Abstract
The upsurge of immunocompromised patients has led to extensive study of fungal infections with Candida albicans being the frontline model of pathogenic yeast in humans. In the quest to find novel antifungal agents, this study reports the potential usage of wild-type C. albicans strain C86 to biosynthesise silver nanoparticles by microwave assisted technique. Visual colour change and UV-spectrophotometer were used for primary detection of silver nanoparticles. Additionally, the FTIR peaks confirm the particles' formation and surface characterisation techniques such as FESEM and EDX suggests that the silver nanoparticles were sized in the range of 30–70 nm. Furthermore, pioneering work of homologous recombination technique was systematically employed to delete uncharacterized gene orf19.3120 (CNP41) in the C86 strain creating the deletion strain C403 of C. albicans. To amalgamate the two significant findings, biosynthesized silver nanoparticles were subjected to antifungal studies by disk diffusion assay on the strain C403 that lacks the gene orf19.3120 (CNP41) of C. albicans. As a synergetic approach, combinational effect was studied by incorporating antifungal drug fluconazole. Both individual and enhanced combinational antifungal effects of silver nanoparticles and fluconazole were observed on genetically modified C403 strain with 40% increase in fold area compared to wild-type C86 strain. This can be attributed to the synergetic effect of the bonding reaction between fluconazole and AgNPs. Taken together, this first-ever interdisciplinary study strongly suggests that the CNP41 gene could play a vital role in drug resistance in this fungal pathogen.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Diverse populations of microbes like bacteria and fungi have advertently inhabited the human race with the passage of time. Although some of the microbial interactions have been beneficial, yet their detrimental effects are numerous. Of late, with increase in the number of immunocompromised patients, the study of fungal infections has gained more prominence in medical research with Candida albicans being one of the frontline model of pathogenic yeast in humans [1]. C. albicans is a diploid fungus, polymorphic in nature having the ability to grow as yeast, pseudohyphae and true hyphae [2, 3]. Several studies demonstrated that C. albicans is the most prevalent fungal pathogen that can cause multiple diseases ranging from superficial infections of mucous membranes to life-threatening systemic candidemia [1, 4].
In the last few decades, several antifungal drugs have been developed to treat Candida infections. Based on their chemical composition and mode of action, antifungals have been classified into different groups such as allylamines, azoles, echinocandins, 5-fluorocytosine and polyenes [5]. Among these groups, azole class of antifungals are preferred due to its broad spectrum activity, high efficacy and low toxicity. However, long-term therapy or repeated use of these antifungals leads to the development of resistance among the candida species which could in turn affect the individuals having weak defence mechanisms [6]. To overcome the current challenges witnessed in the existing conventional antifungal agents, the present work addresses the dire need to explore a novel antifungal agent which would also be cost-effective with lesser side effects.
Several alternative strategies to produce antifungal agents include antifungal peptides, efflux pump inhibitors, essential oils, nanoparticles, oxidative stress markers, phytochemicals and statins to mention a few [7]. Among these, metal oxide nanoparticles have several advantages which include intracellular free radical generation, large surface to volume ratio, membrane damage, metal ion release and multiple cellular targets that can prevent the risk of resistance development [8]. Recent studies suggest that the importance of silver in the health care sector has driven research to shift from ionic or colloidal silver based usage to silver nanoparticles (AgNPs) as they have a better safety profile. Moreover, AgNPs have a broad spectrum of antibacterial, antifungal and antiviral activities [9, 10]. Nevertheless, the production of silver nanoparticles using physicochemical methods would be expensive, dangerous and toxic to human health and environment [11]. Therefore, biosynthesis of silver nanoparticles to combat the environmental hazards sets the premise of the current study.
