Abstract
In order to transform waste tea leaves into a useful/valuable material for removal of Pb2+ ions from wastewater, MnFe2O4/biochar was synthesized. The tea waste was pyrolyzed at 500 °C to obtain the biochar. Effects of the composition of tea leaves on the physicochemical properties of biochar were evaluated. Biochar and MnFe2O4/biochar were mainly organic matter. Regarding inorganic components, aside from Fe and Mn there were considerable albeit small amounts of the mineral elements K and Ca in the MnFe2O4/biochar. The MnFe2O4/biochar is porous with a specific surface area of 24.38 m2 g−1, and the surface is loaded with MnFe2O4 and amorphous MnO2 particles. Also carboxylic acid, hydroxyl, and carbonyl functional groups were formed on the MnFe2O4/biochar surfaces. The surface area and pore volume characteristics of the MnFe2O4/biochar were also increased compared with the baseline biochar, and the prepared MnFe2O4/biochar had mesostructure. The modification of biochar into MnFe2O4/biochar improved adsorption of Pb2+ ions with the removal increased to ∼98%. The Freundlich isotherm and the pseudo-second order kinetic models matched well Pb2+ adsorption onto the MnFe2O4/biochar.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
List of abbreviations
| BET | Brunauer–Emmett–Teller |
| BC | Biochar from waste tea leaves |
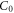
| Initial Pb2+ ion concentration (mg l−1) |
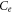
| Equilibrium concentration of Pb2+ ion (mg l−1) |
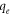
| The amount of Pb2+ ion adsorbed by BC or FMBC at equilibrium (mg g−1) |
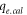
| Calculated amount of Pb2+ ion adsorbed by FMBC at equilibrium (mg g−1) |
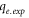
| Experimental amount of Pb2+ ion adsorbed by FMBC at equilibrium (mg g−1) |
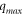
| The maximum amount of Pb2+ ion adsorbed by FMBC adsorbent (mg g−1) |
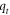
| The amount of Pb2+ ion adsorbed by FMBC at time t (mg g−1) |
| k1 | Pseudo-first order rate constant (1 min−1) |
| k2 | Pseudo-second order rate constant (g mg−1 min−1) |
| kL | Langmuir isotherm equilibrium constant (l mg−1) |
| kF | Freundlich constant representing sorption capacity (l g−1) |
| FMBC | Fe–Mn binary oxide-load biochar material |
| R2 | Correlation coefficient value |
| SEM | Scanning electron microscope spectroscopy |
| XRD | The x-ray diffraction spectroscopy |
| t | Time (min) |
| TOC | The total organic carbon |
1. Introduction
Wastewater discharged from various industries, for example producing electrodes, batteries or paint, can be contaminated by heavy metals at levels that pose potential risks to the environment, including humans and animals in it [1–4]. Lead (II) or Pb2+ ions are among the most toxic heavy metal ions in wastewater, and Pb2+ ingestion via the food chain has proven to be a potential health hazard for plants and humans [1]. With the acceleration of industrialization, Pb2+ ions are discharged into wastewater by lead mining, electroplating industry, manufacture of coatings, and especially battery manufacture [5, 6]. Therefore, it is important to develop effective, sensitive and simple approaches for highly sensitive Pb2+ removal from wastewater, before discharge to drainage systems or waterways. To remove heavy metal ions from wastewater, many conventional techniques such as chemical precipitation, oxidation, ion exchange, electrochemical methods, membrane separation, and adsorption have been employed. However, each technique has its own advantages and constraints, not only in terms of cost but also in terms of efficiency and environmental impact. For these reasons, Ranganathan and Hood [7] and Suwunwong et al [8] suggested that 'the adsorption process has been considered superior to other treatment techniques of heavy metals in wastewater due to its high removal efficiency, lower-cost, rapidness and simplicity of operation'.
Previous studies of Ho et al [9] and Sharififard et al [10] stated that 'the modified waste materials or value-added materials, such as activated carbon and biochar, have become commonly used materials in the treatment of wastewater to remove the heavy metal ions'. According to Ho et al [11] 'among these materials, biochars as alternative materials have been widely applied to Pb2+ ion treatment in wastewater because of their high efficiency, low-cost and eco-friendliness'. 'Biochar is a carbonaceous material produced by the thermochemical conversion of biomass wastes, which is rich in stable aromatic carbon and minerals. Biochar can be also used in various applications, such as carbon sequestration, soil amendment and pollution mitigation', according to Veni et al [12] and Phoungthong et al [13]. Furthermore, Ahmad et al [14] and Pap et al [15] showed that 'various kinds of carbon-rich raw materials, such as banana peels and cauliflower leaves, can be used to produce biochar and biochars derived from different biomass materials have different capacities in the removal of Pb2+ ion from aqueous solution'.
