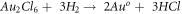Abstract
The present research informs about the synthesis of gold nanoparticles (AuNPs) through Ultrasonic Spray Pyrolysis (USP), which were collected in ethanol with 0.1% Polyvinylpyrrolidone (PVP). Initially, the research focused on two precursors, where the first represented a homemade H-HAuCl4, completed in our own laboratory through the chlorine gas method by using HCl and KMnO4, and the second was the commercial C-HAuCl4, prepared by using Gold (III) chloride tetrahydrate powder and deionised water. The goal was to find any potential precursor differences and their influences on the later use for AuNPs synthesis through USP using almost the same parameters. In the first step of research it was determined that the H-HAuCl4 precursor was similar to C-HAuCl4 in chemical composition, surface tension and pH value. This finding represented the starting point for being able to use H-HAuCl4 in the USP for AuNPs' synthesis. In the second step, AuNPs were synthesised from both types of precursors. Afterwards, characterisation of some functional properties by FTIR and UV–vis techniques was done directly for H- and C-AuNPs in the collecting media. For SEM/EDX and TEM microscopy both types of H- and C-AuNPs were dried, and observation revealed that the morphology, shape and size distribution of dried AuNPs were very similar. Based on the performed laboratory research, it could be concluded that prepared H-AuNPs could represent a new and low-cost effective solution for future USP transfer onto the industrial level, not only in in the process itself, but also in the field of Low-cost Precursor Preparation.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
Abbreviations
| AuNPs | Gold nanoparticles |
| C- AuNPs | AuNPs made from commercial Gold (III) chloride tetrahydrate |
| ICP-MS | Inductively Coupled Plasma-Mass Spectrometry |
| C-HAuCl4 | Commercial HAuCl4 precursor |
| EDX | Energy-Dispersive x-ray spectroscopy |
| FTIR | Fourier-transform infrared spectroscopy |
| HAuCl4 | Gold chloride precursor |
| H- HAuCl4 | Homemade HAuCl4 precursor |
| H-AuNPs | AuNPs made from a homemade precursor |
| PVP | Polyvinylpyrrolidone |
| SEM | Scanning Electron Microscopy |
| SPR | Surface Plasmon Resonance |
| TEM | Transmission Electron Microscopy |
| tUSP | Time period of the USP process |
| USP | Ultrasonic Spray Pyrolysis |
| UV–vis | Ultra-Violet visible spectroscopy |
1. Introduction
Nanotechnology has become a crucial part in various critical research fields, like Material Science, Engineering, Biology, Physics, and Chemistry [1]. The attraction towards Nanotechnology is due to the evolution of nanomaterials and their different shapes, sizes and chemical composition because of their controlled scattering and improvised techniques. Due to these, nanomaterials play a dominating usability in the field of Medical Science, including biological applications viz, photothermal therapy, x-ray image [2–4] etc.
AuNPs have gained attention during the last decades because of their unique properties, like Surface Plasmon Resonance (SPR), non-toxicity, biocompatibility and stability, which have made them a very attractive material for biosensor systems [5–8]. Moreover, many researchers have investigated the role of AuNPs in the areas of electronics [9, 10], biomedical [11, 12], catalytic [13–15], food technology [16–18] and also solar cells for high performance applications [19–21].
To produce AuNPs, two processes are mainly in use, one is bottom up synthesis, and the other is the top down approach. Wet chemical methods [22, 23], radiolysis [24, 25], chemical vapour deposition [26], spray pyrolysis, the UV irradiation technique [27, 28] are the bottom-up approach, and nanolithography, and high-energy milling belong to the top down approach. USP is one of the prominent methods for synthesising nanoparticles with high purity and controllable uniform sizes. The main advantage of the USP method is that it is economical and capable of producing nanoparticles in larger quantities [29].
