240 h of artificial accelerated aging experiment. Thus, the proposed CsxWO3-ZnO-SiO2 coatings are promising for outdoor smart windows.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Energy crisis has become a worldwide topic of widespread concern owing to the rapid development of the industrialization and the increasing energy depletion [1]. According to the International Energy Agency reports, the building energy consumption accounts for 41% of the total global energy depletion. This has caused serious energy burdens and environmental pollution, so improving energy efficiency has become an extremely concerned issue. In the past ten years, solar thermal insulation coatings for building glass have become more and more important for sustainable development from an energy-saving point of view [2–5].
Cs-doped tungsten bronze (CsxWO3) nanoparticles can selectively shield NIR light while keeping high visible transparency. It belongs to a kind of non-toxic and cheap material for solar thermal insulation [6–8]. The near-infrared shielding properties and thermal insulation of the CsxWO3 have been extensively investigated in recent years. For instance, a wide-band two-component near-infrared shielding coating with CsxWO3 and antimony doped tin oxide (ATO) was prepared [9], the coating could shield NIR light as much as 90.9% and maintain high transparency in visible light of 70.6%, as the optimized mass ratio value of CsxWO3 to ATO is 1. Wu et al [10] proposed a novel CsxWO3/ZnO smart coating, whose thermal insulation performance was superior to the indium tin oxide coating and the photocatalytic purification of toxic gas was better than the commercial P25 (TiO2). However, the NIR shielding properties of CsxWO3 nanoparticles exhibited significant instabilities in weathering evaluations [11–13]. One of these serious problems is that when the CsxWO3 is dispersed in resin coatings, photochromism will inevitably occur, leading to a dark appearance of the coating. Another problem is the NIR shielding ability of CsxWO3 easily deteriorates in hot humid and alkaline environment, which hinders further applications of CsxWO3. Thus, Zeng et al [14] made use of the ultraviolet absorber and SiO2 composite resin to enhance the UV resistance of CsxWO3 coating and it showed good near infrared shielding ability as well as excellent optical stability. Meanwhile, CsxWO3@ZnO may improve the stability in the humid alkaline environment, owing to its high performance of NIR shielding and enhanced stability as a core–shell structure nanocomposite [15]. Furthermore, a novel bio-based material which combined CsxWO3 with transparent wood was synthesized. The composite bio-based material showed both excellent NIR shielding ability and good mechanical property [16]. However, the preparation methods of these materials are too complex and costly to realize large-scale industrial application in spite of their improved performances. In addition, dust in the natural environment will decrease the light transmittance of the glass when the solar thermal insulation coating is applied outdoors [17]. So, more resources are needed to clean it. Obviously, the enhancement of anti-dust ability to prevent contamination is indispensable for CsxWO3 served as window glass coatings.
The optical stability of CsxWO3 and the sustainability in outdoor surroundings are crucial for the coating to be applied into the practical applications. Therefore, recent works focus on developing coatings with both solar thermal insulation and anti-dust properties simultaneously. In this study, nano-SiO2 resins were prepared via sol-gel method from MTES and SiO2 sol. Then, the CsxWO3-ZnO-SiO2 coating were prepared by simple co-blending of CsxWO3, ZnO nanoparticles and the previous as-prepared resins. The morphology, chemical structure, optical transparency, thermal insulation, and anti-dust property of the composite coatings were investigated. Besides, the durability of the prepared CsxWO3-ZnO-SiO2 coating were verified. The results indicated that CsxWO3-ZnO-SiO2 coatings are promising for outdoor thermal insulation.
2. Experimental
2.1. Materials
N-butanol, absolute ethanol (EtOH) and zinc oxide dispersed in EtOH (30%, 30–40 nm) are all purchased from Aladdin Chemical Reagent Co., Ltd. (Shanghai, China). Hydrochloric acid (HCl) is provided by Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). SiO2 sol dispersed in isopropanol (30%, 10–15 nm) is purchased from Nissan Chemical Industries, Ltd. Dispersion of CsxWO3 NPs in EtOH, the solid content is 30% is obtained from Nanomaterials Technology Pte. Ltd. (Xianmen, China). MTES is purchased from Qufu Chenguang Chemical Co., Ltd. (Qufu, China) and distillated before use.