Nowadays, the growing tea plantations in Northern Thailand generate a lot of tea leaves waste, and there is a wide variety of tea drinks, such as oolong, white and green tea. Shivaji et al [16] reported that 'The tea drinks were typically produced only from the young tea leaves (bud, first and second leaf) achieved by a pruning process, a maintenance process in tea plantations in order to remove mature branches, leaves, and roots as well as to protect the tea plant from diseases. The matured tea leaves (mother leaf) are considered as waste materials during the pruning process.' Therefore, it is also important to mention that more than half of the tea plantation is unsuitable for beverage production [16]. The purpose of this work is to explore the benefits of recycled tea leaves and transform the tea leaves waste to modified biochar, as a valuable material, and use it for the removal of contaminants in wastewater. However, the problems of disposal or separation of spent biochar powder from the effluent after adsorption process limit its commercial applications. For example, Phoungthong and Suwunwong [17] studied 'doping of some paramagnetic or reducing agents, such as iron ions to produce magnetic iron oxides composites on the biochar surface will facilitate the separation of spent biochar from the effluent and enable this modified biochar in various fields for the removal of heavy metal ions in wastewater because the iron oxides/biochar composites can be easily separated from the effluent using a magnet'.
There are many reports showing the higher thermal stability and the enhanced adsorption of heavy metal ions by composites of well-crystallized iron oxides/biochar compared to the pristine biochar [17, 18]. Nowadays, effective Fe–Mn binary oxide adsorbents have been developed. These binary adsorbents show more advantages than pure iron oxides in reducing the heavy metal ions under test conditions. Lin et al [19] also found that despite 'the high adsorption capacity of Fe–Mn binary oxide adsorbents, their use is still not economically viable because the production of Fe–Mn binary oxide adsorbents is expensive and post-treatment after the process may not be environmentally friendly'. The preparation of the Fe–Mn binary oxides combined with biochar is an innovative alternative to develop low-cost, effective and eco-friendly materials for removal of heavy metals from wastewater. There are some previous reports of the Fe–Mn binary oxides-loaded biochar and the phase of Fe–Mn binary oxide can differ from the reaction condition during the preparation process. Zhang et al [21] 'had developed the Fe–Mn binary oxide-loaded biochar with the phase of Fe–Mn binary oxide is found to be FeO0.331MnO0.669 (FeMnO) were formed on the biochar surface derived from corn straws for adsorption of Pb2+ ion from aqueous solution' and Wang et al [20] 'had developed the Fe–Mn binary oxide-loaded pinewood biochar with MnFe2O4 form for As removal'. Metal (M) ferrite (MFe2O4) has a cubic crystal structure, in which M and Fe occupy tetrahedral and octahedral cation sites, respectively. Recently, bimetal spinel nanocrystals, such as metal ferrites including cobalt ferrite, nickel ferrite and zinc ferrite reported by Vazquez-Olmos et al [21], have been found to have extraordinary performances in treatment of environmental pollution, including Pb2+ ion removal. Even though excellent sorption capacities for Pb ion have been reported, there is no report about manganese ferrite (MnFe2O4) in the removal of Pb ion, so MnFe2O4 is thus proposed to modify pristine biochar to improve Pb removal efficiency. The resulting engineered biochar is usually such composite in which MnFe2O4 is distributed and supported within the carbon matrix. Moreover, we wish to identify the mechanism of adsorption of Pb2+ by the Fe–Mn binary oxides-loaded biochar.
In this work, the treatment of Pb2+ ion in an aqueous solution by MnFe2O4-loaded biochar derived from waste tea leaves was carried out. The waste tea leaves were used as raw material to prepare biochar at 500 °C in this research. The objectives of this study were to: (1) develop the Fe–Mn binary oxide-loaded biochar (FMBC) from the prepared biochar (BC) to facilitate treatment of spent biochar after removal of Pb2+ ions from an aqueous solution; (2) characterize the physicochemical characteristics of the obtained BC and FMBC; (3) investigate the adsorption isotherms and kinetics of Pb2+ adsorption by the FMBC, and (5) study the mechanisms of the adsorption of Pb2+ on FMBC for an assessment the adsorption behaviors of Fe–Mn binary oxides-loaded biochar.