In recent years, literature emphasises the biosynthesis approach for producing silver nanoparticles due to its simple, non-toxic, eco-friendly and cost-effective benefits. Moreover, it does not require any capping agents or surfactants which was required by physicochemical methods [12]. The mode of action of AgNPs using biosynthesis approach is yet to be explored in detail and sporadic distribution of literature is seen regarding the studies of antifungal properties of silver nanoparticles [10–13]. In the current study, novel work has been carried out to synthesise AgNPs from a wild-type Candida strain C86 using biosynthesis approach and microwave assisted technique. The formation of AgNPs was characterized by primary and secondary detection techniques. Primary detection of presence of silver nanoparticles included the observation of the colour change of the solution from pale yellow to brown, followed by FTIR analysis. Secondary detection was carried out by using a double beam UV Spectrophotometer and further confirmed by EDX and FESEM analysis.
From Candida Genome Database (CGD), it has been found that open reading frame (ORF)/gene orf19.3120 located on chromosome 4 on the right arm (chromosomal coordinates 1538056-1539795; ORF size 1740 bp) remains uncharacterized and it has been predicted to be a putative PDR-subfamily ABC transporter using bioinformatics approach [14]. This ORF/gene has been taken in this study as it lacks any experimental evidence to prove its role in C. albicans drug resistance arsenals. Moreover, it encodes a protein of half size of ABC transporters Cdr1p and Cdr2p. Therefore, we have attempted to investigate its role in drug resistance by novel approach using AgNPs. Otherwise its function could have been masked by other stronger ABC transporters encoded by the genes CDR1 and CDR2 and would not be amenable for functional analysis. The nanoparticles obtained were tested on the uncharacterized gene, orf19.3120 (designated as CNP41 gene). Both the copies of the CNP41 gene were deleted in the wild-type strain C86 generating the strain C403 (cnp41Δ/cnp41Δ). Antifungal activity of silver nanoparticles was tested on C403 strain using disk diffusion method. Nanoparticles alone and in combination with fluconazole were used in this study.
2. Materials and methods
2.1. Experimental procedure
The flowchart of the procedures has been presented in figure 1. Briefly, double copy deletion of CNP41 has been done in two phases, Phase I and Phase II. Biosynthesis of silver nanoparticles was followed by its primary and secondary detection and characterization. Lastly, these nanoparticles were tested on CNP41 deleted strain for their antifungal activity.
Figure 1. Flow diagram summarising the experimental studies performed in this work.
Download figure:
Standard image High-resolution image2.2. Strains
Candida albicans strain C86 (Δura3::imm434/Δura3::imm434 Δleu2::FRT/Δleu2::FRT) was derived from CAF4-2 (Δura3::imm434/Δura3::imm434) [15] by deleting both the copies of LEU2 gene using construct pKA76 [16] and evicting URA3 flipper as described by Morschhäuser et al [17]. The plasmid pKA76 was digested with KpnI-SacI to release the deletion cassette and transformed into parental strain CAF4-2 to delete both the copies of LEU2 gene and URA3 flipper was evicted by incubating in yeast carbon base (YCB) media in presence of bovine serum albumin as described by Morschhäuser et al [17]. The resulting strain C86 was used for deleting the gene CNP41 (orf19.3120). For routine amplification of plasmids and subcloning of DNA fragments, Escherichia coli strain XL-1 Blue was used [18]. Table 1 details the strains used in this study.
Table 1. List of strains.
| Strains | Description | Phenotype | Source |
|---|---|---|---|
| CAF4-2 | Wild-type strain | Ura- | [15] |
| C86 | Δura3::imm434/Δura3::imm434 Δleu2::FRT/Δleu2::FRT | Ura- Leu- | This study |
| C386 | Single copy deletant of CNP41 gene | Ura+ | This study |
| C403 | Double copies deletant of CNP41 gene | Leu+ Ura+ | This study |
2.3. Media and growth condition
Yeast extract/peptone/dextrose (YPD) and synthetic dextrose (SD) media were prepared for culturing the yeast strain as described [19]. Uridine 50 μg ml−1 was supplemented additionally as per the requirement. Strains were maintained in 15% v/v glycerol stock and stored in −80 °C. Candida albicans strains were streaked freshly to avoid spontaneous chromosomal instability when required [20]. Bacterial strains were grown in yeast extract/tryptone/sodium chloride (YT) media (0.5% sodium chloride, 0.5% yeast extract and 1% tryptone) at 37 °C. Plasmids were amplified in YT media containing 100 μg ml−1 of ampicillin.