2.2. Synthesis of nano-SiO2 resin
Typically, 35.6 g of MTES, 35.6 g of n-butanol and 66.7 g of SiO2 sol were added into a four-neck flask equipped with a mechanical stirrer, thermometer and reflux condenser. Then, the mixed system was heated to 70 °C and stirred for 30 min. Then, 10.8 g of HCl aqueous solution (pH 3) was added dropwise into the flask at 70 °C for 5 h. Subsequently, the system temperature was raised to 110 °C, and major solvent (about 100 g) in the bottle was removed by atmospheric distillation. After cooling down to 60 °C, the unreacted MTES was removed through vacuum distillation at the pressure of −0.095 MPa. Finally, a nano-SiO2 resin with solid content of about 50% was obtained from the bottom.
2.3. Preparation of the CsxWO3-ZnO-SiO2 coatings
The preparation process of the CsxWO3-ZnO-SiO2 coatings were presented in figure 1. A mixture was formed by directly mixing CsxWO3 dispersion, ZnO dispersion, n-butanol and nano-SiO2 resin. Then, the mixture was sonicated for 30 min to form a uniform colloidal suspension coating. The main components of the coatings are listed in table 1. The solid content of the coating was about 30%. Afterwards, the CsxWO3-ZnO-SiO2 coatings were brushed onto a glass and cured at room temperature for 1 h. And then moved to an oven at 120 °C baked for 2 h to get the cured coatings. The average thickness of the coating was about 7 μm. Herein, they were designated as CXZY (X, Y represents the content of CsxWO3 and ZnO in the solid coating, respectively).
Figure 1. Schematic diagram of the preparation process of CsxWO3-ZnO-SiO2 coatings.
Download figure:
Standard image High-resolution imageTable 1. The different formulations of the solid in CsxWO3-ZnO-SiO2 coatings.
| Sample | C20 | C15Z5 | C10Z10 | C5Z15 | Z20 |
|---|---|---|---|---|---|
| nano-SiO2 resin/wt% | 80 | 80 | 80 | 80 | 80 |
| CsxWO3/wt% | 20 | 15 | 10 | 5 | 0 |
| ZnO/wt% | 0 | 5 | 10 | 15 | 20 |
2.4. Characterization
The transmittance of the coatings was measured by UV–vis-NIR spectrophotometer (Hitachi, Japan). The near infrared (NIR) shielding performance was accurately analyzed by the Solar Energy Transmittance Selectivity (SETS). And the SETS was calculated by the following equation:
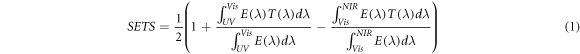
where T(λ) represents the transmittance spectrum, and E(λ) represents the solar radiation spectrum of 1.5 air mass corresponding to the Sun at 37° above the horizon. The chemical structure of the coating samples was characterized by using a Nexus-670 Fourier transform infrared (FTIR) spectrometer. X-ray diffraction (XRD) patterns were obtained through a 3 kW x-ray diffractometer (Smart Lab, Rigaku, Japan), using Cu Kα radiation (λ = 0.154 nm) from 10 to 80° at a constant scanning rate of 10° min−1. The water contact angle (WCA) was performed on a DSA 100 contact angle goniometer tester (KRUSS, Germany) by using a 2 μl deionized water droplet each time. Every coating sample was measured at least five times to minimize the random errors during the WCA test. The micromorphology of the coating was studied by field emission scanning electron microscopy (FESEM) method (Zeiss, LEO-1530VP, Germany). The film thickness was measured by SEM test on the cross section of the coating. Energy dispersive x-ray spectroscopy (EDS) (H-7650, Hitachi, Japan) provided mapping images regarding the cross-sectional surface of the coatings. The thermal performance of the coatings was evaluated by TG-DTA method on a thermo-gravimetric analyzer (NETZSCH STA449C, USA). The hardness test was carried out on CsxWO3-ZnO-SiO2 coating by using Zhonghua® pencils (China) ranging from 6B to 6H.
