Abstract
In order to study the flame retardant properties of hydroxylated multi-walled carbon nanotubes (MWCNTs-OH) modified barium phenolic resin (PR), MWCNTs-OH modified PR composites was prepared by in situ polymerization method. Thermal stability and flame retardancy of PR composites were studied by oxygen index (OI), thermo gravimetric(TG) analyzer, cone calorimeter and thermal field emission scanning electron microscope (FESEM). The results showed the OI increased by 3.6% when 0.2 wt% MWCNTs-OH was added, and the residual carbon increased by about 6.1%. The temperature at weight loss of 20% increased by nearly 5 °C and the apparent activation energy(AAE) increased by 11.052 kJ mol−1 with MWCNTs-OH of 0.2 wt% at high temperature stage. And pHRR of the sample decreased by 21.4%; time to ignition was delayed by 20 s. The proper amount of MWCNTs-OH could promote the formation of carbon layer during the pyrolysis of PR so to strengthen the ability to block heat transfer to improve the flame retardancy.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
PR based materials are widely used in aerospace, automobile, shipping, communications, seal, construction and other fields [1–3]. Although the traditional inorganic fillers as additive flame retardants give PR based materials excellent flame retardancy, their toxicity on the environment and human beings is not considered. With the strengthening regulations of toxic and harmful components in flame retardants, flame retardants not only need to meet the flame retardant properties of materials, but also have the function of green environment protection. The MWCNTs-OH consists of concentric tubes from several layers to dozens of layers, with diameter distribution in the 10–20 nm. The exceptional physical properties combined with high aspect ratios and low density render carbon nanotubes (CNTs) attractive for the realization and application of high efficiency flame retardant composites [4–6]. In addition, its unique structure ensures its excellent mechanical and thermal properties and it is environmentally safe [7–9]. Because of the excellent thermal resistance and thermal conductivity of CNTs, the heat generated in the composites can be derived from it at high temperature. As flame retardant, it can not only enhance the flame retardant properties of composites, but also enhance the mechanical properties of composites. Therefore, it is necessary to study the flame retardancy and smoke emission characteristics of MWCNTs-OH/PR composites [10].
REN et al prepared phenolic resin modified with methylvinylcyclosilazanes (MVSZ), and the results exhibited that the LOI reached to 40.8 with the MVSZ of 26.0 wt% and the mechanical properties were also improved [11]. Zheng studied the fire resistance effect of different flame-retardants such as magnesium hydroxide (MH), Aluminatrihydrate (ATH), intumescent flame-retardants (IFR), zinc borate (ZB) modified phenolic resin. Results indicated that the MH/ATH/IFR/ZB/PF system had the minimum heat release, which is reduced by 65% comparing with pure phenolic resin. The maximum oxygen index was 93.4%, which was 2.2 times higher than that of pure phenolic resin. The vertical burning levels rose from UL94 V-1 of pure phenolic resin to UL94 V-0 [12]. WANG found that the ATH/MH/EG/IFR flame retardant system compounded in a 1:1:1:1 ratio and the ATH/MH/EG/FB flame retardant system compounded in a ratio of 8:8:8:1 had good flame retardant and smoke suppressing effect on phenolic resins. Both of the systems had an oxygen index of nearly 100% added to the phenolic resin at 50 phr and the vertical burning rating was UL94 V-0. The amount of smoke per unit mass of the former was 0.82 g while the latter was 0.79 g [13]. Chin-lung chiang et al blended silica with Novolac type phenolic resin under the coupling agent. The silica was less than 100 nm in the system, and had good transparency. The thermal weight loss was 5% at 281 °C–350 °C; the bending strength was increased by 6%–30%; the oxygen index was 37%; and the flame retardant performance was UL94 V-0 [14]. Researchers adopted various flame-retardants to modify phenolic resin, and the effect is obvious. However, most of them require large amount to take effect and are not environment-friendly. As a result, Nano materials are expected to be the additives to play a role. CNTs as of its special structure are found to be active in modification of many properties of materials, which are also added to modify the PR.