2.4. Molecular biology methods
PCR amplification was performed using a thermal cycler as described [21]. Restriction digestion of plasmids, gel elution of DNA fragments, ligation of DNA fragments into vectors were carried out as described [22]. Plasmids were isolated by alkaline lysis method [23]. E. coli transformation was carried out by calcium chloride method [24]. C. albicans transformation was done by spheroplast method [25, 26].
2.5. Vectors, plasmid constructs and primers
Plasmid pUC19 was used for cloning and sequencing purposes [27]. The plasmid pSFU1 containing URA3 flipper was provided by. Morschhäuser as a gift. The plasmid pKA16 was made by subcloning a 1.4 kb SalI-PstI fragment containing URA3 (digested out from pSFU1) into pUC19 at SalI/PstI site. The plasmid pKA16 was used as basic plasmid to make constructs for gene/ORF deletion. Primers used in this study are listed in table 2. Primers KC16, KC17 (URA3) and KC195, KC206 (LEU2) are made using internal sequences of selection markers [16].
Table 2. List of Primers used in the study.
| Primer no. | Sequence (5'→3') | Purpose |
|---|---|---|
| KC 74 | TCGGGTACCGTTAGTTTCTATTATGGCCGTCAA | Amplification of 990 bp upstream of LEU2, will be used for deletion this LEU2 using URA3 flipper |
| KC 75 | TCGCTCGAGGGATATTGGTTTTAAAAGAAAGGA | |
| KC 76 | TCGGCGGCCGCCAGTAGTTAGCATTTAAATTTCAAATACT | Amplification of 988 bp downstream of LEU2, will be used for deletion this LEU2 using URA3 flipper |
| KC 77 | TCGGAGCTCAATACGTTTATACCACGTGGTGAC | |
| KC 186 | GAGCTCGAAGGCATCAAAGAAGGATTTG | Amplification of 525 bp upstream of orf19.3120, will be used for deleting CNP41 gene |
| KC 187 | GGATCCGACTTATTTGCAATTATTGTTAAAATG | |
| KC 188 | CTGCAGCTCGTACAGAAATGTACTCTTTACG | Amplification of 505 bp downstream of orf19.3120, will be used for deleting CNP41 gene |
| KC 189 | AAGCTTCCCTGCTGCTGATAATCCTTAT | |
| KC 16 | GTCTTGATTAAGCATACATAAGGAC | For verifying deletion of orf19.3120, using URA3 marker |
| KC 17 | TGAAGTTGTTAGCACTGGAACTG | |
| KC 195 | GATGATTTAGCACTTTCAAGAGC | For verifying deletion of orf19.3120, using LEU2 marker |
| KC 206 | AGCGGTATCAGAACAGCAGA | |
| KC 192 | CATGTGACTCAATGGTATAATGATG | For verifying deletion of upstream of orf19.3120, upstream on Chr4 |
| KC 193 | CAAACATGATTGTAGAAGAAGATC | For verifying deletion of upstream of orf19.3120, downstream on Chr4 |
| KC 398 | ATGGCTCTGTTAGTGCTGGAGAA | Internal primers of CNP41 gene to verify double copy deletion. |
| KC 399 | TGTACGCAACTGACATAAACGAC |
The LEU2 deletion construct was made by subcloning flanking sequences of LEU2 gene into URA3 flipper plasmid pSFUI. Upstream and downstream sequences were amplified using the primers KC74/KC75 and KC76/KC77 from CAF4-2 genomic DNA. The PCR products were inserted into plasmid pSFUI at KpnI/XhoI and SacI/NotI sites generating pKA76. The plasmid pKA76 can be digested with KpnI-SacI to release the deletion cassette and transform into recipient Candida strain.