2.5. Anti-dust and anti-fogging test
In a typical anti-dust test, as shown in figure 2(a), the fly ash was used as the model to simulate the dust in natural surroundings. Before the test, all samples, including the fly ash, should be treated in an oven at 100 °C for 30 min to remove the moisture. Cool to room temperature and spray about 100 mg ash uniformly on each sample using a 100-mesh sieve (d = 0.15 mm). Slowly tilt the dust-treated sample until the angle between it and the horizontal plane reached 90°, then stopped and kept for 3 s. The mass percentage reduced by the ash during the process is obtained by the following formula: (1-M1/M0) ×100%, where M0 and M1 represent the weight of the primary and the remaining ash, respectively. The anti-dust performance of the coating under gravity is evaluated by calculating the weight loss of the ash. And each measurement is taken out for three times.
Figure 2. Simulation diagram of the (a) anti-dust test and (b) anti-fogging test.
Download figure:
Standard image High-resolution imageTypically, in an anti-fog test [18–20], as shown in figure 2(b), the sample was placed on the opening of a round-bottom flask filled with boiling water to simulate the foggy conditions. The sample was kept in the opening of the flask for 1 min, and the anti-fog performance was comprehensively evaluated by observing the image clarity under the steam.
2.6. Thermal insulation test
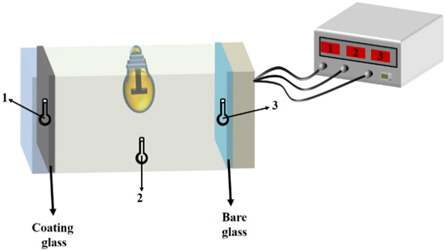
The thermal insulation test was conducted in a designed device as illustrated in figure 3. The device is composed of a xenon lamp, a test chamber and a temperature recording system. The test chamber is divided into three parts by two pieces of installed glass samples. One is coated glass, and the other is bare glass. A xenon lamp with power of 1000 W is located at the middle chamber, which serves as a heat source to simulate sunlight. Upon light irradiation, the temperatures at both ends gradually increased, and the data of temperatures was automatically recorded every 30 s.
Figure 3. Schematic diagram of self-made thermal insulation test device: 1. coated glass temperature sensor; 2. xenon lamp temperature sensor; 3. bare glass temperature sensor.
Download figure:
Standard image High-resolution image3. Results and discussion
3.1. FTIR analysis of the nano-SiO2 resin
The chemical structures of nano-SiO2 resin, the original SiO2 sol and MTES were identified by FTIR spectra in figure 4. In the FTIR spectra of nano-SiO2 resin [21, 22], the band around 3428 cm−1 is assigned to the O-H stretching vibration of hydrogen-bonded water. Correspondingly, the deformation vibration of absorbed water is indicated by band around 1634 cm−1. All samples exhibit two absorption bands at 1107 and 780 cm−1, corresponding to antisymmetric stretching and symmetric stretching vibrations of Si–O–Si bond, respectively. The band at 475 cm−1 corresponds to the deformation vibration of Si–O–Si. The bands around 2972, 2927 and 1272 cm−1 correspond to the C-H symmetric stretching vibration of –CH3, antisymmetric stretching vibration of –CH2 and symmetric deformation vibration of Si–CH3, respectively. The absorption band at 955 cm−1 is ascribed to the hydroxyl group of Si–OH, the intensity of which weakens after reactions. In addition, the area of Si–O–Si peak increased after reaction. The FTIR results demonstrate that the hydroxyl groups on the SiO2 sol particles were replaced and the condensation between MTES and SiO2 sol particles occurred effectively.
Figure 4. FTIR spectra of MTES, the pristine SiO2 sol and nano-SiO2 resin.
