Abstract
In this study, we intend to obtain a novel luminous fiber with colour-tuned luminescence via the photoelectron transfer of a phosphomolybdic acid pigment (PAP). The luminous fiber include the SrAl2O4: Eu2+, Dy3+ materials as the luminescence sources, a solid solution of PAP and polyvinyl alcohol as the photoinitiator, amino silicone resin (ASR) as the surface-modified film-forming material and polypropylene (PP) as the matrix, which are prepared by the melt spinning method. The surface morphology was characterized using scanning electron microscopy (SEM), Fourier transform infrared (FTIR) spectroscopy, ultraviolet-visible (UV–vis) spectroscopy, photoluminescence emission spectroscopy, and CIE-1931 chromaticity coordinates. Thus, promising results were obtained, which denoted that the luminescence colour of the luminous fibers could transform from yellow–green to blue by adjusting the PAP concentration. The PL emission spectra of these luminous fibers consisted of two emission peak regions in the range of 430–530 nm. The intensity of the two emission peaks in the fiber reaches the maximum when the concentration of the PAP was 50%. Further, the fibers gradually turned blue with luminescence decay when the fiber was placed in a dark place. The existence of photosensitive discoloration was found to be directly related to the amino groups embedded in the silicone resin coated with the luminous surface material, which reacted with PAP to develop phosphorous molybdenum blue via the photo-reduction process. These findings provide valuable insights toward developing next-generation luminescent materials and multicolor luminous fibers.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Luminous fiber, which is also referred to as a rare earth light-storing fiber, is a functional material that contains rare earth luminescent material prepared via special textile processes using fiber-forming polymers. Luminous fibers can be classified into four different families depending on the raw material matrix, i.e. aluminate systems [1], silicate systems [2, 3], oxide systems [4], and fluoride systems [5]. Generally, the luminous fibers prepared using aluminate systems are ideal as light-storing functional materials with outstanding afterglow properties, including UV radiation resistance and chemical stability. Further, they are extensively used and exhibit optimal luminous performance. These type of fibers have been widely acclaimed since their introduction and have been widely used in various applications, including home textiles, fluorescent plush toys, light-emitting clothing, and anti-counterfeiting application [6]. A rare earth luminescent material is the light source of luminous fiber. The common rare earth ions Eu2+ and Dy3+ are the activators of the matrix material SrAl2O4 [7], causing crystals to generate defects and multiple energy levels. Furthermore, the Eu2+ and Dy3+ ions generate magnetic moment effects in the electrons during the movement of the electrons. Subsequently, the orbit coupling phenomenon will occur in the entire system, affecting the matrix material and causing the entire material system to emit light.
The prepared luminous fibers can emit red, blue, yellow and other colours under sunlight by adding rare earth luminescent materials and inorganic pigments during the spinning process. However, these fibers still emit yellow-green light under no-light conditions because the outermost electrons of the Eu ions in the rare earth strontium aluminate luminescent material matrix can transition from a 4f65d1 orbit to a 4f7(8S7/2) orbit. The emission spectrum wavelength range of the rare earth strontium aluminate luminescent material is 450–650 nm, and the emission peak can be observed at 525 nm, causing its fluorescent colour to become yellow-green. Therefore, the yellow-green light is mostly applied in fibers, and the luminous hue is not ideal. Moreover, the luminous fiber in single yellow-green colour cannot satisfy people's requirements owing to the continuous enrichment of their material and spiritual life. Thus, colorful luminous fiber need more research and development.
