Abstract
The present study involves the Phyto-synthesis of the colloidal silver nanoparticles (AgNPs) and their applications to biologically control plant bacterial pathogens. The synthesis of AgNPs was monitored by measuring the absorbance and a characteristic surface plasmon resonance (SPR) band was observed at 450 nm. The different reaction conditions such as the temperature, incubation period, the concentration of the silver salt and the pH were optimized using the factorial design of the experiment for the better yield and the synthesis of AgNPs. The microscopic results showed that the AgNPs are anisotropic and nearly spherical and exist in the size range of ∼20–100 nm while the EDX analysis confirmed the presence of the elemental Ag. The x-ray diffraction pattern confirmed that the AgNPs are crystalline. The hydrodynamic diameter of AgNPs has measured in the range of ∼13–35 nm and the average size of a single particle was 15.55 nm. The ability of the AgNPs to biologically control the plant bacterial pathogens was measured in terms of antibacterial activity against gram-negative pathogenic bacterial strains; Pectobacterium carotovorum, Xanthomonas oryzae, Xanthomonas vesicatoria and Ralstonia solanacearum and potential antimicrobial activity were observed between 2–12 μg ml−1. The biocompatibility studies revealed that the AgNPs are highly biocompatible (LD100 208 μg ml−1) against RBCs. These findings endorse the applications of AgNPs to biological control the plant bacterial pathogens and the consumption of the plants treated with NPs is biocompatible for the humans.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
The microorganisms attain resistance against the conventional pesticides and insecticides due to their excessive use and the poor farm management system in most of the developing and underdeveloped countries of the world which allowed the researchers to find alternatives and the most sustainable form of the pesticides to biologically control the pathogens to protect the crop plants. The biological control of the pests has advantages to treat the plant's perilous conditions in a better way without harming other associated living creatures especially humans [1]. The microorganisms have specialized strategies to resist conventional drugs or pesticides that involve enzymatic inhibition of the antimicrobial drugs, change in the target site and the active efflux of drugs from the bacterial cell [2]. Some microorganisms have specific mechanisms to degrade routine antibiotics pertaining to their ability to synthesize the Hydrolytic and the Transferase group enzymes that are encoded by the plasmid genes found freely in the bacterial cytoplasm [3, 4]. The active efflux involves pumping the antibiotics out of the bacterial cells which results in less concentration of the antibiotics inside the cell, giving microorganisms an advantage to survive under antibiotic applications. The genes encoding pumping proteins called 'House-keeping genes' found in the bacterial genome [5]. The chemical modification of the target site involves making antibiotics and their target site less complimentary which results in the less efficacy of the antimicrobial drugs and results in the development of the microbial resistance [6, 7].
The plant pathogens belong to various groups including the bacteria, fungi, protozoans, and algae but the bacterial pathogens are most common and responsible to cause severe actions on the important crop plants. The Xanthomonas vesicatoria is a gram-negative rod-shaped bacterium that forms bacterial colonies on the tomato and the pepper plants. The infection of the plant with the bacterium results in the appearance of the symptoms on the stem canker, leaves and the fruit spots [8]. The Xanthomonas oryzae is another gram-negative pathogenic bacterial strain that causes bacterial blight on the rice plants. This bacterium forms white or grey lesions alongside the leaf veins which affect the rice crop yield greatly in tropical Asia [9]. The Pectobacterium carotovorumis was first identified from the carrot plant as a rod-shape gram-negative bacterium and causes infection on a variety of host plants including the potato, lettuce, onion, tomato, cucumber and many other ornamental plants. It is responsible to cause infection both in situ and on the plants in storage and make plant tissues soft, watery and smelly [10]. The Ralstonia solanacearum colonizes xylem of the host plant body. It is a gram-negative anaerobic plant pathogen. It greatly affects the tomato, potato, banana, ginger, olive, soya bean and the tobacco plants [11].