The syntheses of AuNPs are based mainly on the gold (III) derivatives like chlorides, acetates, bromides, using reducing agents like amines [30], plant extracts [31], polymers [32], and glycerol [33]. The common precursor used for the production of AuNPs is HAuCl4 [34, 35], which is, mostly, soluble in all solvents, including water. In 1951, Turkevich introduced the synthesis of AuNPs with sizes of approximately 20 nm, by reduction of HAuCl4 using the citrate method [36]. Frens et al improved this method further by varying different parameters, and produced different sizes of AuNPs successfully from 15 nm to 150 nm [37]. The literature reveals the synthesis of AuNPs using commercially available HAuCl4 powders [38–41]. However, in the markets, these available precursors are very expensive to make AuNPs for scale-up production.
In previous decades, the extraction of gold was performed from gold ores by using cyanide leaching [42, 43]. Due to the environmental issues of cyanide, of halides, aqua regia, thiosulfate and thiourea are now used as alternative materials [44]. The most common method for extracting gold is dissolving it in aqua regia, but this process has critical steps, like evaporation of HCl and HNO3. The use of halides like the chlorine gas method is used mostly in the industrial application to dissolve gold and produce stable gold chlorides [45, 46]. Shirin et al [47] proposed this method of dissolving gold by using the chlorine gas to synthesise a pure gold chloride solution, which can use the final product directly in the USP process. We adapted this methodology in the present work as a laboratory synthesised homemade H-HAuCl4 precursor for possible synthesis of AuNPs through USP. Based on this, in the first step of research, the aim was to determine the main properties of H-HAuCl4, such as chemical composition, surface tension and pH value with the corresponding analytical techniques, and to compare them with the commercially made C-HAuCl4 precursor prepared from Gold (III) chloride tetrahydrate and deionised water. The second step was preparation of H- and C-AuNPs through the USP process, with as equal and constant technological parameters as possible, and then making a critical assessment of the comparability of both AuNPs' types. For this assessment, various techniques were used, like SEM, EDX, TEM UV–vis and FTIR, in order to find any differences in surface characteristics, size, and morphology between H- or C-AuNPs.
2. Materials and methods
2.1. Materials
Gold (III) chloride tetrahydrate (trace metals basis ≥99.9%, Acros Organics, Geel, Belgium), polyvinylpyrrolidone (PVP40 Sigma Aldrich, Merck, Germany), Gold pellets (99.99%, ∼250 mg, Zlatarna Celje d.o.o, Celje), hydrochloric acid (36% concentrated, Honeywell Fluka GmbH, Germany), potassium permanganate (Carlo Erba Reagents, France), and sodium thiosulfate pentahydrate (Carlo Erba Reagents, France) were all used as received. Milli-Q water and deionized water were used as the reaction solvent.
2.2. Preparation of H-HAuCl4 and C-HAuCl4
H-HAuCl4 was prepared by using simple laboratory apparatus [47]. The thermodynamics and process involved in the preparation of H-HAuCl4 are presented in the supplementary file S1 is available online at stacks.iop.org/MRX/7/055017/mmedia.
The as-received Gold (III) chloride tetrahydrate powder was orange in colour containing micron-sized particles. Deionised water was added to the Au (III) chloride tetrahydrate powder, to obtain C- HAuCl4 precursor with concentration of [Au] = 1 g l−1.
2.3. Characterisation of H-HAuCl4 and C-HAuCl4
Inductively Coupled Plasma-Mass Spectrometry (ICP-MS), while using a spectrometer (HP, Agilent 7500 CE, equipped with a collision cell, Santa Clara, CA, USA), was performed on C- and H-HAuCl4 precursors, in order to measure the concentration of Au (chemical composition). ICP-MS analysis was carried out under the operating conditions: Power = 1.5 kW, Nebulizer-Meinhard, plasma gas flow (l min−1) = 15, Nebulizer gas flow (l min−1) = 0.85, Make up gas flow (l min−1) = 0.28, and Reaction gas flow (ml min−1) = 4.0. Matrix matched calibration solutions were prepared and analysed for calibration. Prior to the analysis, the samples of C- and H-HAuCl4 precursors were dissolved in 10% (v/v) aqua regia. The relative measurement uncertainty was established to be ±3%.