Download figure:
Standard image High-resolution image3.2. XRD analysis of the CsxWO3-ZnO-SiO2 coatings
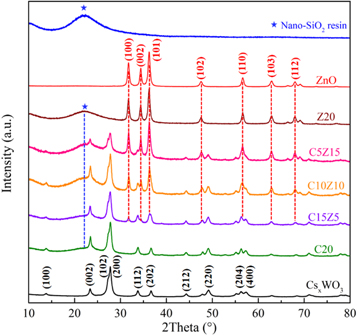
As shown in figure 5, XRD analysis was further used to investigate compositions in the solid coatings. The first diffraction peak around 10° in nano-SiO2 resin can be explained with incomplete hydrolysis of the precursor, while the second diffraction peak around 22° is attributed to the SiO2 network [23]. All diffraction peaks of the CsxWO3 and the ZnO samples could be well indexed to the hexagonal phase of cesium tungsten bronze (JCPSD No. 831334) and the hexagonal phase of zinc oxide (JCPSD No. 890510), respectively [10]. With increasing amounts of ZnO in the composites, the diffraction peaks corresponding to ZnO increased, as expected. These results suggest that the as-prepared CsxWO3-ZnO-SiO2 coatings are composed of nano-SiO2 resin, CsxWO3 and ZnO definitely.
Figure 5. XRD patterns of the nano-SiO2 resin, CsxWO3, ZnO and CsxWO3-ZnO-SiO2 coatings.
Download figure:
Standard image High-resolution image3.3. Morphology of the CsxWO3-ZnO-SiO2 coatings
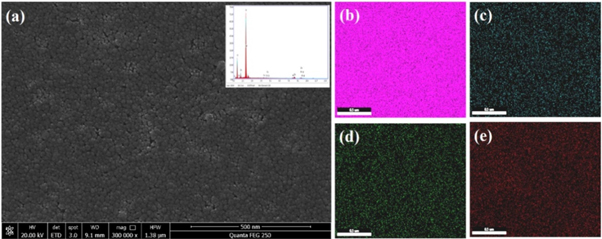
Since the formation of special surface structure is essential for anti-dust properties of coatings, morphology and microstructure of the C10Z10 coating were examined by FESEM and the results are shown in figure 6. The image showed that the surface of C10Z10 coating was covered with nanoparticles with a diameter less than 50 nm on average. These nanoparticles have created the roughness on the surface of coating. Besides, the surface of the coating is continuous and dense, owing to the product after the MTES hydrolysis reaction enhances the attraction force between the polymer and the nanoparticles through generating hydrogen bonds or other long-range forces [24]. Elemental mapping under EDS mode was also employed to further investigate the microstructure and compositional distribution of the composite coating, which is shown from the inset picture in figure 6(a). The elemental analysis result shows that the C10Z10 coating contained O, Cs, W, Si and Zn elements, indicating the as-prepared coating has a high purity, which is in agreement with the results from the XRD analysis. The uniform distribution of CsxWO3 and ZnO in the composites is also observed in elemental mapping from figures 6(b)–(e). The uniform distribution of elements in elemental mapping reflects the nanoparticles dispersed homogeneously.
Figure 6. (a) FESEM image of the C10Z10 coating (the inset is the EDS spectrum of the C10Z10 coating); (b)–(e) Elemental maps of Si, Cs, W and Zn, respectively.
Download figure:
Standard image High-resolution image3.4. TG analysis of the CsxWO3-ZnO-SiO2 coatings
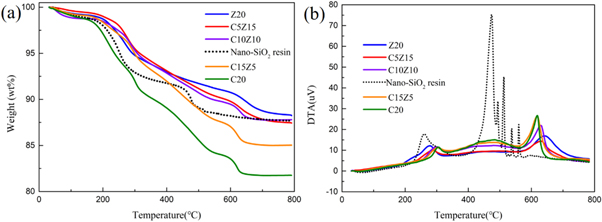
The TG and DSC curves of the nano-SiO2 resin and CsxWO3-ZnO-SiO2 coatings in air atmosphere are shown in figure 7. There are two main degradation stages in TG curves in nano-SiO2 resin. The first step showing a weight loss of 6.25% from 180 to 320 °C corresponds to the loss of water molecules caused by the condensation reaction between Si-OH groups in nano-SiO2 resin. The second step takes place within the temperature range 420 °C–600 °C, accompanied by weight loss of 2.25%, it is assigned to the degradation of Si–O–Si and oxidation removal of the organic species, such as –CH3 and –OH from the Si–O–Si side chains [25, 26]. Similarly, there also two main stages in TG curves for CsxWO3-ZnO-SiO2 coatings. However, with the temperature higher than 450 °C, the thermal stability for CsxWO3-ZnO-SiO2 coatings is much better than that of nano-SiO2 resin. Comparing with nano-SiO2 resin, the decomposition temperature of the CsxWO3-ZnO-SiO2 coatings ascend to around 620 °C after the addition of CsxWO3 and ZnO nanoparticles. A possible explanation is that when nanocomposites are heated, nanoparticles migrate to the surface of the material due to their relatively low surface potential energy, blocking the huge heat transfer to silicon resin to a certain extent [27]. This indicates that the incorporation of the nanoparticles can improve the thermal stability of the CsxWO3-ZnO-SiO2 coatings significantly.