In the past decade, the scientists did several investigations to biologically control the proliferating microbial populations to enhance the growth and the yield of the crop plants. In a modern-day science, the green-nanotechnology provides a great approach to use nanoparticles to kill unwanted microorganisms without giving them time to develop biochemical resistance [12, 13]. The plant-based nanoparticles are eco-friendly, have more surface to volume ratios and are capped by the plant originated secondary metabolites that allow them to appear as the best candidate to use as pesticides. The Ag is a very important noble metal well renowned and used extensively for its antibacterial action and human-friendly nature [14–16]. The exact mechanism of action of silver nanoparticles to fight the dreading pathogens is unknown but the different authors proposed different mechanisms according to their findings. One study explains that the antimicrobial property of the silver is associated with the positive charge on the silver (Ag+). The positive charge on the surface of the silver nanoparticles provides silver with the opportunity to interact with the negative charge on the plasma cell membrane and the nucleic acid which results in the destabilization of the cell membrane and the release of the ROS and the breakdown of the DNA structure [17]. The additional attributes of the Ag+ involve their abilities to interact with the thiol group (–SH group) of enzyme active sites which results in the formation of the stable complex (Ag-S) and enzyme's active site blockage results in cells death due to the poor respiration [14, 18]. The metallic nanoparticles involve physical and chemical targeting to kill bacterial cells while the conventional antibiotics or biocides as explained above manifest biochemical killing of the cells that result in the development of resistance [19].
The Mentha longifolia is a wild mint plant belongs to the family Lamiaceae well known for the presence of essential oils. The sesquiterpenes and monoterpenes are the terpenoids found in essential oils that have been identified as the major agents for the reduction of bulk Ag into Ag° [20]. The pathogenic resistance and the harmful effects of the pesticides and their nature to ameliorate natural habitat and the health risks of the associated organisms, especially humans, lead to the development of the hypothesis that 'The use of the phyto-synthesized silver nanoparticles may have potential to biologically control dreading pathogens, host on economically important crop plants without being harmful to the humans'. The present study aimed to synthesize the silver nanoparticles by using the aqueous extracts of the M. longifolia leaves and determination of their potential as an antimicrobial agent to biologically control the plant bacterial pathogens and the evaluation of their biocompatibility for the human applications (scheme
Scheme 1. The pictorial representation of the study layout.
Download figure:
Standard image High-resolution image2. Materials and methods
2.1. Collection and extraction of the M. longifolia
The M. longifolia leaves were collected from the edges of the Jinnah stream (33.751870 °N 73.135138 °E) located in the Islamabad. The plant material was washed thoroughly with the tap water, snipped into small pieces, dried under the shade and powdered by using the home grinding machine. The plant aqueous extract was prepared by mixing the plant powder and the distilled water in a 1 to 10 ratio respectively and heated on 45 oC–65 oC for 10–20 min. The plant aqueous extract was separated from the powder material by filtration and used as fresh for the synthesis of the silver nanoparticles [21, 22].
2.2. Bio-fabrication of AgNPs
The plant aqueous extract was used to reduce 3 mM of AgNO3 in a 1 to 9 ratio respectively. The temperature of the reaction mixture was maintained at 60 °C (reflux was installed), the pH was adjusted at 9 while the reaction solution was stirred continuously. The synthesis of the AgNPs was monitored by taking an aliquot from the reaction mixture and absorbance was measured between 200–900 nm of the light wavelength by using a UV-Visible spectrophotometer (Shimadzu 1601, Japan). The absorbance of the colloidal mixture was measured after a defined time to determine the reaction kinetics and to confirm the synthesis and the yield of the AgNPs. At the end of the synthesis, the AgNPs were separated from the colloidal mixture by centrifuging at 1000× g (Sorvall RT 7 Plus) for 1 h thrice with the distilled water [1].
2.3. Effect of AgNO3 concentration, pH, incubation time and the temperature on the synthesis of AgNPs
The physicochemical conditions such as the pH, the temperature, the concentration of AgNO3 and the incubation period were optimized in a way that one parameter was changed while the rest of the parameters were kept constant. The reaction mixture was incubated at different temperature 30 °C, 60 °C and 120 °C. The different pH ranges acidic, neutral and the basic were selected (5, 7, 9). The reaction mixture was observed by the UV-Visible spectrophotometer at different time intervals to find the optimum incubation period. The different concentrations of 1 mM, 3 mM and 5 mM of AgNO3 were evaluated. The different combinations of the reaction conditions were devised to study their effects on the AgNPs synthesis and the yield. The effects of different physicochemical conditions on the synthesis of the colloidal AgNPs were studied by using a UV-Visible spectrophotometer [19].