Recently, researchers have attempted to modify the light-emitting properties of luminous materials in case of spinning using photochromic materials such as light-converting agents. Previously, it was found that light-converting organic onium salts can be added to the light-curing adhesive material, modifying the surface of the rare earth material to prepare hydrophobic cotton fabrics and photochromic fibers. Further, the light emission spectrum of the material can be changed to a certain extent, resulting in the blue-shift of the light colour [8–11]. In addition, oxanthene derivatives, coumarin photoconverters, and other thermochromic pigments have been used for modifying the luminescent properties of the rare earth luminescent materials, causing the red-shift phenomenon with respect to the emission spectrum of the unmodified luminous fibers [12–15]. However, the aforementioned types of light conversion agents exhibit a short light absorption wavelength, which cannot achieve the luminous colour of the luminous fibers. Therefore, the expansion and enhancement of the colour-emitting properties of luminous fibers will remain a popular research topic.
Photochromic materials have drawn particular attention due to their potential application in some highly technological fields, such as information displays, chemical sensors, modified electrodes and holographic storage devices [16, 17]. Phosphomolybdic acid pigment (PAP) is a type of multi-nuclear photosensitising complex within caged structures and have been proved to be an attractive class of photochromic materials due to the ability of accepting one or more electrons to yield mixed-valency coloured species (heteropolyblues or heteropolybrowns) [18]. This PAP photoconverter can also be used as an electron acceptor for many hydroxyl (–OH) and amino (–NH2) groups as well as other active groups to form charge-transmitting compounds [19]. These compounds can undergo electron transfer from the ligand to the central metal when exposed to light, electricity or thermal energy, which has drawn considerable attention in the field of photochromics [20–25].
In this study, the SrAl2O4: Eu2+, Dy3+ luminescent materials were synthesised using the microwave calcination method. Subsequently, the as-synthesised luminescent materials were modified and mixed with polypropylene substrates. Then, they were added to the spinning raw materials to prepare a blue-light luminous fiber. Further, the effect of the doping concentration and the PAP photoconverter on the blue-shift of t phenomenon of the luminous fiber spectrum was systematically studied, followed by a discussion on the photochromic mechanisms of the fiber. The fiber exhibits superior luminous performance and an obvious spectral blue-shift effect, which can expand the luminous colour of the rare earth luminous fibers and provide a theoretical basis for the diversification of rare earth luminous fibers.
2. Materials and methods
2.1. Experimental materials
Al2O3(AR), SrCO3(AR), Eu2O3(4N), Dy2O3(3N) and H3BO3(AR) were purchased from Sinopharm Chemical Reagent Co, Ltd. The phosphomolybdic acid pigment (PAP) and amino silicone resin (ASR) were purchased from Shanghai Runjie Chemical Reagent Co., Ltd polyvinyl alcohol was supplied by Beike chemical Co., Ltd. Polypropylene (PP) chips was purchased from Wuxi Taiji Group. All the materials are analytically pure.
2.2. Synthesis of PAP and ASR modified SrAl2O4: Eu2+, Dy3+ materials
SrAl2O4: Eu2+, Dy3+ powders were prepared by microwave calcination. The raw materials SrCO3, Al2O3, Eu2O3, and Dy2O3 were mixed in a mortar in a ratio of Sr: Al: Eu: Dy = 1: 2: 0.025: 0.025, among which the amount of H3BO3 added was 5% (molar ratio) of the total mixture. An appropriate amount of anhydrous ethanol was added for 30 min to make the mixture uniform. After drying at 80 °C, it was placed in an alumina ark and placed in a microwave calciner. The sample was then calcined at 1400 °C for 2 h with carbon powder as the reducing agent. After cooling down, the sample was taken out and ground again. Finally, the rare earth strontium aluminate powders were obtained by screening.
A certain amount of rare earth strontium aluminate powders were added to a solid solution composed of PAP and polyvinyl alcohol (1: 200 molar ratio) (the mass ratios of PAP was 10%-90%), and then added an appropriate amount of amino silicone resin (ASR), which was used as a film-forming substance of the modification solution. After ultrasonic dispersion for 30 min, the samples were stirred in a constant temperature water bath at 60 °C for 4 h, and 2 mol l−1 of acetic acid was added dropwise to adjust the pH to about 3. Finally, they were filtered and washed with absolute ethanol for 3 times, then dried in an oven at 90 °C for 24 h to obtain the modified rare earth luminescent material.
