Abstract
Simple methodology was developed to synthesize copper oxide nanoparticles (CuO NPs) using mucus of Channa striatus (C. striatus). The mucus of C. striatus is known for its biological properties due to the presence of numerous amino acids. This mucus was used as stabilizing agent for CuO NPs synthesis from copper acetate. The prepared CuO NPs were characterized by fourier transforms infrared spectrometer (FTIR), powder x-ray diffraction (XRD), x-ray photoelectron spectroscopy (XPS), scanning electron microscope (SEM), energy dispersive x-ray analysis (EDX) and transmission electron microscope (TEM) coupled with selected area diffraction pattern (SAED). The FTIR study suggested the utilization of mucus in the synthesis of CuO NPs. The XRD data also confirmed formation of pure crystalline phase of CuO NPs. Fish mucus stabilized CuO NPs exhibited significant activity against HeLa cells. The results of cell death clearly indicated that the synthesized CuO nanoparticles could be served as a biomaterial for anticancer treatment.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Various physio-chemical methods have been developed to fabricate metal oxide nanoparticles. Numerous applications of metal/metal oxide nanoparticles have been reported recently [1, 2]. Role of various nanoparticles have been explored in the field of dye degradation [3–5], photocatalytic activity, heavy metal removal, humic acid removal and targeted contrast agent for cancer diagnosis in magnetic resonance imaging [6–10]. Fabrication of metal oxide nanoparticles using plant extract has been considered as eco-friendly and simple method by which, various sizes and morphology of the particles can be produced [11]. It is known that the green synthesis gives the preferred product without producing hazardous byproduct during the reaction. Green synthesis is a safe and low cost method by which, stable nanoparticles can be produced [12]. The biomolecules such as vitamin, carbohydrate, polymers and phenolic compounds present in the plant extract act as reducing as well as a stabilizing agent during the production of the CuO nanoparticles [13]. Aqueous extracts prepared from the plants such as Galeopsidis herba [14], carica papaya [15], Gloriosa superba [16], sugar cane juice [17], Alovera [18], Rauvolfia serpentine [19], Henna extract [20], Syzygium alternifolium [21] and ferulago angulata (schlecht) boiss extract [22] have been used as stabilizing as well as reducing agent to produce CuO NPs [21].
Bio-fabricated CuO NPs have been used as a catalyst in the removal of organic pollutant [23]. CuO NPs are well known for their biological activities. The bactericidal activities of CuO NPs against various pathogenic bacteria have been proved. Antiviral and anticancer activity of CuO NPs has been evaluated by in vitro methods against cell line [24, 25]. CuO NPs have the capacity to bind with proteins, which causes the DNA cleavages and control the free oxygen production proved its antioxidant activity. Industrial applications of CuO NPs in the form of photocatalytic activity in dye removal and as a catalyst for organic coupling reactions have been proved [26–28].
It is recognized as one of the important Asian fish. Its skin consists of various fatty acids. Skin is a vital source of mucus being rich in amino acids [29]. Fish mucus is a source of enzymes [30], proteins [31], numerous crinotoxins [32] and antibacterial peptides [33]. It is well known that fish mucus having various biological activities. Therefore, considering the medicinal value of fish mucus, the present work was aimed to synthesize of CuO NPs using skin mucus of C. striatus. The physico-chemical characterization of prepared CuO NPs was done by a series of techniques. Moreover, the anticancer potential of CuO NPs was assessed in HeLa cells.
2. Materials and methods
2.1. Isolation of skin mucus
C. striatus were purchased from the local market, Cuddalore, Tamil Nadu, India. Mucus was collected according to the procedure described earlier [34]. Before collecting the mucus, fish were kept for one week in a tank filled with fresh water in order to minimize the bacterial pollution and to improve the secretion rate of mucus. Mucus was collected carefully from the skin using a plastic spoon and right away frozen, lyophilized and stored at −25 °C.