2.4. Physical and optical characterization of AgNPs
The AgNPs were evaluated morphologically and optically by using various material characterization techniques. The UV-Visible Spectrophotometer (Shimadzu 1601, Japan) plays a promising role to evaluate the kinetics of the reaction mixture, the reaction solution was evaluated at different reaction intervals to observe the SPR band [17]. The Scanning Electron Microscopy was performed on (SEM) JEOL 7500F HRSEM to collect the micrographic images. The drop-coating method was used to prepare the samples and the images were collected at different voltages and laser intensities [23]. The Energy Dispersive x-ray Analysis (EDX) was performed by using JEOL 7500F HRSEM detectors [1]. The crystalline nature of the AgNPs was confirmed by using the x-ray diffraction (XRD) analysis with the monochromatic Cu- Kα1 radiation at a 2θ angle between 10° and 80° (JEOL, JDX-3532, Japan) [24]. The particle size analysis of the AgNPs was performed by using the dynamic light scattering (DLS) technique (Zetasizer Nano S, Malvern Instruments, UK). The colloidal suspension of the AgNPs was prepared in 1× PBS solution. The temperature of the machine was adjusted at 25 °C and the other experimental parameters were maintained through the automatic machine settings [19].
2.5. Measurement of the antimicrobial activity of AgNPs to biologically control plant bacterial pathogens
The pathogenic bacterial strains (Xanthomonas vesicatoria, Pectobacterium carotovorum, Xanthomonas oryzae and Ralstonia solanacearum) were maintained overnight in the Luria Bertani liquid media (yeast extract = 5 g, tryptone = 10 g and NaCl = 10 g in 1 L of DI water; pH 7.2) and 1 × 108 cells ml−1 were maintained. The cells were plated into a 96-well microtiter plate. The AgNPs of 500 μg ml−1, 250 μg ml−1, 100 μg ml−1, 10 μg ml−1, 1 μg ml−1, 0.1 μg ml−1, and 0.01 μg ml−1 concentration were prepared. The different subsequent concentrations were prepared in response to the bacterial activity against the AgNPs. The bacterial growth plates were incubated at 37 °C on a rotatory shaker for 24 h. Following 24 h the plates were evaluated by a microplate reader (Thermo fisher, USA) and the absorbance was measured at 600 nm. The data was arranged as the % survival of the plant bacterial pathogens in response to the different applied concentrations of AgNPs [1].
2.6. Biocompatibility studies of AgNPs against human erythrocytes (Hemolysis Assay)
The hemolytic assay was employed to study the response of the human red blood cells (RBC) or erythrocytes upon interaction with the AgNPs. The erythrocytes were (collected after approval from the university bioethical board members and oral consent of the participant) washed multiple times by using Hank's Buffer Salt Solution (HBSS). The 96-well plate method was used for the experimentation and 180 μl of the RBCs suspension (in HBSS 4% Volume) was introduced to the different doses of the AgNPs. After preparation, the plates were incubated for 3 h at 37 °C in a 5% CO2 chamber. The cell suspension culture (RBCs) with AgNPs was collected in the Eppendorf tubes and centrifuged at 1000 × g for 5 min. The intensity of the released hemoglobin was measured at 576 nm of the light wavelength by using a microplate reader (Thermo Fisher). The RBCs in the HBSS solution were maintained as the negative control while Triton X-100 (0.1 %) was used as a positive control. The percentage of hemolysis was measured by using the following formula. The GraphPad Prism® software was used to determine the lethality dose (LD50, LD90 and LD100) [23].

2.7. Data processing
The reproducibility of the experiments was confirmed after performing in a triplicate arrangement. The mean and the standard deviation (±) was considered to represent the results. The figures were plotted by using the Microsoft MS Excel® Program. The statistical analysis of the antimicrobial activity and the hemolysis assay was performed by using a student's t-test and P < 0.05 was considered a significant difference.