2.3. Preparation of luminous fiber
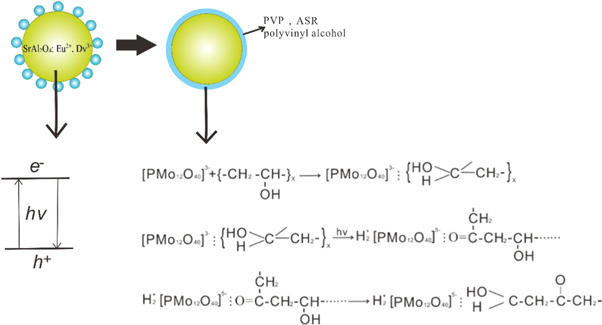
After the polymer PP chips were dried, they were mixed with the modified SrAl2O4: Eu2+, Dy3+ powders in a certain proportion in a melt spinning machine, and extruded by twin-screw extruder at a melting temperature of about 220 °C–250 °C. Finally, the samples were stretched and winded in the melt spinning machine. The preparation process of PAP modified rare earth luminescent material and luminous fiber is shown in figure 1.
Figure 1. The preparation process of photochromic luminous fiber.
Download figure:
Standard image High-resolution image2.4. Instrumental measurements
The surface morphologies of luminous fiber were measured by a scanning electron microscope of the Fei Company of the Netherlands, and all samples were dried and gold-plated before testing with a voltage of 20 kV. Electron spin resonance spectroscopy (ESR) was measured on a Japanese JES-FE3AX electron spin resonance spectrometer. FTIR spectra were determined from samples at room temperature with a Nicolet Impact 410 FTIR spectrometer in the wavenumber range of 500–4000 cm−1. The ultraviolet-visible absorption spectrums of luminous fiber were tested by a Shimadzu UV3600 ultraviolet-visible near-infrared spectrophotometer with the measurement range was from 3000 to 200 nm. Photochromic experiments were carried out using a fluorescence spectrophotometer, the emission spectrums of the scanned sample were tested using a HITACHI 650–60 fluorescence spectrophotometer manufactured by Hitachi, Ltd, Japan with the Xenon lamp as an excitation source. The slit width was 2 nm, excitation wavelength was located at 365 nm, and the scanning speed was 1200 nm min−1 in room temperature environment. The CIE chromaticity coordinates of the luminous fiber were measured using a PR-650 spectral radiation analyser manufactured by Hangzhou Zheda Tricolor Instrument Co., Ltd. The detection light source was a D65 standard light source, and the viewing angle was 10°. The glow color of luminous fiber was measured by Olympus CKX53 fluorescence microscope with 16.5 million pixel digital acquisition device. Before performing all optical tests, leave the samples in the dark for 20 h to rule out errors.
3. Experimental results and discussion
3.1. Electron microscopic analysis of luminous fiber
To compare the SrAl2O4:Eu2+, Dy3+ powder before and after modification and understand the distribution situation of ASR/PAP @ SrAl2O4:Eu2+, Dy3+ composites in the luminous fiber, we provide the SEM images of SrAl2O4:Eu2+, Dy3+ powder before figure 2(a) and after modification figure 2(b), as well as the surface images of luminous fiber in figure 2(c). The powder accumulation phenomenon with uneven particle size can be seen in figure 2(a). Compared with the morphology of the SrAl2O4:Eu2+, Dy3+ powders in figure 2(a), the modified luminous powder has no agglomeration, and the particle size is relatively uniform with approximately 5–8 μm in figure 2(b). It is due to the filtering of composition after SrAl2O4:Eu2+, Dy3+ modification. When the surface of the luminous powder is modified, the solid solution formed by PAP, ASR and polyvinyl alcohol associates and aggregates to form a network structure on powder surface, which make the surface of SrAl2O4:Eu2+, Dy3+ powder smooth and round. Figure 2(c) displays the surface of the luminous fiber with a rough topology. By examining these sporadic particles at a higher magnification, we can verify that these particles have rough surface morphologies because of their attachment to the smaller particles of ASR/PAP @ SrAl2O4:Eu2+, Dy3+ composites extruding from the inside of the PP fiber substrate. During melt spinning, luminous powder and other polymers are extruded by a screw extruder and then are sent to the spinning site. After being fed into the spinning component by a spinning pump for filtration, they are extruded through the capillary holes of the spinneret, thus the particle size of the powder in figure 2(c) attached to the surface of the primary fiber is reduced than the particle size of luminous powder in figure 2(b). Moreover, the thickness of the fiber is relatively uniform with approximately 25 μm in diameter. Therefore, it further shows that the fiber has good silkiness.