2. Experiments
2.1. Biochar and FMBC preparation
The waste tea leaves were received from a tea plantation field in Northern Thailand. The waste tea leaves were washed thoroughly with distilled water to remove dirt, and were then oven dried at 100 °C for 24 h. The surface area of biochar is important because, like some other physicochemical characteristics, it may strongly affect the reactivity and pyrolytic behavior of the biochar. There is a report that waste tea leaves with high specific surface area can be prepared in the temperature range 450 °C–500 °C [22, 23]. 'Comparatively high oxygen contents in biochar were observed with pyrolysis temperatures 400 °C–500 °C', according to Suwunwong et al [8]. Therefore, the temperature for biochar production was fixed at 500 °C in the present experiments. The biomass of waste tea leaves was measured and then they were placed in covered crucibles. Karunanayake et al [24] and Vu et al [25] report that 'the pyrolysis process was carried out by heating the covered crucibles containing waste tea leaves in a furnace under oxygen-limited conditions at the pyrolysis temperatures 500 °C with a heating rate of 10 °C min−1'. To obtain BC material, the residence time was set at 2 h. 'After the pyrolysis process, the BC material was washed several times with distilled water to remove the water-soluble organic residuals, impurities and fine particles' according to Kumar et al [26]. The 20 g of BC was dried in an oven at 80 °C for 2 h. 'The BC was then ground and stored in dried and closed vessels', as reported by Kumar et al [26] and Sun et al [27]. The FMBC was prepared based on a previous study reported by Lin et al [19]. In brief, 5 g of BC was added to an aqueous solution of KMnO4 (0.40 mol l−1, 40 ml) and Fe(NO3)3 (0.50 mol l−1, 40 ml) compounds. The mixture solution was stirred in an ultrasonic bath at room temperature for 2 h, and then was stirred in the water bath at 90 °C for 22 h in order to reduce the volume of the solution. Finally, the material was re-pyrolyzed at 500 °C with the heating rate of 10 °C min−1 and the residence time of 1 h, and then washed several times with distilled water to remove impurities and fine particles. The FMBC material was separated from distilled water using a magnet. The obtained FMBC material was dried in an oven at 90 °C for 4 h.
2.2. BC and FMBC characterizations
The Total Organic Carbon (TOC) analysis was performed using a TOC analyzer (Analytik Jena AG, Germany). The elemental composition was determined using a CHNS/O analyzer (Flash 2000, ThermoScientific, Italy). The ash contents for both BC and waste tea leaves were determined by Wet Lab WI-RES-Wet lab-010 using Gravimetric method (Furnace, Carbolite CWF 11/13). The presence of 174 major mineral components in the samples was analyzed by an x-ray fluorescence (XRF) spectrometer (Zetium, PANalytical, Netherlands). The x-ray diffraction (XRD) pattern (PAN analytical, X' Pert Pro MPD) of FMBC was further recorded for confirmation of the crystalline structure of metal composites on the BC surfaces. The morphologies of BC and FMBC were imaged by a Scanning Electron Microscope (SEM, Czech Republic) and the specific surfaces by the BET (Brunauer–Emmett–Teller) method. The functional groups in the BC and FMBC were determined using a FT-IR (Vertex70, Bruker, Germany) system.
2.3. Adsorption studies
In order to study adsorption of a heavy metal by BC and FMBC, the removal of Pb2+ ions by BC and FMBC was screened with 10 mg l−1 Pb2+ (Aldrich, USA) solution at 30 min adsorption time. The adsorption isotherms and kinetics of Pb2+ removal by FMBC were determined in batch experiments. The final concentrations of Pb2+ ions in the sample solutions after the adsorption process were determined with the ICP-OES technique (ICP-OES PerkinElmer, Optima 2000 DV, USA). The effects of contact time on adsorption of Pb2+ by FMBC were investigated. A 10 ml sample of the 100 mg l−1 Pb2+ solution was transferred to a 15 ml centrifuge tube along with 0.1 g of the FMBC adsorbent. The solution was agitated at 180 rpm in a thermostatic shaker water bath for a controlled time from 5 to 120 min at 25 °C. The FMBC powder was separated from the sample solution using a magnet. The final concentrations of Pb2+ ions in the supernatant were determined with ICP-OES as the Pb2+ contents left in the solution. A 10 ml mixed solution sample compared with Pb2+ solution was transferred to a 15 ml centrifuge tube along with 0.1 g of the FMBC adsorbent with the adsorption time of 30 min, at 25 °C. The % removal and adsorption capacity ( ) of FMBC on removal of Pb2+ was determined with the ICP-OES technique. The equilibrium capacity, to remove Pb2+ ions by FMBC from the aquous solution, can be described by an adsorption isotherm in which the ratio of Pb2+ ion adsorbed or that remains in solution at equilibrium at a fixed temperature' as proposed by Freundlich [30] and Langmuir [31]. In this study, the Pb2+ adsorption was measured at optimum conditions, at 25 °C. Wan et al [28] suggested that 'the Langmuir equation can be used to estimate the maximum adsorption capacity, with complete monolayer coverage of the biochar surface'. In addition, the empirical Freundlich model is used to estimate the adsorption intensity by an adsorbent, in which the model assumes that the adsorbed molecules interact with a heterogeneous multilayer surface [29, 30].