3. Results and discussion
3.1. Optimization of the Phyto-synthesis of AgNPs (UV-Visible Spectrophotometric Studies)
The optimization of the physicochemical parameters plays a very important role to synthesize nanoparticles at a higher rate with better physical, morphological and biochemical attributes. The change in the color of the reaction mixture confirmed the reduction of the mass Ag+ into Ag° by using the Mentha longifolia leaves aqueous extract. The spectrophotometric analysis expressed a characteristic Surface Plasmon Resonance (SPR) band at 450 nm that is the response of the electromagnetic light waves with the vibrating nanostructures in the colloidal mixture [25].
3.1.1. Effect of the concentration of AgNO3 on AgNPs synthesis
The concentration of the silver salt plays a very important role to affect the reaction kinetics which influences the size and the shape of nanostructures. Increasing the concentration of the AgNO3 from 1 mM to 3mM resulted in increasing the synthesis of the AgNPs while the synthesis decreased with the increasing concentration at 5 mM so, 3 mM was selected as the optimum concentration for the synthesis of the AgNPs (figure 1(A)). The higher concentration of the silver salt in the reaction mixture beyond the optimum level results in the alteration of the protein conformations that affect the normal process of the reduction which results in the drop of synthesis [25].
Figure 1. The effects of the different physicochemical parameters on the Phyto-synthesis of AgNPs (a) Concentration of the AgNO3 (b) pH of the reaction medium (c) Temperature of the reaction medium (d) Incubation period of the reaction medium.
Download figure:
Standard image High-resolution image3.1.2. Effect of the pH on AgNPs synthesis
The pH is an important physicochemical parameter that affects the synthesis of the AgNPs and determines the size, shape, and yield. It contributes greatly by increasing or decreasing the concentration of H+ ions in the reaction mixture and changes the electronegative state of the phytometabolites which are mainly responsible for the reduction of AgNO3. Changing the pH from the acidic to the neutral and the alkaline increased the synthesis of the AgNPs and the highest peak was observed at pH 9 (figure 1(B)). The SPR band was shifted toward a lower wavelength from the 450 nm to 405 nm (blue shift) which shows the synthesis of the small size of nanoparticles. According to Mie's theory, small particles absorb light at the lower wavelength while the large particles absorb light at a higher wavelength [19].
3.1.3. Effect of the temperature on AgNPs synthesis
Different temperatures have different impacts on the synthesis of the AgNPs by altering the rate of the synthesis in response to the kinetic energy which changes the shape, size and the biological corona of the nanoparticles. The increase in temperature from 30 °C to 60 °C increased the synthesis but it was dropped by increasing the temperature up to 120 °C. So, 60 °C was selected as an optimum temperature for the Phyto-synthesis of the AgNPs (figure 1(C)). The increase in the temperature beyond the optimum threshold can alter or denature the enzymatic conformations and the protein structure that takes part in the process of the reduction of the metallic silver in AgNPs [25].
3.1.4. Effect of the incubation period on AgNPs synthesis
The change in the color of the reaction mixture was observed after some time of incubation of the reaction mixture. The highest peak (0.507 a.u) was observed after 30 min of the incubation of the reaction mixture and it started declining gradually up to the next 4 h which showed that the incubation time is a vital parameter to steer the reaction conditions to tailor the size and the shape of the nanostructures (figure 1(D)). The increase in the reaction time beyond the optimum level results in the prolonged interactions between the metallic silver, plant secondary metabolites and the synthesized AgNPs in the reaction medium which can greatly affect their size, shape and other biochemical properties of the nanoparticles [19].
3.2. Synthesis of AgNPs under the optimized conditions
The AgNPs were synthesized by the optimization of physicochemical reaction parameters including the temperature, the concentration of AgNO3, the pH and the incubation period. The location of the peak, the absorption unit and the SPR band shift in the spectrophotometric analysis were considered important to determine the optimized conditions for the synthesis of the AgNPs. The spectrophotometric results showed that the mixing of 3 mM of AgNO3 with the plant extract in a 9 to 1 ratio respectively at 60 °C of the temperature and 9 pH, results in the optimized synthesis of the AgNPs (figure 2). The UV-visible spectrums showed the highest peak at 450 nm characteristic of the AgNPs synthesis. Various studies were performed previously to study the effects of different physicochemical parameters on the synthesis of nanoparticles from the Mimosa pigra [26], Ginkgo biloba [27] and Lantana trifolia [28]. The results of these studies support our findings to steer physicochemical parameters to tailor the shape and the size of the nanoparticles under the best possible reaction conditions.