2.2. Fish mucus stabilized synthesis of CuO nanoparticles
Copper acetate (Cu(CH3COO)2) was used as a precursor. The copper acetate solution was prepared using double distilled water. In brief, 1, 2 and 3 ml of fish mucus were added in 100 ml of copper acetate solution (0.1 M) and homogenized thoroughly using a magnetic stirrer at 85 °C for 3 h. The solution was turned yellowish green followed by the brownish black. The precipitate was centrifuged, thoroughly washed with distilled water and used for further study followed by the calcinations at 500 °C for 3 h. The samples prepared using 1, 2 and 3 ml fish mucus was named as C1, C2 and C3. A blank sample (C0) was prepared with the same procedure for the purpose of comparison.
2.3. Characterizations methods
Characterization methods used were FTIR spectrophotometer (Perkin Elmer RX1) by KBr pellet method scanned in the range of 4000–500 cm−1. The crystal structure of the CuO NPs was analyzed by x-ray diffractometer (Shimadzu XRD 6000, Japan) equipped with Cu-Kɑ radiation source recorded in the range of 10°–80° in 2θ value. The formation of nanoparticles was analyzed by scanning electron microscope (CAREL ZEISS SEM EVO, 18) and high resolution transmission electron microscope coupled with selected area diffraction (HR-TEM-EDAX: Bionand's FEI-Tecnai G220 Twin). The x-ray photoelectron spectroscopy (XPS, Thermo Scientific K-Alpha) with Al Kα x-ray source was used to find out the chemical state of CuO NPs.
2.4. Anticancer activity by MTT assay
Toxicity of CuO NPs was carried out by MTT assay against HeLa cells. The cells were developed in DMEM with 1% Penicillin–Streptomycin and 10% fetal bovine serum in standard culture medium condition. Cells were poured into in 96-cell culture dishes (plate count at 5000 cells cm−1). After 24 h of incubation, the cells were fed with fresh medium and treated with CuO NPs and Doxorubicin (control). The medium was kept for 48 h, and then the cells were treated with MTT and further incubated for 1.5 h in culture condition. The formazan crystals produced by mitochondrial reduction of MTT were dissolved in solvent (DMSO) and the optical density (OD) of the solution was read at 490 nm using Elisa Reader (DRG, USA). Cell viability was calculated in percentage after subtracting the background corrections [35].
3. Results and discussion
3.1. Fourier transform infrared analysis
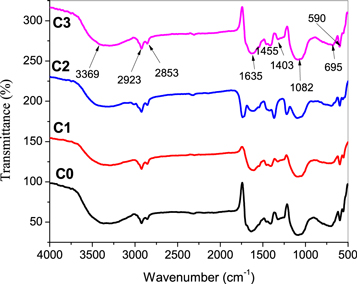
FTIR is a useful tool to assess the functional groups of fish mucus extract involved in the reduction of copper ions into CuO during the synthesis. FTIR spectra of CuO NPs are shown in figures 1(C0)–(C3). Formation CuO is confirmed by FTIR spectrophotometer through stretching vibrations of metal oxygen bond (Cu-O). Characteristic bands of CuO NPs were obtained at 590, 695, 1082, 1403, 1455, 1635, 2855, 2923 and 3369 cm−1, respectively. A band at 3369 cm−1 confirmed the O−H stretching of moisture present in the samples. A narrow band at 1635 cm−1 revealed the −C=C− bending vibrations of conjugated hydrocarbon. The small bands at 448, 590 and 695 cm−1 confirmed the presence of Cu–O vibration of synthesized CuO NPs. The bands at 2853 and 2923 cm−1 revealed the C–H stretching frequency. The bands at 1403 and 1455 cm−1 are due to the N–H stretching of amino group.
Figure 1. FTIR spectra of CuO NPs. (C0) blank CuO NPs; (C1)–(C3) CuO NPs stabilized by 1, 2 and 3 ml mucus.