Puglia et al found the thermal degradation retard of modified composites could be interpreted in terms of the extreme high thermal conductivity of CNTs and the ability by offering a higher surface for heat propagation [15]. Jiang et al optimized the process of PR and systematically studied the direction of thermal stability of PR structure [16]. Li et al introduced the advantages of in situ polymerization, combining the advantages of intercalation and direct dispersion to ensure the dispersion of nanoparticles and the integrity of nanoparticles; also it was suitable for various polymers and inorganic nanoparticles [17]. Feng studied the dispersion effect of MWCNTs in PR, and found that MWCNTs was conducive to improve the heat resistance of PR [14]. Tai, Yeh and others studied the mechanical properties of MWCNTs modified PR. It was concluded that MWCNTs and resin could form better bonding [18, 19]. An et al found that adding proper amount of CNTs was helpful to improve the thermal decomposition temperature of CNTs/PR Composites [20]. Qiulong Li et al studied the thermal properties of PF/MWCNTs via in situ polymerization, and found that the MWCNTs were well dispersed on the surface and the introduction of MWCNTs could increase the thermal insulating properties of PR. In situ polymerization could not only cut down the amount of MWCNTs-OH addition to reduce cost, but also reduce the agglomeration of MWCNTs-OH and enhance its thermal stability [21]. Liang et al used carbon black (CB) and CNTs as additives in phenolic resin and observed that the graphitization degree of carbons containing CNTs was higher than that of those with CB, however the oxidation resistance of the former was lower due to the higher porosity in the microstructure [22, 23].
Current researches focused on the preparation methods, mechanical and thermal properties of CNTs/PR composites. However, there are not enough studies to systematically investigate the flame retardant properties of CNTs/PR composites. This paper presents a study that combines processing and characterization of modified MWCNTs-OH reinforced PR. In-situ polymerization was employed as the PR preparation method, and surface chemical non covalent bond modification and ultrasonic dispersion were used as the primary means to achieve MWCNTs-OH dispersion in the pretreatment. The effects of MWCNTs-OH on the thermal stability and flame retardancy of PR were studied. Finally, the optimal MWCNTs-OH addition was obtained by comparative analysis. The experimental work lays the groundwork for a systematic study that optimizes the process for thermal stability and flame retardant properties of modified PR.
2. Experimental
2.1. Experimental materials and main test instruments
The experimental materials and main testing instruments used in this paper are shown in table 1.
Table 1. Experimental materials and main testing instruments.
| Name | Purity/model | |
|---|---|---|
| Materials | phenol(C6H5OH) | ≥99.0% |
| formaldehyde(HCHO) | 37%–40% | |
| barium hydroxide octahydrate(Ba(OH)2·8H2O) | ≥98.0% | |
| hydroxyl multi-walled carbon nanotubes(MWCNTs-OH) | ≥99.0% | |
| ethanol(C2H5OH) | ≥99.0% | |
| sodium dodecyl benzene sulfonate(SDBS) | ≥98.0% | |
| Instruments | Oxygen index determinator | FTT |
| Cone calorimeter | FTT0242-Standard Cone | |
| Thermogravimetric analyzer | DTG-60AH | |
| Thermal field emission scanning electron microscope | ΣIGMA |
2.2. Preparation and curing of PR
The white solid phenol was heated in a water bath at 45 °C and maintained for a certain time until the solid phenol became colorless and transparent liquid. The electronic balance was used to weigh the quantitative barium hydroxide catalyst with the mass ratio of phenol to barium hydroxide 1: 0.04. As shown in figure 1(a), connect three flasks, mixer, thermometer and other experimental devices. A certain mass of melted liquid phenol and formaldehyde solution were weighed into a three-mouth flask. The molar ratios of formaldehyde and phenol were 1.3:1, respectively, for six comparative experiments. The temperature of the water bath was maintained at 60 °C–65 °C.
Figure 1. Preparation equipment (a) and liquid sample (b) of phenolic resin. (1) agitator, (2) three-mouth flask, (3) dispensing funnel, (4) thermometer, (5) condensing tube, (6) azeotropic separator, (7) electric thermostatic water bath.