Figure 2. The surface feature of (a) SrAl2O4:Eu2+, Dy3+ powder; (b) Modified SrAl2O4:Eu2+, Dy3+ powder; (c) Photochromic luminous fiber with 50% PAP.
Download figure:
Standard image High-resolution image3.2. Infrared analysis
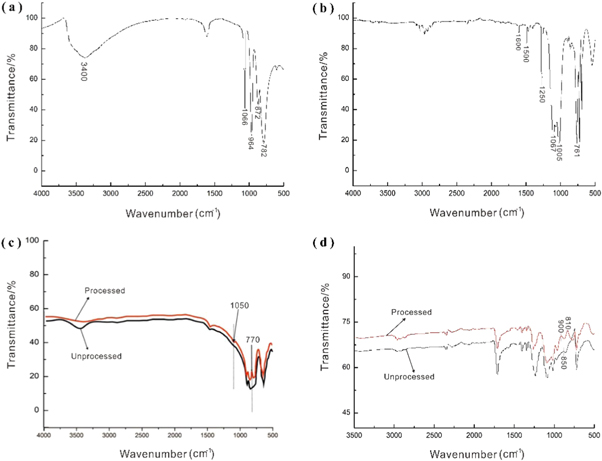
Infrared spectroscopic tests were performed on PAP, silicone resin, and luminous fiber before and after modification to analyse the colour-changing principle of the luminous fiber.
As can be seen from figure 3(a), PAP has four distinct characteristic absorption peaks [26]. The characteristic peak at 1066 cm−1 can be attributed to the anti-symmetrical stretching vibration of the P-Oa bond. Further, the peak at 872 cm−1 can be attributed to the anti-symmetric stretching vibration of the bridged oxygen bond corresponding to the Mo-Ob-Mo, whereas that at 782 cm−1 can be attributed to the anti-symmetrical stretching vibration of the bridged oxygen bond of Mo-Oc-Mo. In addition, the wide peak at 3400 cm−1 is attributed to the –OH stretching vibration, which is not completely removed or exists as a hydrate.
Figure 3. FT-IR spectrum of (a) phosphomolybdic acid pigment (PAP);(b) amino silicone resin (ASR);(c) SrAl2O4:Eu2+, Dy3+ powder before and after modification;(d) luminous fiber with 0% (marked as unprocessed) and 50% PAP (marked as processed).
Download figure:
Standard image High-resolution imageFigure 3(b) shows the infrared spectrum of the silicone resin. Here, the absorption peaks at 1005 cm−1 and 1067 cm−1 correspond to the characteristic absorption peaks of Si–O–Si. The absorption peak at 1250 cm−1 is attributed to the symmetrical deformation vibration of CH3–Si–CH3, whereas that near 761 cm−1 can be attributed to the in-plane bending vibration of CH3–Si–CH3. Typically, these are the characteristic absorption peaks of silicone resin. The spectrum for the modified SrAl2O4: Eu2+, Dy3+ powder is shown in figure 3(c). The absorption peaks of the modified powders between 3000–3600 cm−1 are obviously weakened because the hydrophilic group (–OH group) on the surface of the luminous powder is obviously weakened after being treated with a silane coupling agent. The absorption peak of the treated powder at 1050 cm−1 can be attributed to the stretching vibrations of C–O, whereas this absorption peak at 770 cm−1 can be attributed to the Si–O–Si group. The aforementioned data indicate that the Si–O–Si group of the amino silicone resin has been 'grafted' onto the luminous powder.