Surface tension was measured with a KRU SS K12 tensiometer for H-HAuCl4 and C-HAuCl4 solutions. Three repetitions were made, and ten measurements were performed on each set.
pH was measured with a pH meter (Mettler Toledo, USA) for both precursors.
2.4. Synthesis of AuNPs
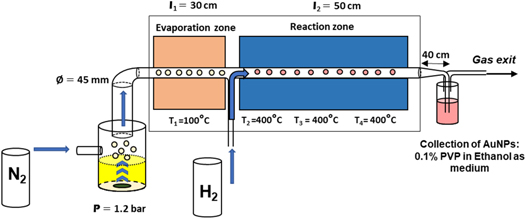
A horizontal type of USP device (Zlatarna Celje d.o.o, Celje) was used for synthesis of AuNPs. The USP device consisted of:
- Ultrasound generator (model PrizNANO Prizma d.o.o. Kragujevac, Serbia, with a frequency 2.4 MHz and aerosol capacity of 500 ml h−1),
- Carrier gas supply unit (N2),
- Heating part (1. Evaporation zone T = 100 °C and 2. Reaction zone divided into 3 parts for better controlling of T in the transport tube with T = 400 °C),
- Reaction gas supply unit (H2),
- System for AuNP collection by using ethanol as a medium and PVP polymer as a binder.
The schematic presentation of the USP device, with dimensions and some important technological parameters for AuNPs synthesis in this research, is shown in figure 1.
Figure 1. Schematic presentation of the USP device for the synthesis of AuNPs.
Download figure:
Standard image High-resolution imageAerosol formation occurred in the ultrasound generator from H- or C-HAuCl4 separately, with the initial concentration of [Au]=1 g l−1. Due to the vibrations of the ultrasound below the precursor surface, the kinetic energy of the precursor molecules increases rapidly. This causes small droplets to overcome surface tension and break away from it. This effect, known as nebulisation, produced micron sized aerosol droplets, which act as individual chemical reactors when subjected to thermal treatment in the heating part of the USP. Droplets in a size distribution from 1 to 15 micrometers were created with a high-frequency ultrasound (0,5–3 MHz), [48]. In our case, the frequency was 2,4 MHz, so it could be assumed that the droplets' sizes were in the same range. With the help of N2, droplets were passed through the heating part, where H2 entered into the reaction zone for the reduction of Au+3 into AuNPs. The detailed parameters used for synthesising of H- AuNPs and C- HAuCl4 precursors are shown in table 1.The steps and chemical reactions involved in formations of AuNPs in USP synthesis were the following [48]:
- 1.Droplet evaporation at

- 2.Thermal decomposition
![${H}^{+}\,+\,{\left[AuC{l}_{4}\right]}^{-}\to \,A{u}_{2}C{l}_{6}\,+\,HCl$](data:image/png;base64,iVBORw0KGgoAAAANSUhEUgAAAAEAAAABCAQAAAC1HAwCAAAAC0lEQVR42mNkYAAAAAYAAjCB0C8AAAAASUVORK5CYII=)
- 3.Hydrogen reduction

- 4.Densification of AuNPs
Table 1. Parameters used for the synthesis of H- and C-AuNPs.
| [Au] (g l−1) | T1 (°C) | T2 (°C) | T3 (°C) | T4 (°C) | N2 (l min−1) | H2 (l min−1) | Collecting medium | tUSP (min) |
|---|---|---|---|---|---|---|---|---|
| 1 | 100 | 400 | 400 | 400 | 4 | 2 | 0.1% PVP dissolved in ethanol | 120 |
2.5. Characterisation of AuNPs
Attenuated Total Reflectance - Fourier Transform Infrared (ATR-FTIR) spectroscopy analysis of the H- and C-AuNPs was performed by using a Perkin–Elmer IR spectrophotometer with a Golden Gate Attenuated Total Reflection (ATR) attachment with a diamante crystal. The spectra were accumulated within 16 scans at a resolution of 4 cm−1 within a range of 4000 cm−1 to 650 cm−1.