Download figure:
Standard image High-resolution imageAfter 30 min, the barium hydroxide catalyst was added into the three-mouth flask. The sample was heated for 4–5 h in water bath at 80 °C–85 °C. It was then heated at 90 °C–95 °C for about 60 min. Finally, PR after initial modification was obtained from being cooled down with cold water. When it drops to 20 °C the brown-red PR liquid was poured into the beaker, as shown in figure 1(b), and was then dried in the vacuum drying oven. The temperature was set at 30 °C–45 °C, and the pressure was maintained at about 0.084 MPa. After vacuum drying for about 30 min, take out the beaker. It was then put into the air drying oven to cure. The temperature of the drying oven was set at 60 °C, and it was heated to 80 °C after drying for about 1 h. After it was maintained at a certain temperature gradient for 10 h, it was taken out of the mold when cooled to room temperature. The final specimens were bagged and recorded.
2.3. Characterization
LOI test is used to characterize the flame retardant properties of the composites. The diffusion ignition method is applied in the test. The flame of the igniter is contacted with the vertical surface through the top surface of the sample and reaches the 6 mm mark. The material is ignited for 30 s continuously and then removes igniter every 5 s. Check the burning condition of the specimen until the vertical surface can be stably ignited. When the burning part reaches the upright line, it is considered that the specimen is ignited.
TG test is to characterize the thermal stability of the material. The specimen is ground into powder about 3–5 mg. In the air atmosphere, the sample is increased from 50 °C to 800 °C at the heating rate of 10 °C min−1 to study the relationship between the temperature and the char content.
The cone calorimeter is to characterize the flame resistance of the material. According to the international standard ISO 5660, the sample size is about 100 mm × 100 mm × 4 mm and the radiation heat flux experimental value is 50 kW m−2 [24]. The room temperature was about 22 °C; the pressure was about 1 atm; relative humidity was 33%; air flow rate was 24 l s−1.
FESEM is to characterize the microstructure of the material. Firstly, ETD-2000 ion sputtering instrument was used to spray gold for samples, and then charring of samples with different proportions was observed by FESEM. FESEM used in the test is ΣIGMA series thermal field emission scanning electron microscope produced by Carl Zeiss company of Germany.
3. Results and discussion
The results of characterization experiments are shown in table 2.
Table 2. Results of characterization experiments.
| MWCNTs-OH (wt%) | 0 | 0.2 | 0.6 | 1.0 | 1.4 | ||
|---|---|---|---|---|---|---|---|
| LOI (%) | 32.9 | 34.1 | 33.7 | 31.1 | 29.9 | ||
| TG | t20%(s) a | 2005 | 2035 | 2036 | 2041 | 2050 | |
| T20%(°C) b | 386.7 | 391.2 | 390.5 | 384.3 | 390.1 | ||
| AAE (kJ mol−1) | n = 1 | 1st stage | 65.455 | 67.083 | 68.985 | 49.376 | 52.685 |
| 2nd stage | 219.845 | 230.897 | 169.409 | 133.070 | 123.077 | ||
| n = 2 | 1st stage | 75.532 | 77.321 | 79.533 | 57.248 | 60.831 | |
| 2nd stage | 332.330 | 336.914 | 288.040 | 223.049 | 211.715 | ||
| TTI (s) | 159 | 179 | 165 | 170 | 53 | ||
| HRR (kW m−2) | 194.9 | 153.1 | 169.9 | 171.5 | 216.4 | ||
| Time to EHC Peaks (s) | 242 | 352 | 266 | 314 | 209 | ||
| Average of EHC (MJ kg−1) | 15.0 | 14.4 | 16 | 15.7 | 19.6 | ||
| Peak of COP (g s−1) | 0.0027 | 0.002 | 0.0026 | 0.0028 | 0.0033 | ||
| Time to COP Peaks (s) | 225 | 338 | 266 | 303 | 193 | ||
| Average of COY (kg kg−1) | 0.028 | 0.022 | 0.028 | 0.027 | 0.038 | ||
a t20% means the time to loss the weight of 20% of the sample. b T20% means the temperature at the weight loss of 20% of the sample.