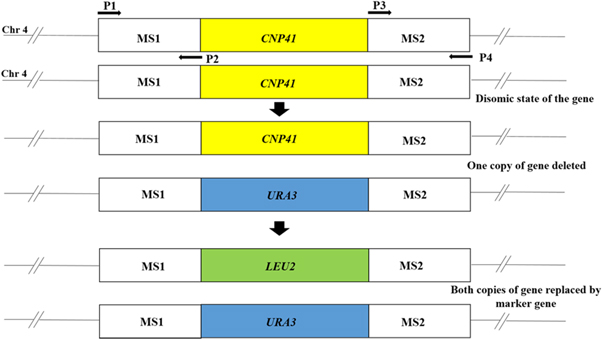
The CNP41 gene deletion was done as shown in figure 2. The CNP41 gene deletion construct was made by subcloning flanking sequences on either side of the selection marker. Briefly, two sets of primers, KC186/KC187 and KC188/KC189 (table 2) were made by using the Candida albicans genome sequence available at Candida Genome Database. PCR products of 525 bp and 505 bp were amplified using genomic DNA of parental strain CAF4-2. These PCR products were cloned into pTZ57R/T vector using InsTA PCR Cloning kit as recommended by the manufacturer (Thermo Fisher Scientific, Lithuania). Subsequently, these two products were digested out from pTZ57R/T and inserted at SacI/BamHI and PstI/HindIII sites of pKA16 generating the plasmid pKA244 carrying URA3 marker for first copy deletion of CNP41 gene. Second deletion construct carrying the LEU2 marker was made by replacing URA3 with LEU2 (taken out from pKA188) gene in pKA244 resulting in the plasmid pKA594. The plasmid pKA244 was digested with SacI/HindIII to release the deletion cassette and transformed into Candida strain C86 for deleting the first copy of the CNP41 gene. Subsequently, the second copy was deleted by transforming a single copy deleted strain with the plasmid pKA594. Plasmids used in this study are listed in table 3.
Figure 2. Schematic diagram of deletion of CNP41 gene located on chromosome 4. MS1 and MS2 are flanking sequences of CNP41 gene. MS1 was PCR amplified using primers P1 and P2; MS2 amplified using P3 and P4. URA3 and LEU2 are selection markers.
Download figure:
Standard image High-resolution imageTable 3. List of plasmids.
| Plasmid Name | Description | References |
|---|---|---|
| pSFU1 | Vector | [17] |
| pUC19 | Vector | [27] |
| pKA16 | URA3 marker cloned in pUC19 | This study |
| pKA188 | LEU2 marker cloned in pUC19 | This study |
| pKA244 | CNP41 gene deletion construct containing URA3 marker | This study |
| pKA594 | CNP41 gene deletion construct containing LEU2 marker | This study |
| pKA76 | LEU2 deletion construct | [16] |
2.6. Confirmation strategy for gene deletion
Deletion of the CNP41 gene was confirmed by PCR with two pairs of primers for checking 5' and 3' junctions. Double deletions were verified with internal primers and the primers used for this purpose are listed in table 2. PCR products of 5' and 3' junctions were also digested with restriction enzymes to ensure that there are no non-specific amplifications.
2.7. Biosynthesis of silver nanoparticles
Nanoparticles were synthesized using biosynthesis approach and microwave assisted technique using wild-type C. albicans strain C86. Procedure for nanoparticles biosynthesis has been briefly described herein. The Candida strain C86 stored in −80 °C ultra-freezer was streaked on YPD plate supplemented with uridine (50 μg ml−1) and incubated at 30 °C for 12–16 h to get barely visible young colonies. The cells were then inoculated into a 100 ml YPD liquid supplemented with uridine (50 μg ml−1). Cells were incubated at 30 °C in shaking incubator at 110 rpm for 16–18 h. The cells were filtered using Whatman grade 01 filter paper [28]. 100 ml silver nitrate solution was prepared using MilliQ water with its concentration (0.1 M). The resultant 50 ml of cell filtrate was taken and mixed with silver nitrate solution in 1:1 ratio [29]. The mixture was then heated at 600 W for 5 min till the mixture reduces Ag+ to Ag0 by changing its colour from pale yellow to brown [29].