Download figure:
Standard image High-resolution image3.2. X-ray diffraction study
The existence of the apparent and sharp peaks with different 2θ values obviously confirmed the crystallinity of CuO NPs. The diffraction pattern of CuO NPs is shown in figures 2(C0)–(C3). The XRD pattern showed diffraction peaks at 2θ of 32.19, 35.22, 38.40, 48.59, 53.05, 57.99, 61.17, 66.11, 67.86, 72.16, and 75.03 which can be designated (110), (−111), (111), (−202), (020), (202), (−113), (−311), (220), (311) and (004) planes, respectively. The analysis of given Bragg reflection of quite prominent peaks can be indexed to the monoclinic phase of CuO (No. 96-410-5686) [36]. Similarly, XRD of CuO NPs prepared using starch solution and banana peel extract showed that the nanoparticles are monoclinic structure [25, 37]. The XRD pattern showed no additional peaks, revealing that the synthesized CuO is highly pure in nature. The average crystallite size of pure CuO and CuO synthesized using 1, 2 and 3 ml fish mucus (C0–C3) calculated using Scherrer formula were 104.62 ± 13.50, 104.62 ± 13.50, 88.97 ± 14.90 and 82.38 ± 8.42, respectively. This is very clearly reveals that as the concentration of fish mucus increases, the size of the particles decreases.
Figure 2. XRD pattern of CuO NPs; (C0) blank; (C1)–(C3) CuO NPs stabilized by 1, 2 and 3 ml mucus.
Download figure:
Standard image High-resolution image3.3. X-ray photoelectron spectral analysis
As an evidence for the formation of CuO NP, C3 was subjected to XPS. Figures 3(a), (b) reveals the XPS of CuO stabilized with 3 ml fish mucus. The XPS spectrum is shown in figure 3(a) with binding energies 931.49 eV for Cu 2p3/2 and 951.38 eV for Cu2p1/2. The binding energy difference between Cu 2p3/2 and Cu 2p1/2 is 19.89 eV, which is an agreement with the normal value of 20.0 eV of CuO [38]. Two shake-up satellite peaks at 941.54 and 960.22 eV are the evidence of a 3d9 shell resembling to Cu2+. The XPS spectrum of O1s is shown in figure 3(b) with the binding energies of 527.48 eV, respectively. No residual Argon and other element are noticed in the spectra. The major peaks of Cu2p3/2 for samples (a) and (b) lie at 931.49 eV. A major peak at the lower binding energies of 527.50 eV is confirmed Cu–O bonds. In O1s spectrum (figure 3(a)), the binding energy of 529.4 eV corresponds to lattice oxygen (O2−) related to pure Cu–O and 528.62 eV for CuO produced with 3 ml fish mucus [39].
Figure 3. (a) Cu 2p, (b) O 1s XPS spectrum CuO synthesized using 3 ml fish mucus, presenting that the surface is covered by CuO NPs.
Download figure:
Standard image High-resolution image3.4. SEM image analysis
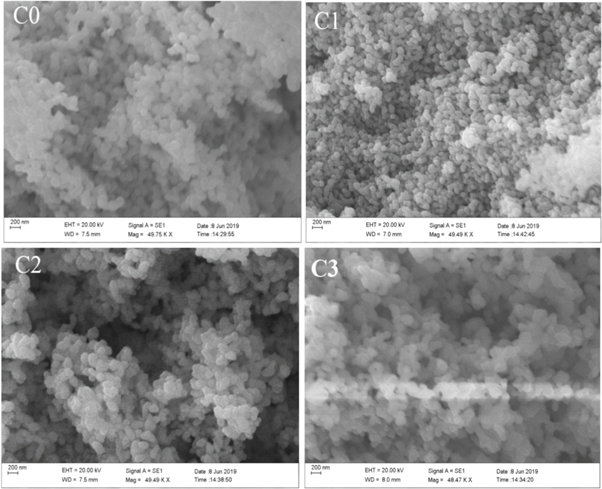
The representative SEM images of CuO NPs are shown in figures 4(C0)–(C3). The SEM results showed the formation of spherical shape particles confirming the development of CuO NPs stabilized by fish mucus. Formation of irregular particles found in the pure CuO (C0). Development of spherical particles clearly appeared in CuO stabilized by 1, 2 and 3 ml fish mucus. However, an agglomerated of particles found in all the samples.
Figure 4. SEM images of CuO NPs: (C0) (a) Blank CuO NPs; (C1)–(C3) (a) SEM images of CuO NPs synthesized using 1, 2 and 3 ml fish mucus.
Download figure:
Standard image High-resolution image3.5. EDAX analysis
Copper, oxygen imaging and elemental composition of the samples was quantitatively estimated by the EDAX analysis as shown in figures 5(a)–(c)). The percentage of copper and oxygen obtained by EDAX of C0, C1, C2, C3 and C4 were 82 and 18%, 89 and 11%, 88 and 12%, and 86 and 14%, respectively. Elemental imaging confirmed the uniform distribution of copper and oxygen as shown in figure 5(c).