Figure 2. The spectrophotometric analysis representing the synthesis of AgNPs at optimum conditions. Inset: change in the color of the reaction mixture after synthesis of the AgNPs is due to the interaction of the electromagnetic light waves with nanoparticles.
Download figure:
Standard image High-resolution image3.3. Physical and the optical characterization of AgNPs
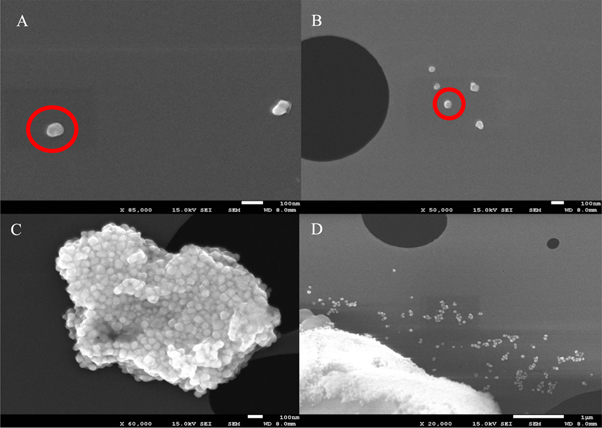
The physical structure of the colloidal AgNPs synthesized by steering the physicochemical parameters was examined mainly by using the SEM. The electron micrographs represented that the NPs are anisotropic and nearly spherical (figures 3(A), (B)) and exist in a size range of ∼20–100 nm. Some small monodisperse nanoparticles were observed (figure 3(D)) while some nanoparticles were found in the form of small aggregates (figure 3(C)).
Figure 3. The SEM micrographs of AgNPs. The red circles in (a) & (b) are showing anisotropic and nearly spherical AgNPs (c) small nanoclusters showing a mixture of nanoparticles of different shapes such as spherical, cubical and triangular (d) small singular monodisperse nanoparticles of nearly spherical shape.
Download figure:
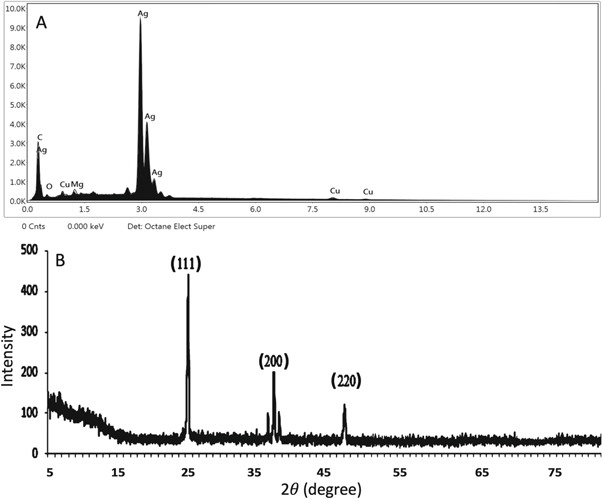
Standard image High-resolution imageThe EDX analysis was performed to confirm the elemental composition of the AgNPs (figure 4(A)). The characteristic peak of the Ag has observed at 3 KeV and the atomic % of the Ag signal was ∼53%. The C and O were measured as 36% and 5% respectively which have their origin from the phytometabolites and help to reduce and stabilize nanoparticles. Previous studies explain the role of various secondary metabolites especially terpenoids and phenolic compounds enriched in C and O as the main agents to carry the oxidative reductive reactions to induce the reduction of the mass silver into Ag° [29]. The presence of the O also contributes toward the cytotoxic potential of the nanoparticles by generating ROS along with their surface to volume ratio. The presence of the Mg and Cu is because of the involvement of the laser signals with the sample carrying grid.