2.8. Characterization of silver nanoparticles
The solution of prepared nanoparticles was subjected to double beam UV spectrophotometer (Systronics 2202, India) for primary confirmation of synthesis of AgNPs. The silver nanoparticles sample was tested along with control microbial suspension. As silver nanoparticles' range of wavelength falls under 400–480 nm, the range was set at 200–800 nm. Furthermore, the synthesised AgNPs were centrifuged to obtain pellets and then characterized by FTIR (JASCO V 600, Japan) for observing the functional groups. EDX and FESEM (Carl Zeiss sigma, Germany) techniques were incorporated to view size and shape of silver nanoparticles.
2.9. Antifungal susceptibility test
Disc diffusion test was performed to evaluate the antifungal activity as described [30]. Sterile 6 mm disks were purchased from Himedia, India. 20 μl of silver nanoparticles was pipetted onto the sterile disk. Standard disks of fluconazole (10 μg, Himedia) was used as positive control and combination of fluconazole with AgNPs was impregnated on the disks for this study. SD plates containing Candida cells and disk were incubated at 30 °C for 24–48 h until a clear zone of inhibition was formed. The diameter of these zones was measured. The protocol was followed as described [30]. Each test was conducted in triplicates to ensure reproducibility.
2.10. Assessment of increase in fold area
The increase in fold area was assessed by calculating the mean surface area of the zone of inhibition of antifungal activity of fluconazole alone and fluconazole along with AgNPs. The percentage fold increase in area of tested antifungal activity was calculated from equation (1):
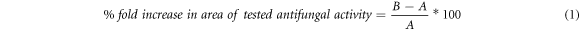
where, A is the zone of inhibition of tested antifungal alone and B is the zone of inhibition of tested antifungal along with synthesized silver nanoparticle [31].
3. Results and discussion
3.1. Deletion of CNP41 gene in Candida albicans
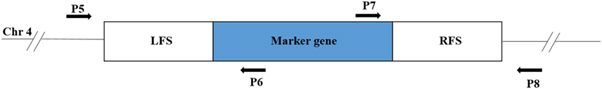
The Candida albicans strain C86 was transformed with the plasmid pKA244 by digesting with SacI/HindIII and ura+ transformants were obtained on plate lacking uridine (ura- plate). The ura+ transformants were re-streaked on the ura- plate to remove any false transformants. Transformants were independently processed for making genomic DNA for PCR verification of CNP41 gene deletion. PCR verification of the junctions was done as shown in figure 3. Both 5' and 3' junctions were verified by primers KC16/KC192 and KC17/KC193 respectively. The expected sizes of amplified products for 5' and 3' junction verification would be 811 bp and 901 bp, respectively. The obtained PCR products were exactly matching with the expected sizes (see figure 4(a). Further confirmation comes from the digestion of these PCR products with diagnostic restriction enzymes producing the fragments of expected size (data not shown). Approximately 15 ura+ transformants were screened by this process and 3 right candidates were obtained for first copy deletion of CNP41 gene. Henceforth, we refer orf19.3120 as CNP41 ( Candida Nanoparticle 41). The strain carrying the first copy deletion was designated as C386.
Figure 3. Schematic diagram of primer design for verifying the CNP41 gene deletion. Chr4, chromosome 4 of C. albicans; LFS and RFS, flanking sequences on the left side and right side of the gene used for making deletion constructs. Primers P5 and P8 are designed from chromosomal DNA sequence outside LFS and RFS whereas P6 and P7 are designed from selection marker which is used for deletion.
Download figure:
Standard image High-resolution imageFigure 4. PCR confirmation of 5' and 3' junctions of CNP41 gene deletion in C403. (a) Deletion using URA3 selection marker: lane 1, 5' landing with primers KC186/16 (775 bp); lane 2, 5' junction with primers KC192/16 (811 bp); lane 3, 1 kb DNA Ladder; lane 4, 3' landing with primers KC189/17 (755 bp); lane 5, 3' junction with primers KC193/17 (901 bp). (b) Deletion using LEU2 selection marker: lane 1, 5' landing with KC186/195 (775 bp); lane 2, 5' junction with primers KC192/195 (811 bp); lane 3, 1 kb DNA Ladder; lane 4, 3' Landing with primers KC189/206 (755 bp); lane 5, 3' junction with primers KC193/206 (901 bp).
