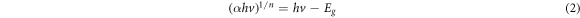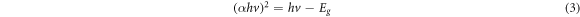Abstract
In this paper, the ZnO–SiO2 was synthesized using ZnO nanopowders and SiO2 developed from coconut husk ash by using conventional solid state method. The ZnO–SiO2 crystal system was heat-treated and the properties was studied. The XRD results showed high intensity peaks due to its high crystallinity when sintered at high temperature. The morphological differences can also be observed through FESEM images as the heat-treated crystal system showed well-distinct boundaries. Meanwhile, the absorbance intensity decreased and shifted to the lower wavelength after heat-treated. The optical band gap value of the ZnO–SiO2 was 3.22 eV before treated and increased to 4.05 eV after heat treated. The presented results showed good properties of zinc silicate and it has a great potential as phosphors in optical application.

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Waste disposal is a complicated methodical process of removing nonessential materials including burial, burning, discharge and recycling. Waste materials were produced from various sources of operations such as factories, industries, mining operation, and agricultural sectors. These waste materials can be hazardous, toxic, corrosive and sometimes can be fatal to human. Thus, these obsolete items require appropriate waste management and disposal technique so that the problems can be contained since most countries have limited number of suitable disposal sites [1]. However, finding the best and innovative solution for effective management of waste require lots of time, energy and expense. Despite that, methods of recycling have been developed to minimize the harmful effects caused by landfill disposal and to convert the waste into variety of useful products.
Among the waste materials being recycled today, bio-waste is one of the most heavily studied because of its potential to be converted into raw materials. Bio-waste is defined as waste from living organisms, animals or plants. It is believed that major problems can arise if it is not disposed properly. Various researches have been conducted to 'recycle' bio-waste into materials that have potential to be used in certain applications. Among them were rice husk [2–6], rice straw[7–10], banana peel [11], corn cob [12, 13], palm waste [14, 15], sugarcane [16], turmeric [17], and coconut husk [18, 19]. Raw materials silica (SiO2) is one of the elements presents in most agricultural waste and commonly used in numerous researches for optical application especially in phosphors materials production. Therefore, production of phosphors materials from waste materials indeed solved the landfill problems at the same time provided a different route with less production cost.
One of the most promising candidates for phosphors materials is zinc silicate. Zinc silicate exhibits good luminescence properties and often used as a phosphors materials in numerous applications including fluorescent lamp, television, and displays materials or lighting appliances [20–22]. There are many advantages associated with the use of zinc silicates as optical host materials since they are able to acquire a wide range of multi-colors from luminescent activators [23]. Recent studies on zinc silicate showed that its absorbance and optical band gap value varied with the method of preparation. In addition, its optical properties can be altered by adjusting few parameters such as heat treatment, sintering temperature and dopant [2, 4, 24–26]. Commonly, zinc silicate was synthesized using sol-gel method or solution precipitation method, which has the limitation due to the complicated process and higher cost of production. To overcome these issues, zinc silicate was synthesized by using solid-state reaction method, where waste coconut husk was used as the alternative silica source. In the nutshell, this research will greatly contribute the society by providing an alternative path of green synthesis method of producing zinc silicate phosphors from waste coconut husk. The optical properties of this novel ZnO–SiO2 product from the green synthesis will be explored.
2. Experimental procedure
2.1. Starting materials and synthesis
ZnO nanopowder (99.99%) (US Research Nanomaterials, Inc., United State of America) with range size of 10–30 nm and coconut husk ash (CHA) was used as the starting materials in this work. CHA was obtained from burned coconut husk at 700 °C for 2 h and after that undergone acid leaching process by sulphuric acid (H2SO4) to increase the silica composition to 91.76% [19]. ZnO and CHA were mixed together for 1 day using ball milling process to ensure the homogeneity of the mixed composition. After that, the mixture was pelleted and undergone heat treatment process at 1000 °C.
2.2. Characterization
X-ray diffraction analysis was carried out by using Phillips X'Pert High Pro PANanalytical Diffractometer (Malvern Panalytical, Almelo (Netherland) and Malvern (United Kingdom)) with set of data range from 2θ = 20° to 80°. Field emission scanning electron microscopy (FESEM) Nova NanoSEM 30 (FEI, Hillsboro, US) was used to study the morphological surface of the samples. The absorption spectra of the sample were determined by using UV-3600 Shimadzu (Shimadzu, Kyoto, Japan) in wavelength range from 220–800 nm. All measurements were carried out in room temperature.
3. Data analysis
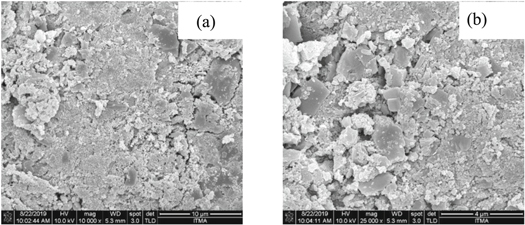
FESEM analysis of the morphological structure of sample before and after heat treatment was performed with magnification level of 10 000 and 25 000. The micrograph in figure 1 clearly revealed that the surface morphology of the sample was not consistent and the shape of grains was irregular before applying the heat treatment. The sample size distribution was sporadic that probably due to the different size of starting materials used in the work. Contrary to sample before treatment, FESEM images of the samples after the heat treatment at 1000 °C (figure 2) have well-distinct boundaries. Undoubtedly, the heat treatment caused the sample to increase in size and produced larger crystal. The increased in grain size following the heat treatment was according to kinetic grain growth phenomenon [27]. With the increased in sintering temperature at constant holding time, the migration of crystal boundaries became more rapid thus increased the average grain size [28]. The sample was also no longer in irregular shape with no porosity observed in the figure due to the increase in the grain growth. This caused the particles to form neck with each other and agglomerated into bigger particles [24, 29]. Based on figures 1 and 2, the grain size was measured, where the average grain size for ZnO–SiO2 was 198 nm for sample before heat treatment, and it increased to 460 nm after heat treatment at 1000 °C.