Figure 7. (a) TG and (b) DTA curves of the nano-SiO2 resin and CsxWO3-ZnO-SiO2 coatings.
Download figure:
Standard image High-resolution image3.5. Optical analysis of the CsxWO3-ZnO-SiO2 coatings
The optical transparency of the CsxWO3-ZnO-SiO2 coatings are presented in figure 8. The UV–vis-NIR spectra of the nano-SiO2 resin and the bare glass are presented in figure S1 (available online at stacks.iop.org/MRX/8/025004/mmedia). From the figure, ultraviolet light with a wavelength of less than 300 nm is shielded due to the unique optical properties of the bare glass. At the same time, the nano-SiO2 coating will not reduce the light transmittance of the bare glass. Figure 8(a) exhibits the appearance of the CsxWO3-ZnO-SiO2 coatings at various mass ratio of CsxWO3 to ZnO in macroscopic scale, showing the tendency to be more transparent with an increase in the ZnO content.
Figure 8. (a) The as-prepared and (b) the UV–vis-NIR transmittance spectra of CsxWO3-ZnO-SiO2 coatings.
Download figure:
Standard image High-resolution imageFigure 8(b) illustrates the UV–vis-NIR transmittance spectra of the CsxWO3-ZnO-SiO2 coatings. It can be observed that the C20 coating shows excellent shielding effect of NIR light ranging from 780 to 2500 nm and good visible light transmittance. While the Z20 coating exhibits almost transparency for NIR light, along with good UV light absorption. The UV shielding ability of CsxWO3-ZnO-SiO2 coatings were improved with an increase of ZnO content while the NIR blocking ability decreased. All the compound coatings of C5Z15, C10Z10 and C15Z5 were prepared by the simple co-blending method and they all exhibit good NIR light shielding ability and excellent Vis light transmittance as well as good blocking of UV light. The excellent NIR shielding performance of these coatings are caused by the absorption of CsxWO3 to near-infrared light, which is derived from the localized surface plasmon resonance (LSPR) and a small-polaron absorption [28, 29]. To compare the NIR shielding performances of these CsxWO3-ZnO-SiO2 coatings quantitatively, some optical indices are listed in table 2. The second column in table 2 presents the transmittance at a wavelength of 370 nm which is an important judge basis of UV blocking capability. It can be seen that Z20 coating exhibits much lower transmittance at a wavelength of 370 nm than that of C20 coating, since CsxWO3 contributes to UV shielding much less than ZnO. In addition, the values in fourth column reflect the effect of CsxWO3 content on the NIR shielding performance of these coatings, solar energy transmittance of NIR lights of C15Z5, C10Z10 and C5Z15 are 0.159, 0.243 and 0.411 respectively, which are significantly enhanced comparing with control sample Z20 (0.829). In conclusion, the C10Z10 coating owes a SETS value of 0.665 and offers excellent resistance to both near-infrared and ultraviolet light, making it the most suitable composition for the CsxWO3-ZnO-SiO2 coating.