Figure 5. EDAX and elemental imaging (C0)–(C3); images (a) and (b) signify the distribution of copper and oxygen in all the samples; (c) EDAX spectrum.
Download figure:
Standard image High-resolution image3.6. TEM images analysis
From the XRD study, size of the CuO synthesized using 3 ml found to be 82.38 ± 8.42 nm, which is significantly smaller than the pure CuO (113.74 ± 11.28 nm). Hence, C0 and C3 were subjected to TEM analysis. TEM images visibly indicate the reduction of CuO NPs synthesized using 1 and 3 ml mucus found to be quite monodispersed, more spherical with few pentagonal, square and hexagonal shape particles (C1 and C3 (a-c)). Shape and the formation of particles in C1 and C3 found to be superior to pure CuO (C0). However, slight aggregation of particles was randomly noticed in the images due to sample preparation. The inconsistency in the shape is quite evident from the SAED pattern (figure 6(d)) reveals the crystalline nature of CuO NPs. Spherical shape particles were observed in the SEM images are consistent with the results of TEM images.
Figure 6. TEM images and SAED pattern of CuO NPs. (C0), (C1) and (C3) (C0) TEM images of pure CuO NPs (C1), (C3) TEM images of CuO stabilized by 1 and 3 ml mucus; ((C0), (C1) and (C3) (d)) SAED pattern of pure CuO, and CuO with 1 and 3 ml of fish mucus.
Download figure:
Standard image High-resolution image3.7. Anticancer activity
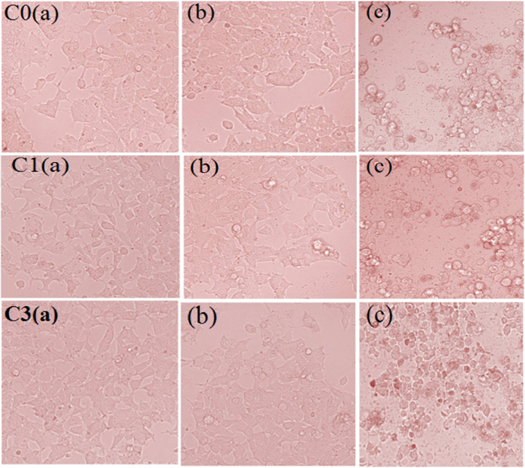
Microscopic imaging of the HeLa cells treated with CuO NPs was carried out. The experimental study showed that the cell monolayer (morphology and structure) did not differ in the control sample group as shown in figures 7(C0), (C1) and (C3). Morphological changes were observed in the cells in higher concentrations of CuO NPs. In lower concentrations of CuO NPs, the cells adhered closely with each other covering all surfaces without any noticeable gaps. Changes in the cellular shape were observed in the population cells. But the cells become spherical from the polygonal with flat shape.
Figure 7. Microscopy images of HeLa cells treated with increasing concentration (0.5–100 μg ml−1) of CuO NPs: C0, C1 and C3 (a) Images of normal HeLa cells without CuO NPs; (b), (c) HeLa cells treated with CuO NPs (0.5 and 100 μg ml−1).
Download figure:
Standard image High-resolution imageFor this experiment, HeLa cells were exposed to CuO NPs at various concentrations (0.5–100 μg ml−1) for 24 h. The cell viability for the concentrations of 0.50 and 100 μg ml−1 observed were 97.77% and 21.66%, respectively. It is found that the cell viability decreased with increasing dosage of the nanoparticles signifying that additional CuO NPs could mount up inside cells, producing the worse stress and eventually the death of the cell (figures 8(C0), (C1) and (C3)). These results noticeably illustrate that the efficacy of the synthesized nanoparticles using the mucus extract of C. striatus against the cancer cells.
Figure 8. Shows the viability of HeLa cells. C0-cells treated with blank CuO NPs; (C1), (C3) Viability of HeLa cells treated with CuO NPs stabilized by 1 and 3 ml mucus. Bar diagram signifies the standard error of three replicate experiments. Concentration of Cu NPs: 0.5–100 μg ml−1. In the cell culture treated with 100 μg l−1 CuO NPs, maximum cells turned to the spherical shape. The monolayer cell structures were disturbed with noticeable gaps separating adjacent cells (C0, C1, C3 (c)).