The spectra of the solution of H- and C-AuNPs were prepared by using quartz cells with a Varian Cary 100 Scan UV/Vis spectrophotometer for the analysis.
A Scanning Electron Microscope (SEM), Sirion 400NC (FEI, USA) with an Energy-Dispersive x-ray spectroscope INCA 350 (Oxford Instruments, UK), was used for identification of AuNPs size and morphology. For this purpose, the rotavapor technique was used for drying the H- and C-AuNPs, in order to achieve the necessary conditions for AuNPs' observation. The AuNPs were put dropwise on SEM holders with conductive carbon adhesive tape and left to dry under vacuum. Energy-Dispersive x-ray spectroscopy (EDX) was used for the determination of the chemical analysis.
TEM (JEOL 2100 JEOL ARM CF), STEM (JEOL ARM CF), Electron Diffraction (ED/TEM; JEOL 2100, JEOL ARM CF), and EDX spectroscopy/TEM (JED 2300), operating at 80 kV electron beam under high vacuum, were used to investigate the deeper morphology of the AuNPs. The collected dried AuNPs were first dispersed in deionised water. A drop of this suspension was put on a carbon coated TEM grid of 200 mesh. The grids were then dried before they were used for TEM investigations.
AuNPs size measurements were performed from the SEM and TEM micrographs with the microscope software, along with manual measurements with the image processing and analysis software ImageJ. The manual measurements from the ImageJ software were used for nanoparticle size distribution representations.
3. Results and discussion
3.1. Characterisation of H-HAuCl4 and C-HAuCl4
The ICP‐MS technique was used to determine the chemical composition of the H-HAuCl4 and C-HAuCl4. In the preparation process (see supplementary information S1), the use of KMnO4 may influence the precursor solution's purity. From the results of ICP analysis, it was found out that there were no traces of Mn in the H-HAuCl4. The concentration of the H-HAuCl4 and C-HAuCl4 precursors was, in both cases, 1 g l−1 Au.
Surface tension measurements were performed by using of the platinum plate, which was suspended vertically and immersed into the H-HAuCl4 and C-HAuCl4. The surface tension was calculated from the Contact Angle and the force, which correlate with the surface tension and the length of wetting. The surface tension values for both the precursors and pH values, are shown in table 2.
Table 2. Surface tension and pH values.
| Surface tension (mN m−1) | pH | |
|---|---|---|
| H-HAuCl4 | 65.60 ± 0.04 | 1.8 |
| C-HAuCl4 | 71.88 ± 0.05 | 3.0 |
From previous research, the precursor solution with an Au concentration of 1 g l−1 had a density (1002 g l−1), surface tension (≈74 mN m−1) and viscosity (≈1 mPa s) similar to that of water, as it is not a highly concentrated solution. This, in turn, ensured that H-HAuCl4 was suitable for aerosol production.
3.2. Synthesis of AuNPs
For the synthesis of AuNPs, optimal parameters with a gas flow of nitrogen at 4 l min−1 and hydrogen at 2 l min−1 were used in the USP process [48]. The synthesised AuNPs were collected in ethanol as a medium [49]. PVP was used as the stabilising agent and to minimise the aggregation of AuNPs. PVP was also used as a shape-controlling agent, and for preventing the agglomeration of AuNPs during the synthesis [50].
The droplet sizes depend on the ultrasonic frequency of the generator and on density, surface tension and concentration of the dissolved Au in the precursor solution, as seen in the distributions measured by laser diffraction [51]. The separate evaporation zone ensured the formation of spherical dried particles of gold chloride before the reaction zone with H2—this was accomplished by using low temperature, T = 100 °C required for slow evaporation of the solvent- deionised water. Theoretically, this enabled more stable conditions for evaporation. When the solvent evaporation was slow enough, the solute (gold chloride) diffused into the centre of the droplet, forming spherical dried AuNPs, and without the reaction gas present at this stage, the particles cannot form on the surfaces of the droplets from gold chloride.