3.1. Limited oxygen index (LOI)
LOI is a way to evaluate the relative flammability of polymer composites. From table 2, it can be seen that the LOI of PR is 32.9%, and the LOI are increased by 3.6% and 2.4% respectively after adding 0.2 wt% and 0.6 wt% MWCNTs-OH. The flame retardant effect is more obvious than the pure PR. When MWCNTs-OH is 1.0 wt% and 1.4 wt%, LOI of the samples are decreased by 5.5% and 9.1% respectively. With the increase of MWCNTs-OH, the LOI of PR decreases gradually. When PR is curing, its molecules are randomly arranged and the collision is random. The addition of MWCNTs-OH can induce the accumulation of PR molecules in the surroundings and increase the probability of intermolecular collision. When the proportion is appropriate, the combination of them is sufficient, and its flame retardant will increase. Excessive MWCNTs-OH will cause agglomeration, resulting in specimen voids and roughness, affecting the integrity of the carbonized carbon layer and weakening the role of blocking heat transfer. Finally, heat can be transferred to the carbon layer, causing more intense combustion.
3.2. Thermo gravimetric analysis
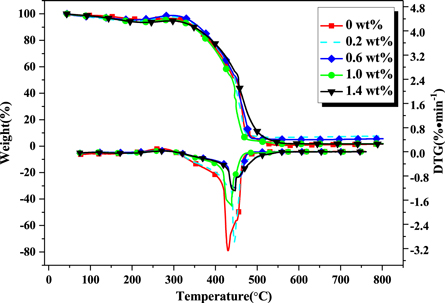
Figure 2 shows the thermo gravimetric curve (TG curve) and the weight loss rate curve (DTG curve) of different samples. There are three stages of degradation in the process of heating. The first stage is from 50 °C–300 °C. In the initial stage, as the temperature increases, the physical bulk density changes, and the unreacted formaldehyde and other substances volatilize. At about 200 °C, the ether bond begins to undergo thermal degradation, and small molecular groups such as carboxyl groups and methyl groups are removed (about 300 °C), and its weight loss is relatively small. The second stage is the thermal decomposition of methylene and the dehydration condensation of hydrogen between phenolic hydroxyl and benzene ring (about 450 °C). Further crosslinking reaction of PR results in the formation of cyclic carbon with increasing temperature; methylene-bridged is converted to hydroperoxide, further triggering decomposition to produce alcohol and ketone compounds [25, 26]. In the third stage, PR occurred dehydrogenation and carbonization, and decomposed to CO, CO2, CH4, H2 and other small molecules. When the temperature is higher than 570 °C, the weightlessness is basically stable. Comparing the TG curves of MWCNTs-OH/PR and PR, the PR curve varies obviously, while the MWCNTs-OH/PR curves of 0.6 wt% and 1.4 wt% are relatively flat and smooth. It is due to that MWCNTs-OH could promote the condensation and cyclization reaction between hydroxyl and PR to make MWCNTs-OH insert into the PR molecules by chemical bond and form a highly stable heterocyclic structure. Meanwhile, it improves the aromatic of resin to reduce the emission of H2, CO, CH4, CO2 and other small molecules, thereby reduces the amount of decomposition of phenolic resin in high temperature, and effectively improves the carbon residue rate of PR. It will lead to the aggregation of MWCNTs-OH and result in reducing flame retardant effect with the increasing of the amount of MWCNTs-OH. However, when reaching a large number MWCNTs-OH, the flame retardant of PR will increase again because of the nature of MWCNTs-OH. According to the data of TG curve, it is found that the carbon residue rate of MWCNTs-OH/PR is increased at 800 °C, and it is increased by 6.1% when MWCNTs-OH is 0.2 wt%. Compared with PR, the addition of MWCNTs-OH was beneficial to reduce the thermal decomposition rate of PR and improve the carbon residue ratio. The temperature (T20%) and time (t20%) of the weight loss obtained from figure 2 are shown in table 2. The results show that the t20% of MWCNTs-OH/PR is prolonged. When MWCNTs-OH is 1.4 wt%, the t20% of modified resin is extended by 45 s compared with PR. It coincides with the analysis of TG. The peak of the DTG curve indicates the temperature at maximum weight loss rate. According to the curve, the temperature at maximum weight loss rate of the 0.2 wt% MWCNTs-OH curve is about 440 °C. The time to reach the temperature of samples of 0.6 wt%, 1.0 wt% and 1.4 wt% MWCNTs-OH are significantly delayed, in which 1.4 wt% MWCNTs-OH is delayed the most. The main reason is that MWCNTs-OH is beneficial to delay the thermal decomposition of PR and effectively decrease the temperature at maximum weight loss rate, reducing the decomposition of PR at high temperature, and thus improving the flame retardant performance of PR.