Figure 4. (a) The elemental composition analysis of the AgNPs representing the peak of the Ag at characteristic region 3.0 KeV. Other elements e.g. C, Cu, O and Mg were found in the trace amount (b) the x-ray diffraction pattern of the synthesized AgNPs.
Download figure:
Standard image High-resolution imageFigure 4(B) manifested the x-ray diffraction peak pattern and the phase angle of the synthesized AgNPs. Three different x-ray diffraction peaks were observed at 25.23°, 38.50° and 48.39° which were having lattice plane value and analogous to (111), (200) and (220) Miller indices respectively. The presence of the (111), (200) and (220) lattice planes correspond to the crystalline structure of the silver. The intense and well-resolved XRD pattern shows that the nanoparticles are crystalline. The crystalline size of AgNPs was estimated by using Debye-Scherrer's formula (D = Kλ/β cos θ). Where K is the Scherrer's constant (0.94), λ denotes the wavelength of the x-ray used, β represents the full‐width at half‐maximum (FWHM) of the x-ray diffraction peak in radians while θ represents the half of the x-ray diffraction angle. The estimated mean crystalline size of AgNPs was measured at ∼13.69 nm. Our recorded XRD diffraction peaks are according to the previously published literature [30].
Figure 5. shows the hydrodynamic diameter of the AgNPs and was measured by using the DLS machine. It collectively represents the size of the metallic core and the biological corona that protect the AgNPs from agglomeration and also provide surface modifications. The size distribution of the AgNPs by intensity was measured ∼16–300 nm (figure 5(A)) which represents the size of some aggregated nanoparticles and small nanostructures while it was observed that most of the AgNPs in the colloidal sample were in the range of ∼13–35 nm (figure 5(B)). The polydispersity of the nanoparticles was recorded at 0.302 which is representing that these nanoparticles are well separated and have narrow dispersity to be used as biologically effective agents to manage or control plant pests [6, 31, 32]. The extremely small size of the nanoparticles and their narrow dispersity (0.302) reported in this study shows that the nanoparticles are well capped by secondary metabolites which prevent agglomeration and provide them with the characteristics such as large surface area and the potential biochemical attributes that help nanoparticles to enter the dreading microorganisms such as the bacterial pathogens and kill them eventually [15, 16, 33].
Figure 5. The particle size analysis of AgNPs measured by using the DLS technique. (a) Size distribution by intensity is showing that the hydrodynamic diameter has a range of ∼16–300 (b) Size distribution by a number represents that the average size of most of the nanoparticles in sample exists in between ∼13–35 nm.
Download figure:
Standard image High-resolution imageThe morphological and optical characterization showed that the colloidal nanoparticles are small in size and are less than 100 nm which makes them a suitable candidate for various biological applications. Huang et al [27] reported the use of small size nanoparticles as effective antimicrobials that supports our findings. The characteristic features of the nanoparticles reported in previous studies synthesized by using the plant extracts involve their small size, different physical and the chemical attributes, electromagnetic potentials and the surface functional groups that help them to perform various biological functions [32, 34–38].
3.4. Antimicrobial activity of AgNPs to biologically control the plant bacterial pathogens
The plant bacterial pathogens have the potential to harm important vegetative crop plants by forming the bacterial leaf spots on the tomato and pepper plants (Xanthomonas vesicatoria), bacterial wilt (Ralstonia solanacearum), a bacterial blight on the rice (Xanthomonas oryzae), and cause decaying of important food plants including the carrot, lettuce, onion, cucumber, potato and tomato (Pectobacterium carotovorum). The use of chemical pesticides deteriorates human health, damages the habitat of various organisms living in the same niche and cause environmental hazards. The Phyto-fabricated AgNPs provide an ecofriendly approach to biologically control the plant bacterial pathogens [8–11].