Table 2. The optical indices of the CsxWO3-ZnO-SiO2 coatings.
| Coatings | Transmittance at 370 nm (%) | Solar energy transmittance of visible lights | Solar energy transmittance of NIR lights | SETS |
|---|---|---|---|---|
| C20 | 48.2 | 0.591 | 0.111 | 0.740 |
| C15Z5 | 12.1 | 0.569 | 0.159 | 0.705 |
| C10Z10 | 2.13 | 0.572 | 0.243 | 0.665 |
| C5Z15 | 0.513 | 0.576 | 0.411 | 0.583 |
| Z20 | 0.017 | 0.617 | 0.829 | 0.394 |
3.6. Ultraviolet aging analysis of the CsxWO3-ZnO-SiO2 coatings
CsxWO3 nanoparticles are relatively stable in the natural environment, but when they are dispersed in organic resins to prepare coatings, significant photochromism appears in the weathering evaluation [6, 12, 15]. A large amount of hydrogen atoms on alkyl groups are existed in the resin. The typical reactions of RH → R·+H· and H → H+ + e− in organic resin are initiated under ultraviolet light. In general, hydrogen atoms on alkyl groups in organic resin tend to generate H+ and e− under ultraviolet light, and the generated H+ and e− can further be inserted into the crystal lattice of CsxWO3 and make the coating darken [14]. A 45-day outdoor exposure experiment to be verified the sustainability of CsxWO3-ZnO-SiO2 coatings. The optical properties of the as-prepared coatings did not change significantly after a 45-day outdoor exposure experiment, which was shown in figure S2, indicating that the coating has excellent sustainability.
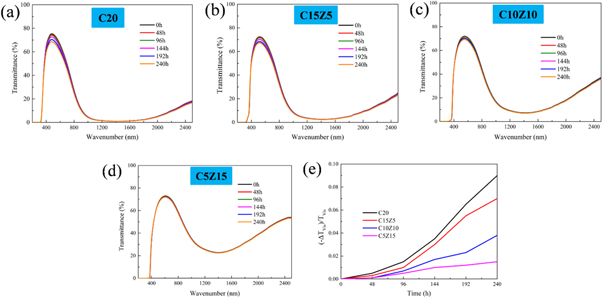
The ultraviolet aging test was performed to evaluate the optical stability of the prepared CsxWO3-ZnO-SiO2 coatings. Changes in the transmittance spectra of different coatings before and after UV illumination are shown in figures 9(a)–(d). The TVis of C20 coating dropped obviously under UV illumination. After 240 h of UV light illumination, TVis for C20 coating decreased by 9.0% compared to the initial value, while for C15Z5 and C10Z10, that decreasing values are 7.1% and 3.8%, respectively. Figure 9(e) shows the curves of the net change of TVis of the coatings over time, it can be clearly seen that with the addition of ZnO, this value tends to decrease under an equal intensity of UV illumination. Since ZnO can effectively absorb ultraviolet light [30], leading to the less production of H+ and e− in resin, the photochromic effect can be suppressed to a certain extent.
Figure 9. Changes in transmittance curves of (a) C20, (b) C15Z5, (c) C10Z10, (d) C5Z15 coating on glass illuminated by two lamps filtered for UV lights from 340 to 400 nm at 40 W. (e) Curves of the net change in TVis of the above four coatings.
Download figure:
Standard image High-resolution image3.7. Thermal insulation analysis of the CsxWO3-ZnO-SiO2 coatings
The self-made device shown in figure 2 was used to characterize the thermal insulation performance of the coatings. Two side chambers were designed to imitate enclosed rooms with the coated window and the blank glass window, respectively. For better comparison of thermal insulation performance between different coatings, the variation of temperatures as a function of radiation time in two chambers were recorded simultaneously. As shown in figure 10(a), under the luminous xenon light, the chamber temperature gradually increases with the lapse of time, and reaches peak value within 1200 s. Apparently, all the temperatures of the chamber with CsxWO3-ZnO-SiO2 coated glass after 1200 s light irradiation are lower than with nano-SiO2 resin coated glass. The highest temperature difference between chambers with C20 coated glass and with nano-SiO2 resin coated glass is 9.4 °C. While C10Z10 coating, with well overall optical performance, also obtains a good temperature difference of 7.0 °C. As indicated in figure 10(b), it is observed that temperature difference increases with the concentration of the CsxWO3 in the coating. Considering that NIR light accounts for as high as about 45% of total solar energy, CsxWO3 obviously plays a dominant role in achieving well thermal insulation effect owing to its proved excellent NIR shielding performance. Consequently, this observation is in good accordance with optical measurement results.