Figure 2. TG and DTG curves for different samples.
Download figure:
Standard image High-resolution image3.3. Pyrolysis kinetics analysis
The thermal stability of the composites was calculated by Coats-Redfern method. The thermal stability of the composites was analyzed from the mathematical point of view by the apparent activation energies. The Coats-Redfern method is a classical integral method for calculating the single heating rate in pyrolysis kinetics. And when the material consists of one or more irreversible reactions, the pyrolysis kinetic expression is divided into two types [27].
When n = 1,
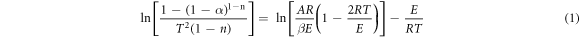
When n≠1,
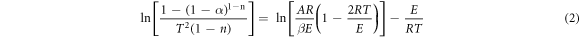
Where α is the decomposition rate, T is the temperature corresponding to the decomposition rate, A is the preexponential factor, E is activation energy, β is the heating rate, and R is the gas constant (8.314 J · mol−1 · K−1), n is the reaction order.
Since the thermal decomposition of PR and MWCNTs-OH/PR samples varies greatly from 10% to 80%, the weight loss rate of 10%, 20%, 30%, 40%, 50%, 60%, 70%, and 80% were selected according to the DTG curve. The first four components are in the low temperature phase and the last four components are in the high temperature phase. Select the two levels of reaction stages n = 1 and n = 2, and use DTG data for efficient calculation mapping. As a result of the thermal decomposition of PR is a relatively complex process, two temperature intervals were chosen in this study, namely low temperature stage (1st Stage) and high temperature stage (2nd Stage). Figure 3 shows the curves obtained by fitting the pyrolysis data by the Coats-Redfern method, where n = 1 and n = 2, respectively. When n = 1, ![$\mathrm{ln}\left[-\,\mathrm{ln}\left(1-\alpha \right)/{T}^{2}\right]$](https://content.cld.iop.org/journals/2053-1591/7/8/085602/revision3/mrxabad06ieqn1.gif) and
and  are plotted to produce a straight line with a slope of
are plotted to produce a straight line with a slope of  When n = 2, the straight line can be got in the same way.
When n = 2, the straight line can be got in the same way.
Figure 3. Pyrolysis Kinetics fitting curve of different samples.
Download figure:
Standard image High-resolution imageThe apparent activation energy of different specimens can be calculated as shown in table 2. The value of α is based on TG and DTG curve data. The polymer activation energy represents the minimum energy required for a chemical reaction of a polymer, and it indicates how easy the polymer pyrolysis process is. According to table 2, when n = 1, the activation energy can be increased by 1.628 kJ mol−1 and 3.530 kJ mol−1 respectively when MWCNTs-OH is 0.2 wt% and 0.6 wt% at low temperature stage. And the activation energy is increased by 11.052 kJ mol−1 with MWCNTs-OH of 0.2 wt% at high temperature stage. When n = 2, the activation energy is increased by 1.739 kJ mol−1 and 4.001 kJ mol−1 when MWCNTs-OH is 0.2 wt% and 0.6 wt% at low temperature stage. The activation energy is increased by 4.584 kJ mol−1 when MWCNTs is 0.2 wt% at high temperature stage. It shows that when MWCNTs-OH is 0.2 wt%, the activation energy of both MWCNTs-OH is improved in the low temperature stage and high temperature stage, which is beneficial to enhance the thermal stability of PR. When MWCNTs-OH is 0.6 wt%, the activation energy increases most at the low temperature stage, but decreases at the high temperature stage.