Figure 3(d) shows the infrared spectrum of the luminous fiber with 0% and 50% PAP (unprocessed and processed, respectively). Compared to the infrared spectrum in figure 3(a), the curve of figure 3(c) luminous fibre with 0%. The four characteristic absorption peaks of PAP are observed to be present in the modified fiber. However, some characteristic peaks were slightly shifted. Generally, Mo=Od is considered to denote a pure vibration, and its position of vibrational peaks is considerably affected by the interaction between adjacent anions [27]. After the colour changes, the anti-symmetric stretching vibration peak of Mo=Od is red-shifted from 964 cm−1 to 938 cm−1, becoming Mo–Od. Therefore, the interaction becomes weaker with the increasing distance between the anions in molybdic acid. The degree of interaction between the organic and inorganic components could also be evaluated based on the Mo-Ob-Mo and Mo-Oc-Mo vibrations peaks [28]. Here, the Mo-Ob-Mo vibration peak shifted from 850 cm−1 to 900 cm−1 (figure 3(d)), indicating that the interaction between the anions of PAP in the modified luminous fiber deteriorated during the lighting process. The anion interaction is also enhanced in case of PAP, silicone resin and polyvinyl alcohol [29].
3.3. ESR atlas analysis
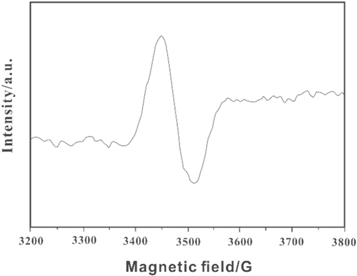
The ESR spectra of the fibre before and after discoloration were tested to further determine the discoloration mechanisms of the luminous fiber. As shown in figure 4, an ESR signal could be observed at 1.9395, indicating that Mo5+ was present in the luminous fiber with PAP under ultraviolet irradiation. This can be attributed to the transformation of Mo5+ ions from yellow to blue under the irradiation of ultraviolet light, i.e. Mo6+ + e → Mo5+. The structure of the Mo5+ electronic layer was 1s22s22p63s23p63d104s24p64d15s0, which contains unpaired electrons in such a manner that an ESR signal will appear. Therefore, g = 1.959 was caused by Mo5+ formation during the phosphorescence of luminous fiber. This value is consistent with the results of literature [30], and consistent with the ESR signal data of Mo5+ (i.e., g = 1.931) [31]. Thus, the luminous fiber with PAP could generate Mo5+ under irradiation of ultraviolet light.
Figure 4. ESR spectrum of the luminous fiber with 50% PAP (84 K).
Download figure:
Standard image High-resolution image3.4. Photochromic behavior of luminous fiber
3.4.1. UV-visible absorption spectrum
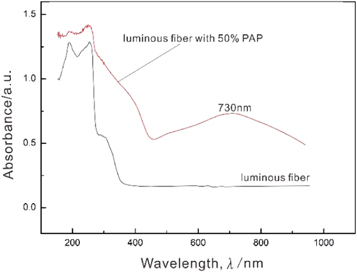
The spectra of the luminous fiber without PAP and with 50% PAP were analysed using a UV–vis near-infrared spectrophotometer to investigate their photochromic properties. From figure 5, we can see that the luminous fiber without PAP has an absorption peak of 200–400. Further, there is no absorption peak in the range of 400–1000 nm. However, after ultraviolet irradiation, the luminous fiber with 50% PAP has an absorption peak in the range of 400–1000 nm and a new strong characteristic peak can be observed near 730 nm. This peak can be attributed to the characteristic absorption peak of the valence charge transfer transition (Mo6+ → Mo5+) [32]. The aforementioned results demonstrate that the luminous fiber without PAP undergoes photo-induced charge transfer within the molecule under ultraviolet light irradiation [33].