Figure 10. (a) Insulation curves (temperature versus time) of nano-SiO2 resin, CsxWO3-ZnO-SiO2 coatings; (b) The temperature difference between the CsxWO3-ZnO-SiO2 coatings and nano-SiO2 resin after 1200s.
Download figure:
Standard image High-resolution image3.8. Anti-dust and anti-fogging analysis of the CsxWO3-ZnO-SiO2 coatings
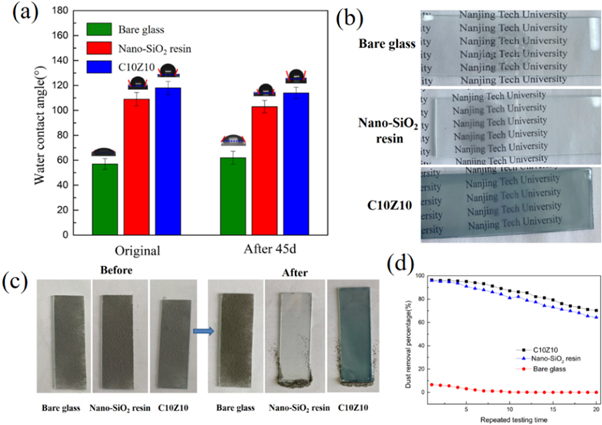
The wettability of the bare glass, nano-SiO2 resin coating, C10Z10 coating were measured by static WCA measurement. All the error bars represent the standard deviation of at least five replicate measurements. Studies have shown that low surface energy and surface roughness are the key factors that determine the hydrophobicity of the coating [31, 32]. Concerning nano-SiO2 resin was obtained by the hydrolytic condensation reaction of MTES and silica sol in this study, it is supposed that the hydrophobic group (–CH3) reduces the surface energy of the coating, and the addition of SiO2 nanoparticles improves the surface roughness. As shown in figure 11(a), the WCA of the nano-SiO2 resin coating is 109°, much higher than 57° of bare glass. Besides, with the incorporation of the CsxWO3 and ZnO nanoparticles, the WCA of the coating increases to 118°. Since both the CsxWO3 and ZnO nanoparticles are irrelevant to low surface energy, their participation probably benefited the construction of enhanced surface roughness in this case. Meanwhile, after a 45-day outdoor exposure experiment, the WCA of nano-SiO2 resin and C10Z10 coating slightly descended to 103° and 112° respectively, indicating the excellent sustainability of the as-prepared coatings.
Figure 11. (a) Water contact angle of the bare glass, nano-SiO2 resin and C10Z10 coating before and after a 45-day outdoor exposure experiment. (b) The bare glass, nano-SiO2 resin and C10Z10 coated glass were placed on 90 ℃ water for one minute respectively. (c) Photos of the bare glass, nano-SiO2 resin and C10Z10 coated glass with 100 mg fly ashes and their corresponding ones after anti-dust tests. (d) The anti-dust percentage of the bare glass, nano-SiO2 resin and C10Z10 coated glass as the functions of repeated testing time.
Download figure:
Standard image High-resolution imageTo investigate the anti-fogging ability of the nano-SiO2 resin coating and C10Z10 coating, the corresponding samples were placed on the opening of a round-bottom flask filled with boiling water to simulate the foggy conditions. Figure 11(b) showed the simulation diagram of the anti-fogging test. From the Video 1, it only takes 4 s for the water droplets to completely cover the surface of the bare glass, the words under the bottle cannot recognized through the bare glass apparently. With respect to nano-SiO2 resin coating and C10Z10 coating, as can be seen in Video 2 and Video 3, the words under the bottle were still seen after a 1-minute hot water vapor experiment. Figure 11(b) shows the photos of three kinds of coated glass after a 1-minute hot water vapor experiment. Many large mist droplets attached to the surface of the bare glass, while only tiny droplets attached to the surface of the nano-SiO2 resin coating and C10Z10 coating. The tiny mist droplets entirely evaporated on the nano-SiO2 resin coated glass and C10Z10 coated glass after about 3 min while it took about 10 min on the bare glass.