3.3. Characterisation of AuNPs
3.3.1. FTIR studies of H- and C-AuNPs in collecting media
Figure 2 shows FTIR spectra for PVP functionalised AuNPs. The absorption band at 3400–3300 cm−1 for Hydroxy group was 1290 cm−1, which exposed the symmetric stretching of the C–N or C–O bond [52]. In the case of PVP capped AuNPs, a shift of C–O stretching from 1650 cm−1 to 1630 cm−1 confirms the functionalisation of AuNPs with PVP via intermolecular hydrogen bonding [53].
Figure 2. FTIR spectra of pure PVP, H-AuNPs, and C-AuNPs.
Download figure:
Standard image High-resolution image3.3.2. UV–vis measurements of H- and C-AuNPs in collecting media
Figure 3 shows the UV–vis spectra of AuNPs in collecting media from (i) C- HAuCl4 and (ii) H- HAuCl4. The spectrum of PVP functionalised AuNPs in collecting media from both precursors show absorption peaks at ∼548 and ∼536 nm, which are characteristic peak of AuNPs arising due to SPR [54].
Figure 3. UV–vis spectra of C-AuNPs and H-AuNPs, having a maximum absorbance wavelength at 548 nm and 536 nm.
Download figure:
Standard image High-resolution image3.3.3. SEM/EDX analysis
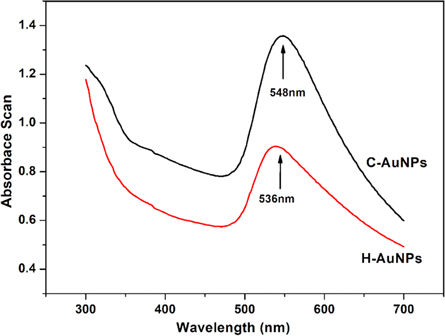
Figure 4 shows the SEM analysis of dried C-AuNPs and H-AuNPs. In both cases, the formed AuNPs were a combination of irregular shapes and round nanoparticles, and their chemical composition confirmed the presence of Au with a percentage of 99.7%; chlorides and oxides were in very low quantities, which could be attributed as a residue of the USP process. The formed AuNPs have a typical size of C-AuNPs and H-AuNPs, as are shown in figures 4(A) and (B). The shapes of AuNPs are mostly spherical, and no agglomeration was observed, due to the presence of PVP polymer as a stabilizer [55]. There were no visible surface defects on the surfaces of the AuNPs.
Figure 4. SEM micrograph of dried: (A) C-AuNPs, (B) H-AuNPs.
Download figure:
Standard image High-resolution image3.3.4. TEM analysis
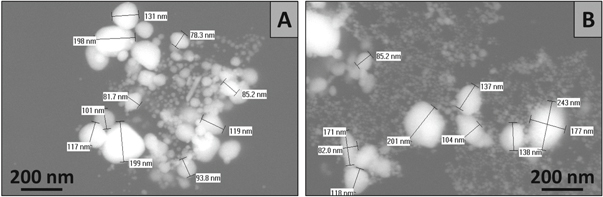
Figure 5 shows typical TEM images for the smallest group of dried C-AuNPs and H-AuNPs.
Figure 5. TEM images of (A) C-AuNPs (B) H-AuNPs.