3.4. Cone calorimeter analysis
Figure 4 shows the heat release rate (HRR), total heat release (THR), mass loss rate (MLR) and effective heat combustion (EHC) curves of PR and MWCNTs-OH/PR. Heat release rate (HRR) indicates the fire intensity. According to figure 4(a), HRR curve is single peak type. When the sample is burning, the carbon layer formed prevents the heat transfer to reduce the heat transfer rate and slows down the decomposition rate of the material. It leads to the inhibition of the heat release rate to flame retardant effect. It can be seen from table 2 that when MWCNTs-OH is 0.2 wt%, 0.6 wt% and 1 wt%, the ignition time is delayed by 20 s, 6 s and 11 s respectively. And the peak of HRR (pHRR) is reduced by 21.4%, 13% and 12% respectively compared with pure PR. However, when the addition is 1.4 wt%, the ignition time is shortened by 106 s and pHRR is risen by 11%.
Figure 4. HRR, THR, MLR and EHC contrast curves of PR and MWCNTs-OH/PR. (a) THR, (b) HRR, (c) MLR, and (d) EHC for different samples.
Download figure:
Standard image High-resolution imageTotal heat release (THR) is the total amount of heat released from the material per unit area. The greater the THR, the greater the danger of fire. As shown in figure 4(b), when MWCNTs-OH is 0.2 wt%, 0.6 wt% and 1 wt%, THR is decreased by 0.5%, 6.7%, 1.3% respectively compared with pure PR. When MWCNTs-OH is 1.4 wt%, THR is increased by 13.4%. Mass loss rate (MLR) is the rate of change in mass loss of material during combustion. It reflects the pyrolysis speed and behavior of materials at a certain fire intensity. Figure 4(c) shows that when the MWCNTs-OH is 0.2 wt%, 0.6 wt%, 1.0 wt%, the time to the peak of MLR (pMLR) is delayed for 62 s, 9 s and 34 s respectively. The average of MLR in the first 120 s is 0.068 g s−1, 0.062 g s−1, 0.068 g s−1, 0.066 g s−1 and 0.081 g s−1 respectively. Effective heat of combustion (EHC) indicates the heat generated by the combustion of the combustible component in the volatiles formed by the thermal decomposition of the material during combustion. It reflects the degree of gas combustion in the gas phase flames, the higher the ratio, the higher the risk of fire. From figure 4(d), it is found that time to peak of EHC (PEHC) of the samples with 0.2 wt%, 0.6 wt% and 1.0 wt% MWCNTs-OH are delayed to 110 s, 24 s and 72 s compared to PR. When MWCNTs-OH is 0.2 wt%, the average of EHC is reduced by 4.0%.
In conclusion, the effect on the thermal stability and flame retardancy of the material is the most obvious when the MWCNTs-OH is 0.2 wt%. When adding the appropriate proportion of MWCNTs-OH, it can better prevent the heat transfer, slow down the HRR and thermal degradation rate, and effectively reduce THC and EHC under heat radiation. The proliferation of the inhibition plays a role in enhancing the flame retardant and thermal stability of the sample. And the following thermogravimetric analysis shows that when MWCNTs-OH is 1.4 wt%, the thermal stability is relatively better because MWCNTs-OH/PR shows more MWCNTs-OH characteristics after PR is grinded into powder. However, the whole resin composite is analyzed in cone calorimeter experiment, which is closer to reality application. And the results indicate that excess MWCNTs-OH will affect the dispersion of MWCNTs-OH in PR and destroy the structural properties of PR, thus PR thermal stability and flame retardancy will be reduced.