Figure 5. UV-visible absorption spectrum of luminous fiber with 0% and 50% PAP.
Download figure:
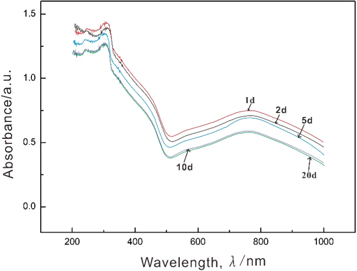
Standard image High-resolution imageA UV irradiation test was also performed on the luminous fiber with 50% PAP. As shown in figure 6, the charge migration transition peak at approximately 730 nm does not weaken or disappear with the extension of the storage time when the luminous fiber is kept in a dark place for an extended period of time. The peak values similarly stayed between 0.565 and 0.515, proving that the luminous fiber cannot transform from yellow to blue after the addition of PAP. Thus, the charge transfer in case of Mo6+ → Mo5+ in the fiber, and the redox reaction between the silicone coated on the surface of the luminous powder and phosphomolybdic acid is irreversible. Thus, the colour of the luminous fiber does not become lighter with time after the addition of PAP.
Figure 6. Time-varying UV-visible absorption spectrum of the luminous fiber in the dark.
Download figure:
Standard image High-resolution image3.4.2. PL emission spectra
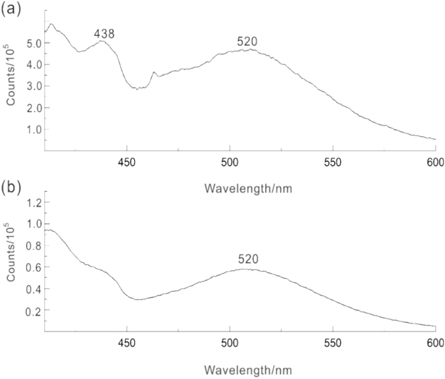
Figure 7 shows the emission spectrum charts of the luminous fiber both without PAP and with 50% PAP, at a excitation wavelength of 365 nm. It can be seen from the figure that the luminous fiber with and without PAP denote broadband spectra, covering ultraviolet and visible spectrum. Here, the emission peak at 520 nm originate from the 5d electron energy level of rare earth Eu2+, this energy level can form many metastable energy levels with different trap depths. Moreover, previous literature found that different trap depths can absorb photons with corresponding energy to generate multiple continuous absorption bands from the broad-spectrum excitation pattern [34]. The luminescent fiber with PAP in figure 7(a) has an extra emission peak at 438 nm than the luminescent fiber without PAP in figure 7(b), which can be mainly attributed to the charge transfer transition peak of the Mo6+ →Mo5+ valence layer after PAP absorbs energy under UV light exposure. This peak belongs to the characteristic band of heteropoly blue [35], Therefore, the emission peak of the modified luminous fiber exhibits a blue-shift phenomenon.
Figure 7. The emission spectrum curves of luminous fibers at 365 nm: (a) luminous fiber with 50% PAP; (b) luminous fiber without PAP (λex = 365 nm).