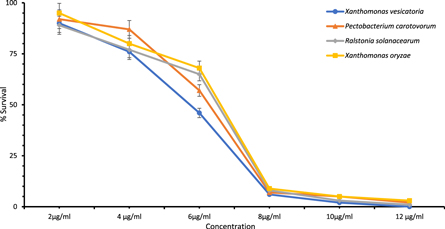
The antimicrobial potential of the AgNPs was measured against R. solanacearum, P. carotovorum, X. oryzae and X. vesicatoria that cause various pathophysiological conditions to the important food crop plants. It was observed experimentally that the colloidal AgNPs have promising dose-responsive antibacterial activity against the studied bacterial pathogens (figure 6). There was no growth of the bacterial pathogens at the higher concentration and survival % increased with the decreasing dosage from 500 μg ml−1, 250 μg ml−1, 100 μg ml−1, 10 μg ml−1, 1 μg ml−1, 0.1 μg ml−1 to 0.01 μg ml−1. The statistical analysis showed that the results are highly significant (P < .05). The dose-dependent response of the AgNPs was measured below 15 μg ml−1 against all bacterial pathogens. The promising antimicrobial activities of the AgNPs at the lower dosage shows their potential to be used as pesticides to biologically control plant pests after extensive field experiments. The 500 μg ml−1, 250 μg ml−1 and 100 μg ml−1 inhibited the whole bacterial population and no survival was reported while 1 μg ml−1, 0.1 μg ml−1 and 0.01 μg ml−1 were not found effective concentrations (100% survival, P < .05). The dose-responsive antibacterial activity of the AgNPs was recorded in between 2–12 μg ml−1, while the MIC was observed at 12 μg ml−1. The 10 μg ml−1 of ampicillin dose (positive control) killed 89%, 67%, 71% and 70% of X. vesicatoria, P. carotovorum, R. solanacearum, and X. oryzae population respectively and the results were highly significant (P < .05) while there was no decrease in the % survival by using water as a negative control.
Figure 6. Antimicrobial activity evaluation of biogenic colloidal AgNPs to biologically control plant bacterial pathogens. The growth inhibition was observed between 2 and 12 μg ml−1.
Download figure:
Standard image High-resolution imageThe antagonistic response of AgNPs synthesized by the Mentha longifolia leaves aqueous extracts is attributed to the diverse physicochemical characteristics of the colloidal AgNPs. The presence of the elemental O may contribute to generating the Reactive Oxygen Species (ROS) that disrupt the membrane ionic balance and eventually results in cell death. The small size nanostructures have the advantages to cross the plasma cell membrane of the bacterial cells and disrupt the osmotic balance of the cells which eventually leads cells to death [3, 15, 16]. The physical and the chemical properties of the AgNPs feature special interaction mechanisms that help them to interact with the bacterial cell membrane and differ significantly from the Ag+ ions. The small size nanoparticles have advantages to cross the plasma cell membrane barrier of the bacterial pathogens and enter the nucleus through the cytoplasmic channels which finally lead to the fragmentation of the DNA double helix and the death of the cell. The special biochemical features of the AgNPs embellish them with the abilities to bind with the thiol group (-SH) of the respiratory enzyme's active sites and the subcellular organelles such as the mitochondria which results in the disruption of the cell's homeostasis and cell's death [3, 39, 40].
3.5. Biocompatibility studies of AgNPs (Hemolysis)
Biocompatibility plays a promising role to use nanoparticles for human applications and was measured in terms of the hemolytic assay. The blood is the main connective tissue in the human and the animal body; it supplies nutrients, transport hormones, delivers oxygen, maintains the pH, transmits an immune response, biochemical signals, defense mechanisms, acts as a site for many biochemical reactions and transfer drugs [41, 42]. The biocompatibility of the nanoparticles makes them safe for human applications. The application of the nanoparticles to the plant bodies to control the plant bacterial pathogens and the consumption of the nanoparticles treated plants by humans is a great concern. The hemolytic abilities of the AgNPs against human RBCs provide information about the biocompatibility of the nanoparticles.