) of FMBC on removal of Pb2+ was determined with the ICP-OES technique. The equilibrium capacity, to remove Pb2+ ions by FMBC from the aquous solution, can be described by an adsorption isotherm in which the ratio of Pb2+ ion adsorbed or that remains in solution at equilibrium at a fixed temperature' as proposed by Freundlich [30] and Langmuir [31]. In this study, the Pb2+ adsorption was measured at optimum conditions, at 25 °C. Wan et al [28] suggested that 'the Langmuir equation can be used to estimate the maximum adsorption capacity, with complete monolayer coverage of the biochar surface'. In addition, the empirical Freundlich model is used to estimate the adsorption intensity by an adsorbent, in which the model assumes that the adsorbed molecules interact with a heterogeneous multilayer surface [29, 30].
The adsorption kinetics experiments were run in order to investigate the adsorption dynamics of the Pb2+ ion uptake rate, which is the rate limiting step in adsorption of metal ions by FMBC surfaces. In this work, 'pseudo-first order and pseudo-second order model types were selected for representing the adsorption kinetics of Pb2+ ion by FMBC, at 25 °C', as proposed by Mahmoud [31] and Simonin [36].
The pseudo-first order kinetic model is given by equation (1).
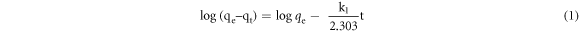
The adsorption kinetics data were also analyzed in terms of the pseudo-second order model, given by equation (2).
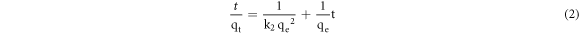
The initial adsorption rate h (mg/g·min) is given in equation (3).
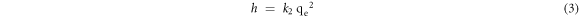
And the equations (2) and (3) can be written as equation (4).
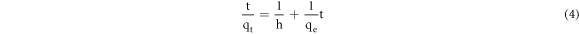
3. Results and discussion
3.1. Characterizations of BC and FMBC
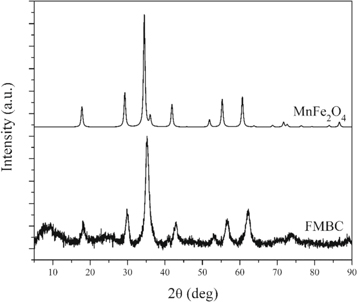
In order to test whether the BC and FMBC derived from waste tea leaves are suitable for use as eco-friendly adsorbents that remove Pb2+ ions from wastewater, or even for soil amendment and fertilizer, the chemical components, especially the heavy metal components in BC and FMBC derived from waste tea leaves were characterized before adsorption studies. The total organic carbon (TOC), ash and the elemental (CHNOS) components in waste tea leaves, BC, and FMBC, were determined and are presented in table 1. Moreover, the physical characteristics and chemical compositions of BC and FMBC materials were determined with SEM, elemental analysis, XRF and FT-IR techniques. The mineral components in FMBC compared to the raw material waste tea leaves are presented in table 2. The results in tables 1 and 2 show that organic matter (C, H, N, O and S) is the main component in both BC (93.31%) and FMBC (61.17%). The obtained BC material derived from waste tea leaves prepared at 500 °C pyrolysis temperature at the heating rate 10 °C min−1, is suitable for carbon sequestration process or for use as fertilizer with the carbon content of 64.14% and the total organic carbon content of 57.89%. Regarding FMBC, the carbon, hydrogen, and oxygen contents were decreased due to dehydration and decarboxylation. Some carbon, hydrogen and oxygen can be lost by releasing volatiles CxHyOz and CxHy. According to Liu et al [32] 'the high oxygen contents in biochar were observed which may benefit the surface complexation of oxygen-containing group, on biochar, with Pb2+ ions in adsorption process'. Figure 1 shows the XRD pattern of FMBC compared to the XRD pattern of MnFe2O4 spinel structure [33, 34] corresponding to the structure reported by Vaez-Zadeh and Mohammadi [35]. Based on the high contents of Mn and Fe in XRF results, at 17.11 and 16.73% contents, and the XRD pattern of FMBC, the Fe–Mn binary oxide as a MnFe2O4 spinel phase particles was successfully deposited on the surfaces of BC.