Download figure:
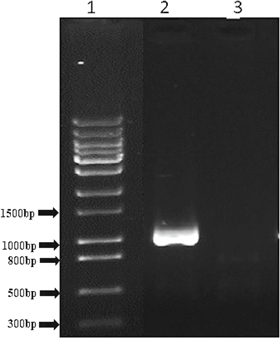
Standard image High-resolution imageFor the deletion of the second copy of the CNP41 gene, the strain C386 was transformed with the plasmid pKA594 and leu+ transformants were obtained on the SD plate lacking leucine. The transformants were processed for genomic DNA preparation and subsequently PCR was done to screen the right candidates. The primers KC195/KC192 and KC206/KC193 were used for verifying 5' and 3' junctions. Expected size of the PCR products are 811 bp and 901 bp, respectively. The sizes of the PCR products obtained are matching with the expected sizes (see figure 4(b)). PCR products were further digested with diagnostic restriction enzymes to ensure that they are not non-specific bands (data not shown). Approximately 40 leu+ transformants were screened and 8 right candidates were obtained. Deletion of CNP41 gene was further confirmed by designing a pair of internal deletion primers KC398/KC399 which would fail to amplify any product in the double copy deleted strain whereas parental strain would produce a band of 1035 bp (see figure 5). Thus, the CNP41 gene was successfully deleted, generating the strain C403 (cnp41Δ/cnp41Δ).
Figure 5. PCR verification of CNP41 gene null mutant. Lane 1, 1 kb DNA DNA ladder; lane 2, control PCR with CAF4-2 genomic DNA (1035 bp); lane 3, null mutant (C403), no PCR product amplified.
Download figure:
Standard image High-resolution image3.2. Biosynthesis of silver nanoparticles
Biosynthesis of silver nanoparticles using C86 strain of C. albicans was done by microwave assisted technique. The primary confirmation of the synthesised AgNPs was authenticated as the solution colour changed from pale yellow to brownish (see figure 6). Although some studies hypothesised that silver ions require NDPH dependent nitrate reductase enzyme for the reduction to AgNPs [28, 32], the exact mechanism of formation of AgNPs remains unanswered. One of the possible explanations for this study involving C. albicans is that the fungal cells secrete NDPH dependent nitrate reductase enzyme in their extracellular environment [29, 33].
Figure 6. Synthesis of silver nanoparticles. (a) Transparent AgNO3 solution (left) and pale yellow liquid culture (right) and (b) brown in colour synthesised AgNPs solution.
Download figure:
Standard image High-resolution image3.2.1. Double beam UV spectrophotometer
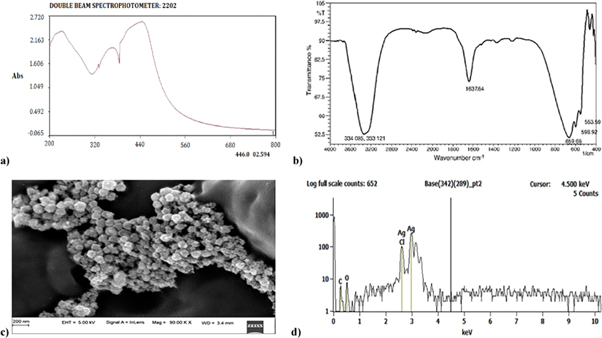
Optical measurements for the primary detection of silver nanoparticles was done using UV-Visible spectrophotometer (Systronics 2202, India) and the range of wavelength was set between 200–800 nm. As depicted in figure 7(a), the maximum absorbance peak observed at wavelength 446 nm was within the AgNPs peak range [30]. This is in accordance with several studies that reported the highest absorbance peak between 400–480 nm [28, 29].
Figure 7. Characterization results of the biosynthesized AgNPs. (a) UV spectra of biosynthesized AgNPs. (b) FTIR spectra recorded from solid sample of synthesized AgNPs. (c) FESEM image of synthesized AgNPs. (d) EDX analysis plot of AgNPs from wild-type Candida albicans strain.