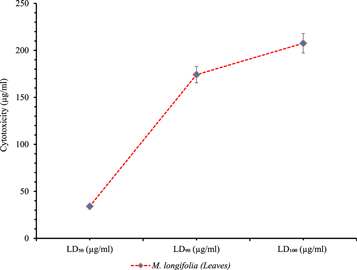
The biocompatibility of the AgNPs against human RBCs is a result of the interactions of the nanoparticles with the plasma cell membrane of the RBCs. The small size of the particles and the ionic nature of the surface capping metabolites provide nanoparticles with the abilities to disrupt the ionic potential and rupture the plasma cell membrane which consequent in the release of the hemoglobin in the medium and explained in terms of biocompatibility of the nanoparticles. Figure 7 represents the biocompatibility results of the AgNPs and was measured by performing a hemolytic assay. Very high doses of the AgNPs were found to be required, expressed in terms of the LD50, LD90 and LD100 to induce lysis in human RBCs. 208 μg ml−1 was reported as a lethal dose to lyse 100% population of the erythrocytes followed by 174.22 μg ml−1 as LD90 and 34.1 μg ml−1 was reported as LD50. The colloidal AgNPs were found non-cytotoxic at a very small dose < 15 μg ml−1 but the toxicity against RBCs increased by increasing the dose. They appeared moderately toxic at > 20 μg ml−1 and highly toxic at a higher dose. The statistical analysis shows the significance of the results (P < .05).
Figure 7. Biocompatibility measurement. LD50, LD90 and LD100 of AgNPs against RBCs.
Download figure:
Standard image High-resolution imageTherefore, the high dose of the nanoparticles drug is required to affect the proliferation and to cause disruption of the RBCs while a very small dose of the nanoparticles drug at 12 μg ml−1 was reported as the MIC to effect the perilous plant bacterial pests. At a very high dose (208 μg ml−1) AgNPs react with the membrane of the RBCs which results in the disruption of the membrane osmotic potential, leads to the breakdown of the membrane and eventually the release of the hemoglobin in the medium. The biocompatibility of the AgNPs depends greatly on the type of the reducing and stabilizing agents of the NPs while the nanotoxicity of AgNPs to RBCs depends on their size, adsorption and uptake, which determine the fate of nanoparticles across the plasma membrane of the RBCs. The positively charged AgNPs can interact with the negatively charged plasma cell membrane. The difference in the electronegative forces results in the destabilization of the membrane structure which leads to its fragmentation and finally leads to the RBCs death [43, 44].
The physical and chemical methods of the synthesis result in the attachment of hazardous chemicals to the central metallic core and make them unsuitable for biological applications and as pesticides. A comparative study was performed earlier to check the hemolytic potential of chemically and biologically synthesized AgNPs. It was evaluated that the chemically synthesized nanoparticles have toxicity at lower doses while green synthesized nanoparticles show toxicity at higher doses against the RBCs which is in favor of our results [45]. The biocompatibility of AgNPs against human erythrocytes or RBCs makes them suitable for human consumption. The eco-friendly one-pot synthesis of the colloidal AgNPs and their diverse morphological, optical and biological attributes make them suitable to biologically control plant pests and biocompatible for human consumption of the plants treated with the nanoparticles.
4. Conclusion
The above experiments confirm the use of the Mentha longifolia leaves aqueous extracts for the eco-friendly one-pot synthesis of the silver nanoparticles and to use them as biologically control agents against the plant pathogenic gram-negative bacterial strains. The morphological characterization of the silver nanoparticles manifested that they exist in the size range of ∼20–100 nm while the particle size analysis showed that most of the nanoparticles are in the range of ∼13–35 nm. The biological assays confirmed that the silver nanoparticles are highly biocompatible and required a very high dose to be toxic against the RBCs while a very small amount of the silver nanoparticles drug measured between 2–12 μg ml−1 was enough to halt the growth of Pectobacterium carotovorum, Xanthomonas vesicatoria, Ralstonia solanacearum and Xanthomonas oryzae. These results greatly endorse the use of the plant synthesized silver nanoparticles to biological control the proliferation of the plant pathogenic bacterial strains and the consumption of plants, received treatment may not have any harmful effects on humans because of their biocompatibility against RBCs.
Acknowledgments
We are very thankful to the Higher Education Commission of Pakistan's, International Research Support Initiative Program (IRSIP) to support Bilal Javed at the University of Pennsylvania for experimentation.
Conflict of interest
The authors declare no conflict of interest.