Download figure:
Standard image High-resolution image3.8. Formation mechanism of CuO NPs
Figure 9 reveals the possible route for the mucus mediated synthesis of CuO NPs. It involves initiation, development and termination steps in mucus mediated CuO NPs biosynthesis. In the bioreduction, amino acids present in mucus possibly play as a stabilizing agent. These results apparently induce that organic molecules are much engaged in the reduction of Cu2+ to CuO NPs [23]. In the nucleation stage, Cu2+ produced from the from copper acetate precursor when it dissolved in double distilled water. During the reaction, the Cu2+ reacts with amino acids such as Aspartic acid, Glutamic acid, Asparagine, Serine, Glutamine, Glycine, Threonine, Arginine, Alanine, Cystine, Tyrosine, Histidine, Valine, Methionine, Isoleucine, Phenyl alanine, Leucine, Lysine, Proline, Tryptohan and Taurine [34]. Electron-rich amino acids donate electrons to Cu2+ ions, which become Cu0 followed by the conversion of CuO lead to the formation of nanoparticles. It is known that amino acids are rich in electrons due to the presence of –OH and –NH2 groups with extensive reduction capabilities would reduce Cu2+ ions into metallic copper (Cu0), which instantaneously converts to CuO NPs as a consequence of the reactivity of the surface of the of the metallic copper nanoparticles. In nucleation stage, the segregated copper atom slowly pooled to form CuO NPs. Stabilization of CuO NPs is preserved in the termination stage. It is believed that amino acids present in the fish mucus would surround the nanoparticles, forming a protective shield and restricting CuO NPs from the growth [39].
Figure 9. Tentative formation mechanism of CuO NPs stabilized by fish mucus.
Download figure:
Standard image High-resolution imageThose biomolecules preserve the capped nanoparticles and thus prevents them from agglomeration by separating from each other [40–42]. It is well-known that amino acids are water soluble compounds, hence it form homogeneous medium with a copper precursor acetate solution. It was reported that the reducing potential is related to the amount of water soluble compound present in the extract [43]. Many experiments have been reported on green-synthesized CuO NPs as potential anticancer agents. Sargassum polycystum mediated CuO NPs inhibited the growth of MCF-7 at the concentration of 61.25 μg ml−1 [44]. Other experimental results showed that the CuO NPs synthesized using aqueous black bean extract can induce apoptosis and suppress the proliferation of HeLa cells [45]. Similarly, CuO NPs synthesized using an aqueous extract of Eclipta prostrate and Prosopis cineraria have exhibited anticancer activity against cell lines [46]. The CuO NPs prepared from Quisqualis indica have shown a dose-dependent cytotoxic activity against melanoma cells at the rate of 40–120 μg ml−1 [47]. The CuO NPs synthesized using using Zingiber and Allium sp have shown anticancer activity against HeLa cell at the concentration of 100 μg ml−1 [48]. These results and explanation confirm that the CuO NPs may scare the cellular integrity of the cancer cells by injury its DNA and other important molecules needed for survival and progression.
4. Conclusions
Biofabrication of CuO NPs using mucus of C. striatus were effectively developed. The fish mucus, methodology and characterization techniques followed in this experiment helped to produce desired CuO NPs. Biosynthesized CuO NPs were found to be monoclinic nature with various shapes and free from agglomeration. From the MTT assay, it was noticed that the CuO NPs have significant anticancer activity against HeLa cells. The results derived from this work confirmed the biosynthesis of CuO NPs and it could be used as material in combating cancer diseases. The XRD and TEM study clearly revealed the role fish mucus in the formation of CuO NPs. From the overall discussion, it is proposed that the protein-rich fish mucus can be used as an organic modifier in the fabrication of CuO NPs.
Acknowledgments
The authors are grateful to the Researchers Supporting Project (RSP-2020/129), King Saud University, Riyadh, Saudi Arabia for supporting this research work.
Conflict of interest
The authors declare that there are no potential conflicts of interest associated with this research