Table 1. Characteristics of BC and FMBC derived from waste tea leaves.
| Parameter, unit | Waste tea leaves | BC | FMBC |
|---|---|---|---|
| TOC (%wt) | 39.88 | 57.89 | 23.97 |
| Ash, (%wt) | 5.77 | 15.28 | 36.93 |
| C | 45.34 | 64.14 | 28.53 |
| O | 38.94 | 16.32 | 29.71 |
| H | 6.00 | 2.83 | 1.06 |
| N | 2.60 | 3.13 | 1.77 |
| S | 0.18 | 0.21 | 0.10 |
Table 2. Mineral contents by weight in waste tea leaves and in FMBC.
| Mineral | Waste tea leaves (%wt) | FMBC (%wt) |
|---|---|---|
| K | 2.867 | 2.471 |
| Ca | 1.619 | 1.006 |
| Al | 0.940 | 0.888 |
| Mn | 0.510 | 16.734 |
| Mg | 0.291 | 0.240 |
| P | 0.230 | 0.254 |
| Cl | 0.113 | 0.028 |
| Si | 0.065 | 0.079 |
| Fe | 0.027 | 17.107 |
| Cu | 0.016 | 0.010 |
| Sr | 0.004 | 0.005 |
| Zn | 0.004 | 0.005 |
| Rb | 0.004 | 0.003 |
| CHNOS | 93.310 | 61.170 |
Figure 1. XRD pattern of FMBC compared to the pattern for MnFe2O4.
Download figure:
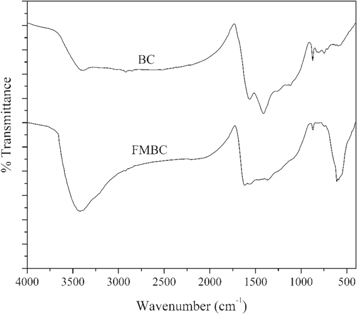
Standard image High-resolution imageSEM images of BC and FMBC are seen in figure 2. For BC the SEM images exhibit rough surface morphology with porosity (figures 2(a) and (b)), and figure 2(c) shows a mesoporous surface with various sizes of small canals in the BC material. In figure 2(d), the SEM image shows a composite with MnxFe3−xO4 powder on BC surfaces. Moreover, various sizes of spinel phase crystals of MnxFe3−xO4 compound are observed on the surface of BC, corresponding to the structure reported by Vernekar et al [36]. The surface areas and the porosity types of FMBC and BC are summarized in table 3. BC and FMBC have mesoporous structures with average pore diameters of 4.44 and 9.59 nm, respectively. Based on the N2 adsorption, the BET mesopore volume estimates for BC and FMBC were 0.012 and 0.058 cm3 g−1, respectively. The surface area was increased to 24.38 m2 g−1 by the modification of BC to FMBC, from that for baseline BC (8.06 m2 g−1). Li et al 2018 [19] indicate that this benefits Pb2+ ion adsorption on adsorbent, and that the surface chemistry of functional groups on the modified biochar is significantly related with the heavy metal ion adsorption [24, 25, 37]. According to Guo et al [38] and Chen et al [39] 'the main functional groups in FMBC are quite similar to those of BC, but some functional groups, such as Fe–O and Mn–O bonds, were formed during the development of Fe–Mn-biochar process by adding the MnFe2O4 particle composites on the biochar surface'. Based on the peak at around 540–680 cm−1 as shown in figure 3, that was identified as the peak of Mn–O and Fe–O bonds from Fe–Mn–O composite, or attributed to the bonds that formed between the oxygenic functional groups on the biochar surface and iron or manganese, which might be considered as the Fe–O–H or Mn–O–H bonds [40–42]. The C–H bending peak for BC and FMBC were observed at 876 and 869, respectively. The C–O stretching vibrational peak at approximately 1404 and 1260 cm−1 of BC was weaken and shifted to 1378 and 1144, respectively, after the MnFe2O4 was successful composited to BC surface. From the literature, a broad stretching vibrational peak at approximately 3429 cm−1 was attributed to the hydroxyl groups (–OH) on the FMBC surfaces, and a larger amplitude of stretching vibrations was observed in Fe–Mn-biochar due to the binding of Mn or Fe ions with –OH group [40–42].