When the material burns, the amount of toxic gas is an important parameter to measure the fire safety of materials. PR produces CO when insufficient combustion occurs. Most of the people who died in the fire were suffocated by inhaling excessive toxic gas, so the use of PR in the combustion process must take full account of the toxicity of the material. The production of CO (COP) can be seen from table 2. The time to reach the peak of COP of the composites with 0.2 wt%, 0.6 wt% and 1.0 wt% MWCNTs-OH are all delayed compared to PR which is 225 s. The modified PR with the addition amount of 0.2 wt% is obviously changed, and the peak time is delayed to 338 s; the peak value is decreased by 25.9%. At the same time, the average yield of CO (COY) is decreased by 21.4%. It displays that MWCNTs-OH have a certain effect on inhibiting the production of toxic gas CO and reducing the release rate of CO. The peak and average production capacity of the 1.4% MWCNTs-OH added increase more than pure PR's. The rate of COY during combustion is faster that is easier to cause personal security threats and it is not conducive to evacuation of the people after the fire.
3.5. FESEM analysis
Figure 5 shows combustion residues of different samples. There are different degrees of cracks in the residue of the sample, in which PR cracks are the most, and the sample is divided into irregular small pieces. The samples with 0.2 wt% and 0.6 wt% MWCNTs-OH have relatively few cracks and good residue integrity. It can be seen that there are circular residues of different sizes on the surface of the residue. This is due to the generation of carbon dioxide, water vapor and a small amount of carbon monoxide during combustion. When the sample is carbonized to form a carbon layer, it cannot be released in time to form a cavity. The presence of the cavity can reduce the heat transfer and reduce the burning rate of the material, and also reflects the relatively complete carbonization of the sample from the side. The residues of the samples with 1.0 wt%, and 1.4 wt% MWCNTs-OH have a good degree of fragmentation compared to the PR. It indicates that the addition of MWCNTs-OH is beneficial to the integrity of PR as a whole.
Figure 5. The residue of combustion products. (a) PR; (b) 0.2 wt% MWCNTs-OH/PR; (c) 0.6 wt% MWCNTs-OH/PR; (d) 1.0 wt% MWCNTs-OH/PR; (e) 1.4 wt% MWCNTs-OH/PR.
Download figure:
Standard image High-resolution imageIn order to confirm the effect of MWCNTs-OH on carbonization of PR, the residue after cone calorimeter is observed under thermal field emission scanning electron microscope (FESEM). Because PR is an insulator, the sample must be sprayed with metal film before it is observed. It can prevent the accumulation of charge, thus increasing the stereo sense of the image and reducing the thermal damage.
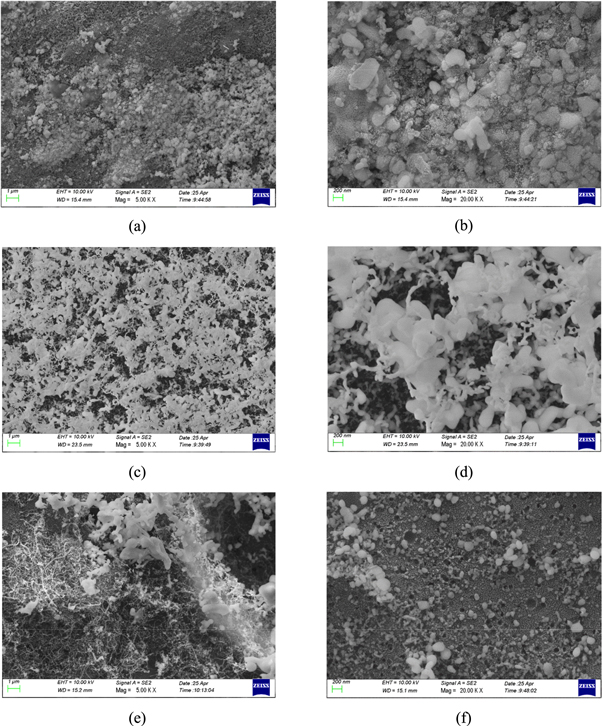
Pyrolysis of resin produces a carbonaceous residue entitled 'char' [28]. Figures 6(a) and (b) indicates the surface of pure PR is relatively symmetrical; white carbonized char is relatively intact that can play a crucial role in ablation behavior because it reduces the pyrolysis rate by emitting considerable incident radiation into the gas phase [29, 30]. The heavier color parts are not completely carbonized which represents that the effect of blocking heat transfer is not obvious. Figures 6(c) and (d) show MWCNTs-OH/PR carbonized image after adding 0.2 wt% MWCNTs-OH. On the surface there are many uneven size of the raised particles wrapped in a thick layer of carbon. This carbon