Download figure:
Standard image High-resolution imageFigure 8 shows the emission spectrum of the luminous fibers, which exhibit different PAP concentrations. We can see that as the concentration of the PAP increases, the electronic energy level transitions of the luminescent fibers did not change. The two emission peaks are still observable with unchanged or shifted, despite the intensity of the emission peaks increases first and then decreasing as the concentration increases. When the concentration finally reached 50%, the maximum intensity of the two emission peaks can be observed, subsequently, the intensity of these peaks gradually weakens. This is because PAP considerably influences the rare earth strontium aluminate luminous fiber. Because the phosphomolybdic acid and polyvinyl alcohol can absorb light energy and convert energy, thus the initial brightness of the luminous fibre increase with the increasing doping amount of PAP. After irradiation, the hydrogen-bonded association can be attributed to phosphomolybdic acid and polyvinyl alcohol interacts with the electromagnetic field of light, causing the electronic transition and structure to be changed and excited. Molecules were generated in the excited state. Some excited molecules can emit fluorescence or phosphorescence via radiative transitions, where some energy is transferred to the activator (the activator refers to the SrAl2O4: Eu2+, Dy3+ luminescent material). The sensitized rare earth ion can absorb more excitation energy, subsequently, it can emit fluorescence and return to the ground state. Therefore, there may be two excitation light sources of the PAP and the luminescent material in the luminous fiber. The light intensity is obtained based on the superposition of the two lights. Consequently, the initial afterglow brightness of the luminous fiber continuously increases with the increasing doping amount of PAP.
Figure 8. The emission spectrum curves of luminous fibers with different PAP concentration (λex = 365 nm).
Download figure:
Standard image High-resolution imageWhen the amount of PAP exceeded 50%, the afterglow intensity of luminous fibers begins to decline. This is because excessive PAP may increase the doping concentration of spinning raw materials. Thus, the fluorescence quenching phenomenon can easily occur with respect to the luminous fiber centre. Another explanation is that some part of the PAP can absorb photons to generate fluorescence, whereas the remaining part of PAP dispersed the number of photons in the excitation light source. The SrAl2O4: Eu2+, Dy3+ material in the fiber cannot be sufficiently excited, which may reduce the the absorption of light energy by the luminescent material. Thus, the luminous intensity of the luminous fibres with PAP is reduced during the initial stage.
3.4.3. Luminescent color
Table 1 presents the values of the light-coloured properties of the rare earth luminescent materials with and without PAP. Here, the positions of the two fibers in the CIE 1931 standard chromaticity diagram are marked based on the chromaticity coordinates X and Y in figure 9. The colour emission of the luminous fiber without PAP is located in the yellow-green light region with a dominant wavelength around of approximately 520 nm. The luminescence colour of the PAP is observed in the blue-violet light region, and the main wavelength is distributed at 438 nm. Further, the luminous colour area of a luminous fiber with PAP is located in the blue range. It exhibits an obvious blue-shift phenomenon when compared with the luminous fiber without PAP.
Table 1. Chromaticity coordinates and dominant wavelength of samples.
| CIE Chromaticity coordinates | |||
|---|---|---|---|
| Sample | x | Y | Dominant wavelength/nm |
| a- luminous fiber without PAP | 0.2354 | 0.5532 | 520 |
| b- luminous fiber with 50% PAP | 0.1893 | 0.2087 | 480 |
| c- The PAP | 0.1776 | 0.0459 | 438 |
Figure 9. CIE 1931 chromatogram of (a) Luminous fiber doped without PAP; (b) Luminous fiber doped with PAP; (c) PAP and (d) Luminous effect of luminous fiber (PLF) under fluorescent microscope.
Download figure:
Standard image High-resolution imageThe emission wavelength of the luminous fiber with PAP shifts to a short wavelengths direction, and the emission peaks are blue-shifted. Simultaneously, the light colour exhibited a blue-shift effect. These results can be explained as follows. The light colour of the modified rare earth strontium aluminate luminous fiber is obtained by superimposing the light-emitting colours of the yellow–green light-emitting centre of the rare earth material and the blue-violet light-emitting centre of PAP. According to the superposition principle of colour, the blue colour comes from the combination of yellow-green and blue-violet lights. Therefore, the spectral colour of the luminous fiber after photosensitivity treatment is blue.