Download figure:
Standard image High-resolution image3.2.2. FTIR studies
For further characterisation of AgNPs, the solution was centrifuged to obtain pellets. As depicted in figure 7(b), FTIR solid sample analysis highlights the 6 bands involved, namely, 553.59, 598.92, 659.68, 1637.64, 3331.21 and 3340.85 cm−1. This confirms the presence of AgNPs along with microbial enzymes. A similar study was carried out by Ukkund et al wherein the FTIR plots of biosynthesised AgNPs had peaks in the range of 4000–400 cm−1 [29]. This led to the understanding of the interactions between silver ions and enzymes in microbial solutions to stabilise the AgNPs. Figure 7(b) further highlights the presence of microbial proteins with the twisted shape of amide bonds. The peaks at 3340.85 (N–H stretch amide), 3331.21(stretching vibrations of primary amines), 1637.64 (C–O amides), 659.68 (C–N stretch amines), 553.59 (Ag–O) and 598.92 (C–Cl stretching) cm−1 verifying the presence of Ag nanoparticles. These peaks signify the involvement of N–H elongated vibrations, N–H bending vibrations and C=O elongating vibrations leading to microbial-silver nanoparticles aggregates [34]. The presence of the nitrate reductase enzymes on the surface of AgNPs was confirmed with the C–N and C–O–C stretching vibration [29]. The findings of Gole et al support this work with similar FTIR peaks describing the presence of AgNPs accompanied by enzymes and microbial proteins [35].
3.2.3. FESEM and EDX analysis
The topology and size of AgNPs were characterised by FESEM and as seen in figure 7(c), the AgNPs synthesised by using C86 strain of C. albicans was spherical in shape and its size ranged from 30–70 nm which is in accordance with the previous studies wherein 66 nm spherical shaped silver nanoparticles were reported [33]. Moreover, there results obtained are also comparable with those that incorporated other fungi like penicillium spp. [28, 29, 36]. Furthermore, EDX results are depicted in figure 7(d) and they are in accordance with the results observed for the AgNPs peaks exhibited in the FTIR studies. Lastly, EDX results confirm that 85.7% of AgNPs are present in the given sample and the results of this work is comparable with several studies reported in the literature [28–31, 34].
3.3. Antifungal activity of AgNPs
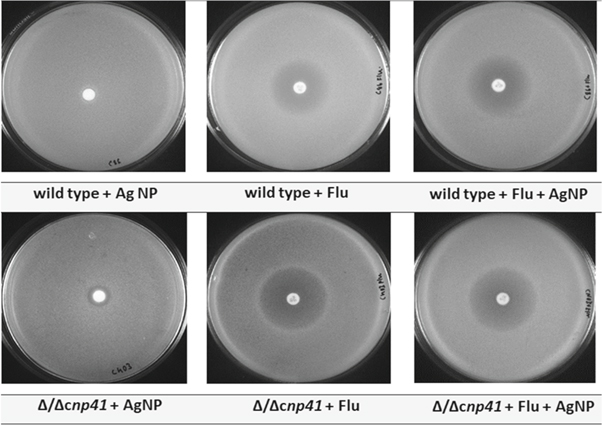
The antifungal activity of AgNPs was carried out by incorporating antifungal agent fluconazole as it shows fungistatic activity against most of the candida strains and helps in curing fungal infections [37]. Moreover, fluconazole belongs to azole class of antifungals and is more preferred due to its broad spectrum activity, high efficacy and low toxicity [6]. The zone of inhibition of AgNPs was studied against Candida strain C403 having both the copies of CNP41 gene deleted. The wild-type strain C86 has been used as a control. As seen in figure 8, the C86 strain of C. albicans showed no zone on addition of AgNPs whereas the C403 strain showed a 10 mm zone around the 6 mm disk containing AgNPs. This finding is comparable with the works published on antifungal action on AgNPs by using ciprofloxacin [38], erythromycin [28] and fluconazole [35, 36, 38].
Figure 8. Zone of inhibition studies for analysis antifungal activity. Flu, fluconazole antifungal agent.