The anti-dust performance of the two above mentioned coatings under gravity were investigated, along with the bare glass served as the comparison sample. Figure 11(c) shows the photos of the anti-dust effect of different samples. Evidently, most of fly ashes slipped from the coated glass slides while only a little fly ash fell off from the bare glass. The anti-dust percentage of the coating was calculated and presented in table 3 with the corresponding WCA. The results show that the dust-reducing performance is associated with the WCA values, as exemplifying by C10Z10 coating that a large WCA couples with a better anti-dust performance. In repeated anti-dust tests, as can be seen in figure 11(d), the anti-dust efficiency of all three samples decreases as the repeated times increases, other researchers have also reported the similar phenomenon [32]. Nevertheless, 70.1% of anti-dust efficiency was kept even after twenty repeated tests, indicating that the C10Z10 coating has a durable anti-dust performance. In comparison, the nano-SiO2 resin coating could maintain 64.3% of anti-dust efficiency after multiple cycles, while the bare glass almost lost its anti-dust performance.
Table 3. The anti-dust property of different coated glass.
| Glass | WCA/° | M0/mg | M1/mg | Anti-dust average percentage/% |
|---|---|---|---|---|
| bare glass | 57° | 93.2 | 87.2 | 6.6 |
| 62° | 86.7 | 76.4 | ||
| 59° | 79.9 | 74.7 | ||
| nano-SiO2 resin | 109° | 86.1 | 2.1 | 95.9 |
| 107° | 91.4 | 4.3 | ||
| 106° | 83.2 | 4.2 | ||
| C10Z10 | 115° | 89.2 | 3.2 | 96.4 |
| 116° | 90.1 | 2.1 | ||
| 118° | 85.2 | 4.2 |
The hardness test was carried out on CsxWO3-ZnO-SiO2 coating by using Zhonghua® pencils (China) ranging from 6B to 6H, from the softest to the hardest, correspondingly. The grade of the hardest pencil that did not cause surface lacerations was taken as the pencil hardness. The test results show that all the CsxWO3-ZnO-SiO2 coatings are rather hard with pencil hardness as high as 4H, due to their dense structure, which is similar with and even a little superior to the previous published works [9, 32, 33].
4. Conclusions
The nanocomposite materials of CsxWO3-ZnO-SiO2 coatings were developed to be used as smart windows. The transparent hybrid resin was prepared from MTES and SiO2 sol by the sol-gel method is used as the substrate, CsxWO3 is used as the infrared shielding phase, and ZnO is used as the ultraviolet blocking agent. The composite coating has excellent anti-dust and anti-fogging properties due to the hydrophobicity of the nano-SiO2 resin. The addition of SiO2 nanoparticles improved the surface roughness and the hydrophobic group (-CH3) reduced the surface energy of the coating. The efficient thermal insulation performance of the composite coating due to the wonderful NIR shielding properties of the CsxWO3 nanoparticles. The composite coating could shield most of the ultraviolet light and effectively inhibit the photochromism of CsxWO3, which is related to the proper band gap of ZnO. Meanwhile, the addition of nanoparticles improved the thermal stability of the composite coating and the pencil hardness could reach 4H. Moreover, the CsxWO3-ZnO-SiO2 coatings showed an amazing temperature difference of 9.4 °C in the thermal insulation test. The dust reduction rate of the C10Z10 coating still maintain 70.4% after multiple cycles. Due to the combination of its excellent UV/NIR blockage ability, anti-dust and anti-fogging performance, the CsxWO3-ZnO-SiO2 nanocomposite is expected to further applied as smart window coatings for energy-saving materials.
Acknowledgments
We thank the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) to support this work and thanks eceshi (www.eceshi.cn) for the FESEM analysis.
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).