layer is PR wrapped outside of MWCNTs-OH that formed by high temperature carbonization. The carbon layer is tightly connected. Thus, the sample with 0.2 wt% MWCNTs-OH plays a significant role in the carbonization during the combustion of PR. Also it shows that MWCNTs-OH has a good combination with PR which is helpful to improve the flame retardancy. It will generate conjugation of two π bonds with adding MWCNTs-OH in PR. It is helpful to promote the carbonization of carbon layer to improve the integrity and tightness of the composites, thus improving the ability of the sample to block heat transfer and fire reaction compared with PR. Figures 6(e) and (f) show MWCNTs-OH/PR carbonized image after adding 1.4 wt% MWCNTs-OH. The carbon layer is obviously uneven, and MWCNTs-OH appears obvious agglomeration that damages the compactness and integrity of the carbon layer, reduces the blocking heat transfer effect.
Figure 6. Carbonization image magnification. (a) PR (5KX); (b) PR (20KX); (c) 0.2 wt% MWCNTs-OH/PR (5KX); (d) 0.2 wt% MWCNTs-OH/PR (20KX); (e) 1.4 wt% MWCNTs-OH/PR (5KX); (f) 1.4 wt% MWCNTs-OH/PR (20KX).
Download figure:
Standard image High-resolution image4. Conclusions
MWCNTs-OH modified PR composites are prepared by in situ polymerization method. The thermal stability, flame retardancy of PR composites are studied by oxygen index (OI), thermo gravimetric analysis, cone calorimeter and FESEM. The results show the proper amount of MWCNTs-OH can strengthen the ability of the sample to block the heat transfer to improve the flame retardancy of PR. some findings are as follows:
OI of the sample with 0.2 wt% MWCNTs-OH is increased by 3.6% compared to PR. Thermo gravimetric analysis shows that MWCNTs-OH can reduce the amount of decomposition of PR in high temperature and effectively improve the carbon residue rate of PR. It will lead to the aggregation of MWCNTs-OH and result in reducing flame retardant effect with the increasing of the amount of MWCNTs-OH, but when reaching a large number MWCNTs-OH, the flame retardant PR will increase because of the intrinsic property of MWCNTs-OH. The residual is the highest when MWCNTs is 0.2 wt% at 800 °C. The temperature at the weight loss of 20% increases by nearly 5 °C with MWCNTs of 0.2 wt% and the activation energy increases by 11.052 kJ mol−1 at high temperature stage when n = 1.
Cone calorimeter shows that adding appropriate proportion of MWCNTs-OH can better prevent the heat transfer and slow down the HRR effectively, thus reducing THR and EHC under heat radiation. The ignition time delays by 20 s and the pHRR decreases by 21.4% when MWCNTs-OH is 0.2 wt%. And THR is decreased by 0.5%; the average of EHC is reduced by 4.0%. With the addition amount of 0.2 wt% MWCNTs-OH, the time to the peak COP is delayed to 338 s, and the peak value decreases by 25.9%. Moreover, the average of COY decreases by 21.4%. It can be concluded that proper amount of MWCNTs-OH can well be combined with PR and helpful to promote the carbonization of carbon layer to improve the integrity and tightness of the composites, thus improving the ability of the sample to block heat transfer and fire reaction compared with PR.
Acknowledgments
This work was supported by National Key Research and Development Plan (Project NO. 2016YFC0802900).
Conflicts of interest
The authors declare no conflict of interest.
Data availability statement
The burning data of Cone calorimeter, TGA and LOI used to support the findings of this study are available from the corresponding author upon request