Download figure:
Standard image High-resolution imageIt has been shown earlier that metal and metal oxide nanoparticles have always stood apart for being an effective entities as antifungal agents [28, 29, 31]. Particularly, silver nanoparticles of triangle shaped and 75 nm sized were demonstrated effectively for presenting antifungal activity with Erythromycin against penicillium spp. [28], whereas spherical shaped silver nanoparticles of size 30–45 nm along with Erythromycin exhibited three-fold antifungal activity against Fuzarium Oxysporum [29]. Additionally, copper oxide nanoparticles of size 7 nm along with fluconazole were proven as effective antifungal agents against C. albicans [39]. These results are comparable with the current antifungal study wherein spherical biosynthesized silver nanoparticles of size 30–70 nm were tested positively against Candida albicans.
An enhanced microbial activity was seen as a promising result of having fluconazole antifungal agent with the synthesised AgNPs and supported by the works published by Balaji et al and Gajbhiye et al [36, 38]. Table 4 illustrates the increase in fold area activity. It was further observed that C403 strain had an increase in fold area by 10.3% when compared to the wild-type strain C86 with its fold area being 7.4%. This increase in the fold area can be attributed to the synergetic effect of the bonding reaction between fluconazole and AgNPs. Interestingly, AgNPs did not have any effect on wild-type C86 strain with its genes intact and the presence of AgNPs were insufficient to kill the strain. However, AgNPs had shown antifungal property on C403 strain having both the copies of CNP41 gene deleted. Thus, this study strongly suggests that the CNP41 gene could play a vital role in drug resistance in C. albicans.
Table 4. Antifungal activity with respect to AgNPs and combination effect of AgNPs with respect to antifungal Fluconazole (Flu) on wild-type strain C86 and modified strain C403.
| Candida albicans strain | Zone of inhibition with AgNP [mm] | Zone of inhibition with Flu (A) [mm] | Zone of inhibition with AgNP + Flu (B) [mm] | % Increase in Fold Area B-A/A*100 |
|---|---|---|---|---|
| C86 | 0 | 27 | 29 | 7.4% |
| C403 | 10 | 29 | 32 | 10.3% |
4. Conclusion
The biosynthesis of silver nanoparticles (AgNPs) was carried out using wild-type Candida albicans strain C86 by adopting a novel approach. The formation of AgNPs was confirmed by several methods such as FTIR, UV spectrophotometer, EDX and FESEM. The results obtained from these analyses clearly indicated the formation of AgNPs. For testing the antifungal activity of this nanoparticles, a novel Candida strain C403 (cnp41Δ/cnp41Δ) has been generated by deleting both the copies of the CNP41 gene making the strain null mutant for this gene. The antifungal effect of AgNPs was studied on C403 (cnp41Δ/cnp41Δ) individually and in combination with antifungal drug fluconazole by the disk diffusion method whereas wild-type strain C86 serves as a control. An individual and enhanced combinational antifungal effect of AgNPs and fluconazole was observed on C403 strain with an increase in fold area of 10.3%. On the other hand, C86 strain remains unaffected for antifungal activity by AgNPs and a marginal increase was observed when in combination with fluconazole. This can be attributed to the synergetic effect of the bonding reaction between fluconazole and AgNPs. Thus, this study strongly suggests that CNP41 gene plays a critical role in drug resistance in C. albicans. Lastly, this work opens a plethora of opportunities to study the efficacy of AgNPs in various pathogenic fungi. Additionally, extensive experimental and clinical trials could be carried out to comprehend better use of AgNPs as a potential antimicrobial agent.
Acknowledgments
We thank Joachim Morschhäuser (Wurzburg, Germany) for providing us Candida plasmid. We thank also Judith Berman (University of Minnesoata, USA) and William Fonzi (University of California, USA) for Candida strain. We are thankful to Paike Jayadeva Bhat (IIT Bombay) for XL-1 strain and plasmid pUC19. We are thankful to National Institute of Technology Calicut to give Faculty Research Grant to Dr M Anaul Kabir.