Figure 2. SEM images on the scale of 100 (a) and 10 (b) microns, and of FMBC on the scale of 30 (c) showing the surface structure and a view the composite spinel MnFe2O4 at 0.5 microns (d).
Download figure:
Standard image High-resolution imageTable 3. BET based estimates for FMBC and BC.
| Sample | Surface area (m2 g−1) | Pore volume (cm3 g−1) | Average pore diameter (nm) | Reference |
|---|---|---|---|---|
| BC | 8.06 | 0.012 | 4.44 | [23] |
| BC | 2.81 | 0.010 | 14.81 | This work |
| FMBC | 24.38 | 0.058 | 9.59 | This work |
Figure 3. FT-IR spectrum of the FMBC compared to BC material.
Download figure:
Standard image High-resolution image3.2. Adsorption of Pb2+ ions by FMBC
The removal efficiency by FMBC was screened with Pb2+ ions in an aqueous solution. The percentage removal of Pb2+ by FMBC as adsorbent was compared to BC for various masses of the adsorbent, as shown in figure 4(a). The increasing removal of Pb2+ by FMBC (to 98%) confirms that Fe–O and Mn–O bonds in Fe–Mn binary oxide-loaded biochar play an important role in the chemical adsorption of Pb2+ ions by FMBC.
Figure 4. Percentage removal of Pb2+ by BC and FMBC, from 10 ml aqueous solutions containing Pb2+ ions at the concentration of 10 mg l−1 in 30 min of adsorption, on using 0.02–0.1 g of adsorbents (a), the percentage removal of Pb2+ by FMBC at the concentration of 10 mg l−1 in 30 min of adsorption, at pH 3–9 (b), the percentage removal (c) and adsorption capacity (d) of Pb2+ by FMBC from 10 ml aqueous solutions containing Pb2+ ions at the concentration of 100 mg l−1, at 0–120 min).
Download figure:
Standard image High-resolution imageThe pH of the adsorption solution affected the removal of Pb2+ ions by Fe–Mn binary oxide/biochar. The pH can modify adsorbent surface charge and ionization of heavy metal. Figure 4(b) shows the change of the adsorption efficiency of Pb2+ ions with pH gradually increased from 3 to 7, and it reached a steady value at pH 6 until pH 7. At pH 6 to 7 the removal efficiency of Pb2+ ions was by around 95%. However, the adsorption of Pb2+ ions gradually decreased with further increasing pH (>pH7). An increased pH is useful to hydrolysis, since the electronic structure of Pb2+ ions is more prominent than those produced by free hydroxides. At pH 7 the adsorption sites of carbon are saturated. On increasing the solution pH, the Pb2+ adsorption competes with the ion exchange of protons to binding sites, and the precipitation or creation of hydroxide complexes of Pb2+ ion might improve removal efficiency. Thus, the highest adsorption capacities were observed in solutions of pH 7.0 (figure 4(b).
The contact time is an important factor influencing the adsorption of Pb2+ from solution onto FMBC at 25 °C as shown in figures 4(c) and (d). The removal in terms of adsorption capacity (qe
) of Pb2+ ions by FMBC increased consistently with time and increased rapidly at the initial time of adsorption (0–15 min). From the kinetics study, the removal of Pb2+ by FMBC reached equilibrium at around 30 min of adsorption. 'The adsorption isotherm models can be used to describe the interaction of the Pb2+ ion on the FMBC surface, the maximum adsorption capacity ( ) of the FMBC material as well as the dynamic equilibrium of adsorption system' according to Tharaneedhar et al [43]. From the experimental results in table 4, the Freundlich isotherm is gave the best fit with the R2 value of 0.99. According to this fitted model, the Pb2+ ions are possibly adsorbed in multiple layers on FMBC under a non-ideal reversible adsorption process, based on the derivation/assumptions of the Freundlich isotherm [44]. The kinetics of Pb2+ ion adsorption by FMBC were well fit with the pseudo-second order kinetic model with R2 value of 1, as shown in table 4. Fan et al [45] suggested that 'the pseudo-second order model can confirm the process of Pb2+ ion chemisorption on the FMBC surface in which the liquid film diffusion, intra-particle diffusion and surface adsorption are contributed in the adsorption mechanisms involving electrostatic attraction, hydrogen bonding, ligand exchange in complexation'.