Download figure:
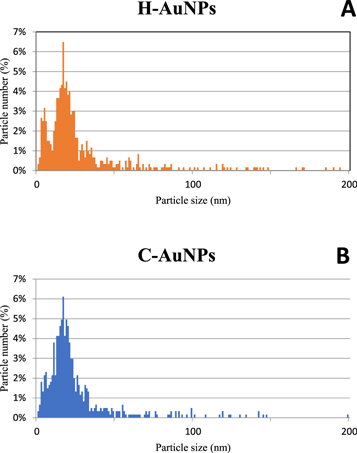
Standard image High-resolution imageAuNPs' size measurements were performed, giving the nanoparticle size distribution in figure 6. 607 nanoparticles were measured for C-AuNPs, and 610 nanoparticles were measured for H-AuNPs. The binning for the distribution was 1 nm. It is evident that a larger number of very small nanoparticles from 2 to 8 nm are present in both samples, while, for the sample of AuNPs from H-HAuCl4, the smaller nanoparticles are even more numerous, as seen in the distribution. The calculated mean nanoparticle size for C-AuNPs is 25.5 nm, while for H-AuNPs it is 32.6 nm. The maximum measured nanoparticle size for C-AuNPs is 199 nm, and 405 nm for H-AuNPs, indicating that H-AuNPs have a broader distribution, with a higher number of very small nanoparticles (2–8 nm) and a higher number of larger sub-micron particles (above 200 nm). These larger particles are also more irregular in shape. It seems the rheological properties of the H- HAuCl4 used in USP favour smaller AuNPs' formation, along with larger irregular nanoparticles, which is probably due to the lower value of pH in comparison with C-HAuCl4. Further investigations into different precursor Au concentrations and USP parameters need to be performed for optimising the formation of more uniformly sized and shaped AuNPs.
Figure 6. AuNPs' particle size measurements: (A) H-AuNPs, (B) C-AuNPs.
Download figure:
Standard image High-resolution imageFrom the FTIR studies, the presence of PVP and the shifting of C=O stretching confirms the functionalisation of AuNPs. It confirms that molecular interactions occurred between the PVP and PVP functionalised AuNPs. UV–vis studies show the successful preparation of AuNPs from both precursors. From figure 3, there is a shift in the absorbance wavelengths from 536 nm to 548 nm. The shift indicates the greater size of the AuNPs [56]. From this, H-AuNPs have a larger number of smaller sized nanoparticles when compared to C-AuNPs. From figure 4, there are smaller spherical size nanoparticles observed in the H-AuNPs when compared to C-AuNPs. This could be attributed to the lower surface tension value for the H- HAuCl4 (table 2); also, these results are in good agreement with the UV–vis measurements. The lower surface tension affects the droplet formation by ultrasound, resulting in smaller droplets and consequently smaller AuNPs.
Previously, we have shown comparisons between different precursor acids, such as the chlorides, acetates and nitrides used with USP [57, 58]. However, the comparisons between different acidity levels of the same acid (chloride) have not yet been shown, nor are they readily available in literature. The possibilities to determine the usability of the H- HAuCl4 with USP were investigated and confirmed with this work.
4. Conclusions
Several main conclusions can be drawn from this study:
- (1)The H- HAuCl4 precursor produced by the chlorine gas method is suitable for USP synthesis.
- (2)FTIR studies of H-AuNPs show the intermolecular hydrogen bonding between PVP and AuNPs in collecting media.
- (3)UV–vis spectra confirm the presence of H-AuNPs at 536 nm, which exhibits the optical feature.
- (4)SEM and TEM analysis identified that H-AuNPs had similar size, size distribution and shape as C-AuNPs.
From all these, it is possible to conclude that H- HAuCl4 has somewhat different rheological properties and acidity, producing H-AuNPs with a different morphology and size distribution as compared to the C-AuNPs. For future works, the concentrations and acidity of the precursor will be modified, for determining their effect on the nanoparticle formation. This could prove to be a helpful type of modification for producing AuNPs with desired properties with USP.
Acknowledgments
We would like to thank Lidija Rozman Zorko for performing the SEM analysis on the gold nanoparticles and Dr Darja Feizpour from the Institute of Metals and Technology, Ljubljana Slovenia for TEM analysis.
Funding
This research was funded by the Ministry of Education, Science and Sport, Republic of Slovenia and European Union, The European Regional Development Fund (ERDF), Early research careers 2.1. and by the Slovenian Research Agency, Infrastructure Core Funding No. I0–0029.
Conflicts of interest
The authors declare no conflict of interest.



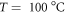
![${H}^{+}\,+\,{\left[AuC{l}_{4}\right]}^{-}\to \,A{u}_{2}C{l}_{6}\,+\,HCl$](https://content.cld.iop.org/journals/2053-1591/7/5/055017/revision2/mrxab80eaieqn2.gif)