Figure 1. FESEM images of crystalline ZnO–SiO2 before heat treatment under magnification level of (a) 10,000 (b) 25,000.
Download figure:
Standard image High-resolution imageFigure 2. FESEM images of crystalline ZnO–SiO2 after heat treatment under magnification level of (a) 10,000 (b) 25,000.
Download figure:
Standard image High-resolution imageThe XRD pattern of ZnO–SiO2 samples is shown in figure 3. All the diffraction peaks were matched with the standard value from Joint Committee on Powder Diffractions Standards (JCPDS). Before heat treatment, ZnO (JCPDS 14-0653) and SiO2 (JCPDS 82-0511) were identified by the diffraction peaks emerged on the spectrum. On the other hand, different peaks started to emerge after heat treatment that can be assigned to zinc silicate (Zn2SiO4) (JCPDS 37-1485). This result is in good agreement with previous reported studies [30, 31]. Also, the peaks with higher diffraction intensity that can be observed after heat treatment depicted as high crystallinity of the zinc silicate. This is due to the high heat treatment temperature increased the atomic mobility that cause the grain growth and thus results in better crystallinity [32, 33].
Figure 3. XRD spectra of ZnO–SiO2: (a) untreated (b) heat treated.
Download figure:
Standard image High-resolution imageThe optical characterization was conducted to investigate the performance of ZnO–SiO2 as potential phosphors materials. The optical properties of a metal nanostructures are determined by the collective oscillation of free electrons within the metal nanostructures, or known as plasmon. Its corresponding phenomenon is called surface plasmon resonance (SPR) [34]. The absorbance spectra of ZnO–SiO2 before and after heat treatment are shown in figure 4. From the spectra, it can be seen that ZnO–SiO2 showed the characteristic of SPR peak at around 364 nm and also a broad band at wavelength around 550–600 nm [35, 36]. This result is in good agreement with thus obtained by Bajpai et al [34] who found that ZnO nanoparticles exhibit a SPR peak around 364 nm. Apart from this, the absorption edge observed at wavelength 400 nm suggested that the absorption properties of the sample occurred in the UV region. It can also be observed that the absorbance intensity decreased and the absorbance edge was blue-shifted after the heat treatment at 1000 °C. The decrease in absorbance intensity at high temperature 1000 °C was due to the deformation of ZnO that leads to the formation of zinc silicate or ratio of crystallized/disordered ZnO [37]. It is known that the intensity of absorbance band was highly dependent on ZnO content [38]. The blue-shifted on the spectrum occurred due to high crystallinity of the zinc silicate sample when at high temperature of 1000 °C.
Figure 4. Optical absorbance spectra of ZnO–SiO2 before and after heat treatment.
Download figure:
Standard image High-resolution imageBased on the absorbance spectrum, the relation between the absorption coefficient, α and photon energy, hv can be obtained by using the equation [39, 40]:
The equation (1) were rearranged into (2)
Where n, is the type of transition, h is the Planck's constant, ν is the frequency and Eg is the optical band gap. Different studies used different value of n, e.g. n = 2 used for amorphous semiconductor and for n = 1/2 was used in most crystalline semiconductor [41]. In this work, n = 1/2 was used in equations (2) and (3) to determine the optical band gap of the sample based on the direct allowed transition. The band gap can be found by extrapolating the linear portion of the absorption curve until the x-intercept as shown in figure 5. From the figure, the optical band gap of untreated sample was found to be 3.22 eV. However, the band gap value of heat-treated sample at 1000 °C were calculated at 4.05 eV. The improvement of the sample crystallinity as proven in the XRD analysis caused the reduction of delocalized states and thus making the sample with less defects resulting in increase of the optical band gap value [42].
Figure 5. Optical band gap of ZnO–SiO2 before and after heat treatment.
Download figure:
Standard image High-resolution image4. Conclusion
In this work, ZnO–SiO2 crystal system was synthesized and investigated. Effect on heat treatment on the sample was discussed. The particle size of the sample measured from the FESEM analysis was 198 nm but increased to 460 nm after the heat treatment. In addition, the grains boundaries become more distinct after being sintered at 1000°C. Formation of zinc silicate crystalline system was determined by the diffraction peaks found on XRD spectrum after the sample was heat-treated. Lastly, the absorbance intensity of the sample decreased after the heat treatment. The optical band gap of untreated sample was 3.22 eV but increased significantly to 4.05 eV after the heat treatment.
Acknowledgments
The authors gratefully acknowledge the financial support for this study from the Malaysian Ministry of Education (MOE) and the Universiti Putra Malaysia (UPM) through the Putra Grant. The laboratory facilities provided by the Institute of Advanced Technology and Faculty of Science, Universiti Putra Malaysia, are also acknowledged.
Funding
The work was funded by Malaysian Ministry of Education (MOE) and the Universiti Putra Malaysia (UPM) through the Putra Grant 9642800. The fund was used for purchasing materials, apparatus and the characterization process fee.
Conflicts of interest
The authors declare no conflict of interest.