) of the FMBC material as well as the dynamic equilibrium of adsorption system' according to Tharaneedhar et al [43]. From the experimental results in table 4, the Freundlich isotherm is gave the best fit with the R2 value of 0.99. According to this fitted model, the Pb2+ ions are possibly adsorbed in multiple layers on FMBC under a non-ideal reversible adsorption process, based on the derivation/assumptions of the Freundlich isotherm [44]. The kinetics of Pb2+ ion adsorption by FMBC were well fit with the pseudo-second order kinetic model with R2 value of 1, as shown in table 4. Fan et al [45] suggested that 'the pseudo-second order model can confirm the process of Pb2+ ion chemisorption on the FMBC surface in which the liquid film diffusion, intra-particle diffusion and surface adsorption are contributed in the adsorption mechanisms involving electrostatic attraction, hydrogen bonding, ligand exchange in complexation'.
Table 4. Isotherm and kinetic constants of different models for the adsorption of Pb2+ ions by FMBC at 25 °C.
| Isotherm model | Kinetics model | ||||
|---|---|---|---|---|---|
| Langmuir |
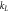 (l mg−1) (l mg−1) | 21.495 | Pseudo- | qe, exp (mg g−1) | 9.85 |
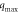 (mg g−1) (mg g−1) | 25.00 | first order | qe, cal (mg g−1) | 2.35 | |
| R2 | 0.91 | k1 (1 min−1) | 0.0976 | ||
| R2 | 0.89 | ||||
| Freundlich |
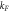 (l g−1) (l g−1) | 1.1148 | Pseudo- | qe, cal (mg g−1) | 9.85 |
| N | 1.0953 | second order | k2 (g mg−1 min−1) | 0.1455 | |
| R2 | 0.99 | h | 14.1243 | ||
| R2 | 1 | ||||
3.3. Proposed mechanisms of Pb2+ absorption on FMBC surfaces
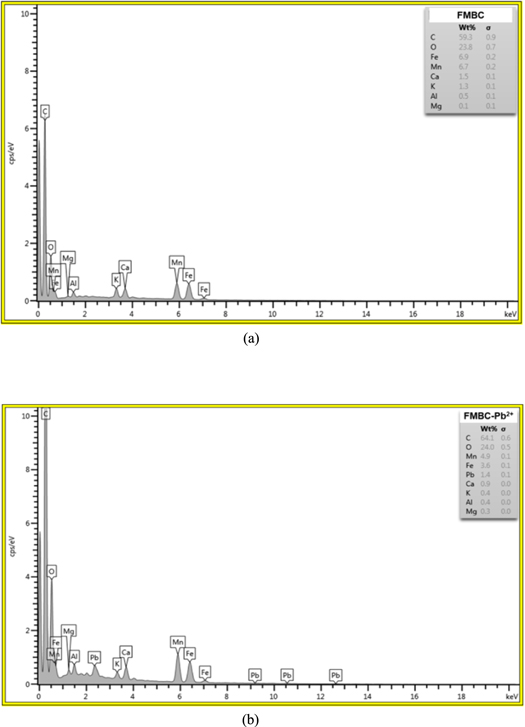
The adsorption of Pb2+ on FMBC surfaces takes place with various mechanisms and in multiple steps with both physical and chemical interactions. The adsorption process is mainly involving external liquid film diffusion, surface adsorption, intraparticle diffusion and complexation. In this work, the mechanism of adsorption process can be characterized and explained based on the SEM-EDS related with the FT-IR pattern. The kinetic and isotherm adsorption results can also explain the adsorption mechanisms of electrostatic interaction, surface complexation, and ion exchange for the removal of Pb2+ ions by FMBC. The potential mechanism between FMBC and Pb2+ includes electrostatic interaction, surface complexation, ion exchange and other processes. The EDS analysis indicated that Mn, Fe, and O were present in the FMBC powder after Pb2+ adsorption compared to pristine surface of FMBC. The Pb was presented in the FMBC powder after Pb2+ adsorption. The mass percentages of elements were recorded at three points. According to the results of EDS spectrum (figure 5), the means of mass percentages (%) of C, O, Mn, and Fe of the FMBC before and after adsorption Pb2+ were 52.78, 31.47, 7.50, 5.58 and 56.76, 28.49, 6.42, 4.94, respectively, and the means of mass percentages (%) of Pb of the FMBC after adsorption of Pb2+ was 1.20%. This pattern confirmed that Pb ions were adsorbed on FMBC surfaces.