3.5. Photochromic mechanism
The surface of the SrAl2O4:Eu2+, Dy3+ material is coated with a solid solution of PAP, ASR, and polyvinyl alcohol. Further, PAP may react with ASR in the fiber. PAP is an oxidant and a photosensitizer during the photoelectron transfer reactions. There are four types of oxygen atoms, i.e. Oa, Ob, Oc, and Od in phosphomolybdic acid, among which the density around the Ob electron clouds is the highest and the Coulomb repulsion between the electrons is the biggest. The oxygen atoms-Ob in PAP can attract the hydrogen-bonded protons on the amino group in the amino silicone resin, and then transfer to itself. The Od achieves a highly photosensitive active site when PAP is added to the SrAl2O4: Eu2+, Dy3+ material using ASR as the film-forming substance. As an electron donor, PAP also serves as an electron acceptor. With the transfer of electrons, the amino group on the silicone resin is oxidised to a nitro group, and Mo (VI) is reduced to Mo (V).
After PAP and ASR reactions, the main situation that followed is the photo-reduction reaction of the PAP and polyvinyl alcohol on the surface of the rare earth luminescent material. The first step of the reaction is the association that can be attributed to hydrogen bonding, which perturb the relevant electronic energy levels. The second step involves the absorption of light quantum by the association of PAP and polyvinyl alcohol, which can change the electronic structure of the secondary alcohol group of PVA during association, and cause the excited transition of the electron and a redox reaction. This association produces three molybdenum-phosphomolybdenum blue emissions with irreversible photochromism. The third and final step is the re-formation of the hydrogen bonding associations between the PAP and polyvinyl alcohol. Three different valence states (VI, V, and IV) of molybdenum are exhibited in the phosphorus molybdenum blue molecule. In case of appropriate positioning, the association can be attributed to a hydrogen bond between multiple hydroxyl groups on the one molecule or the hydrogen of multiple hydroxyl groups on a molecule or oxygen in phosphoro-blue. The reaction equation is shown in figure 10.
Figure 10. Photochromic mechanism of the luminous fiber after the photosensitivity treatment.
Download figure:
Standard image High-resolution imageIn addition, it can be observed from figure 10 that there is a partially filled 4f shell in case of the other light-emitting centre of the fibre with respect to the rare earth element of the SrAl2O4: Eu2+, Dy3+ materials based on the relevant energy metre level. When compared with the environmental factors, the confinement effect between electrons is smaller. Therefore, multiple energy levels can be observed in case of rare earth elements. During electron motion, a magnetic moment effect will be generated within the electrons. The orbit coupling phenomenon will occur during the operation of the entire system. Therefore, for the entire system, the number of energy levels generated is large and the energy absorbed or generated by the levels will impact the matrix material and cause the entire material system to emit light.
4. Conclusions
In this study, a blue-light luminous fiber was prepared based on introducing PAP particles and rare earth material into PP matrix. The luminous fiber exhibits excellent photochromic properties under ultraviolet and visible light. The emission spectrum showed that under a 365 nm excitation light, two types of fluorescence characteristic peaks could appear in the fiber. The first peak at 438 nm corresponded to the fluorescence emission of phosphorous molybdenum blue in PAP, and the second peak at 520 nm corresponded to the emission spectra of SrAl2O4: Eu2+, Dy3+ materials due to the 4f65d1–4f7 transition of Eu2+. When the doping concentration of the PAP reached 50%, the intensity of the two emission peaks in the fiber reaches the maximum. Then, subsequently weakened. Moreover, the spectral colour of the luminous fiber after photosensitivity treatment is blue, which comes from the combination of blue-violet lights generated by the PAP and yellow–green light generated by the rare earth luminescent material.
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (No. 51803096), the Open Project Program of Key Laboratory of Eco-textiles, Ministry of Education, Jiangnan University (No. KLET2004), the Open Project Program of Fujian Key Laboratory of Novel Functional Textile Fibers and Materials, Minjiang University (No. FKLTFM1911) and K C Wong Mangna Fund in Ningbo University.
Disclosure
All authors confirm that no conflicts of interest exist.