Figure 5. EDS analysis of FMBC before and after Pb2+ adsorption.
Download figure:
Standard image High-resolution imageWith the results of experiments, the mechanism can be elucidated as follows:
3.3.1. Surface complexation and ion exchange
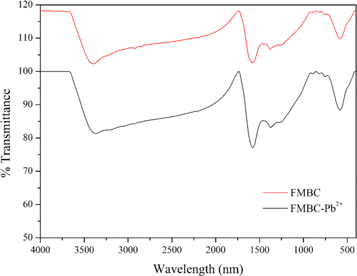
The binding of metal ion Pb2+ on FMBC can be explained by surface complexation. Many oxygen-containing groups existed on the surface of FMBC, such as MnFe2O4 and MnO2. The FT-IR analysis confirmed that the interaction between FMBC and Pb2+ involved surface complexation (figure 6). For the FMBC sample, the bands of Fe–O and Mn-O (from MnFe2O4 and amorphous MnO2) are from stretching vibrations of MnFe2O4. The functional groups on FMBC such –COOH, –OH and –C=O participate in Pb2+ absorption related with the shift of wavenumber as shown in table 5. This mechanism was confirmed by the decrease of pH after adsorption process. Ion exchange is an important mechanism participating in the chemisorption of Pb2+ ions on FMBC surfaces. The ions of Fe3+ and Mn2+ were observed in the leachate water after adsorption process confirming that the adsorption of Pb2+ by FMBC is also related to ion exchange and complexation, which led to the release of the observed cations.
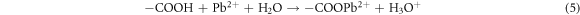
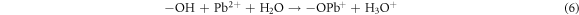
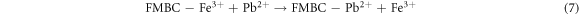
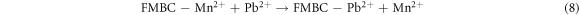
Figure 6. FT-IR spectra of FMBC before and after Pb2+ adsorption.
Download figure:
Standard image High-resolution imageTable 5. Functional groups of FMBC before and after adsorption of Pb2+ ions.
| FMBC, cm−1 | FMBC-Pb2+, cm−1 | ||
|---|---|---|---|
| Assignment | Before adsorption | After adsorption | Difference, cm−1 |
| Bonded –OH | 3398 | 3385 | −13 |
| C=O stretching, aromatic C=C, C=O | 1580 | 1578 | −2 |
| C–O | 1380 | 1367 | −13 |
| Fe–O | 586 | 572 | −14 |
4. Conclusions
- (1)Fe–Mn binary oxide-loaded biochar (MnFe2O4) was successfully synthesized by doping the MnFe2O4 composite on the biochar surface in re-pyrolysis of biochar derived from waste tea leaves at 500 °C (heated at 10 °C min−1 rate).
- (2)The Fe–Mn binary oxide-loaded biochar from waste tea leaves pyrolyzed at 500 °C can be used as an alternative low-cost and efficient adsorbent in wastewater, because of its high content of organic matter (mass fraction of C, H, N, O and S was 61.17%).
- (3)The physicochemical characteristics and the chemical composition of Fe–Mn binary oxide-loaded biochar were investigated. Fe–Mn binary oxide-loaded biochar has improved characteristics for adsorption, in terms of the higher surface area compared to pristine biochar and increased active sites of functional groups and morphology.
- (4)The MnFe2O4-loaded biochar is effective for removal of Pb2+ ions from aqueous solutions with a high percentage of removal. Adsorption isotherm and kinetics for Pb2+ removal by the Fe–Mn binary oxide-loaded biochar were well fit with a Freundlich model and a pseudo-second order model, indicating that the Pb2+ ions were adsorbed reversibly on the biochar surfaces with multilayer adsorption, in which the chemisorption was involved in the adsorption. The mechanism of Pb adsorption by MnFe2O4/biochar is especially related with ion exchange and surface complexation.
Acknowledgments
The financial supports from Mae Fah Luang University and Graduate School, Prince of Songkla University are gratefully acknowledged.