Abstract
This study examined the oxidation properties of rare earth steel at high temperatures (600 °C–900 °C) in the air atmosphere by using Scanning Electronic Microscope (SEM) with an energy dispersive spectroscopy (EDS), x-ray diffraction analyzer (XRD) and atomic force microscopy (AFM) analysis method. High temperature oxidation experiment showed that rare earth element Ce played a very good antioxidant effect with the increasing oxidation temperature. After continuous oxidation at 600 °C, 700 °C, 800 °C and 900 °C for 150 h, the mass gain of the rare earth steel were 1.25 mg cm−2, 2.41 mg cm−2, 12.34 mg cm−2, and 13.37 mg cm−2, respectively. In comparison, the matrix steel under the same conditions were 1.31 mg cm−2, 2.94 mg cm−2, 13.98 mg cm−2, and 15.63 mg cm−2, respectively. AFM features of the oxidized surface confirmed that the rare earth element Ce could inhibit the growth rate of the oxide in the early stage of oxidation, and preferentially promote the formation of the spinel oxide. The surface-dense spinel oxide (Fe, Cr, Ni, Mn)3O4, the enrichment of Cr-containing layer in the middle, and the formation of the Si-rich oxide in the inner layer all affected the high-temperature oxidation resistance of the rare earth steel.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Heat-resistant steel are widely used in various heat-resistant structures in electric power, metallurgy and petrochemical industries due to the stable high temperature properties [1–3]. It is easier to fail that the materials working in a complex environment, thereby it causes the operation interruption of the equipment [4]. Equipment malfunction does not only arouse inconvenience to people's production and life, but also causes the direct or indirect economic losses [5, 6]. High temperature oxidation is one of the main factors for high temperature failure of materials. The oxidation behavior of materials at high temperatures is a very complex process of change [7]. In recent years, the research on high temperature oxidation of heat resistant steels has been the focus of attention [8–10].
Some studies have found that the addition of alloying elements can significantly improve the high temperature oxidation resistance of heat resistant steel [7, 9]. According to our knowledge, the rare earth elements have a significant effect on the high temperature oxidation capacity of heat resistant steel [8, 10]. Generally, the research on high temperature oxidation resistance of rare earth steel are mainly discussed from the aspects of oxide morphology, oxide growth rate, oxide phase composition and oxide film structure under high temperature [11–13]. Besides, the addition of rare earth elements could cause preferentially selective oxidation of certain oxides [10]. To the best of our knowledge, although there are some studies about the effect of rare earth elements on high temperature oxidation resistance of heat resistant steel, they are all aimed at the special application conditions. For example, Liang et al [14] studied the oxidation resistance of rare earth steel under high temperature steam conditions. This material is mainly used in ultra-supercritical boiler tubes for power plants [15]. Yan et al [16] discussed the effect of yttrium on the cyclic oxidation behaviour of HP40 heat-resistant steel at 1373 K. Others have also analyzed the properties of heat-resistant steel under continuous oxidation at elevated temperatures [17, 18]. These theories mainly indicated that the rare earth elements were enriched at the grain boundaries, thereby prevented the diffusion of anions and cations [14, 16]. At the same time, the adhesion of the oxides could be increased during the high temperature oxidation process, and reduced the oxidation rate [15–18].
The object of this work is to investigate the effect of rare earth element Ce on the high temperature oxidation behavior of austenitic heat-resistant steel at different temperatures. Oxidation kinetics and surface oxide formation characteristics of the experimental materials, as well as the precipitates composition near the oxide film, were systematically investigated at 600 °C–900 °C.
2. Experiment methods
The actual chemical composition of the experimental steels for energy spectrum detection is shown in table 1. In order to eliminate or at least minimize the difference on other main compositions except Ce in tested steels, a furnace of molten steel was poured into two casting ladles for solidification. One added the rare earth element Ce during pouring, and the other one without. The oxidized specimens with dimensions of 10 mm × 10 mm × 5 mm, and they were ground, polished and put in dryer.
Table 1. Chemical composition of the experimental steels (wt%).
| Amount (wt%) of elements | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Specimens | C | Cr | Ni | Si | Mn | Mo | V | Ce | Fe |
| Matrix | 0.11 | 20.88 | 31.07 | 0.74 | 1.06 | 0.12 | 0.10 | — | Bal. |
| Matrix + Ce | 0.11 | 20.86 | 31.05 | 0.75 | 1.03 | 0.10 | 0.10 | 0.24 | Bal. |
Prior to the oxidation experiment, all crucibles were dried multiple times in an oven at 50 °C above the experimental temperature. The oxidation samples were put into crucibles when there was no further change in crucible weight. Each crucible held only one sample. A box-type resistance furnace (KSS-1400) was used to perform the oxidation tests. Constant temperature oxidation experiments were conducted at 600 °C, 700 °C, 800 °C and 900 °C for 150 h, followed by air cooling. The weight measurements were performed before and after different oxide time, by using GL623-1SCN analytical balance with an accuracy of 0.1 mg. Measurement time was set to 20 h, 40 h, 60 h, 80 h, 100 h, 120 h and 150 h, respectively. The microstructure of the oxidized surface of specimens were characterized by different analytical methods. A scanning electron spectroscope (SEM; Nova Nano 430) incorporated with an energy dispersive spectroscopy (EDS) was employed for the oxide morphology and cross-sectional analyses, and an x-ray diffraction (XRD-D8; Advance diffractometer) was used for characterizing the phases. In addition, after the short-term exposures (1 h and 3 h) at 900 °C, the surface roughness and three dimensional morphology of the oxide film were analyzed with an atomic force microscopy (AFM; Park Systems).
3. Results and discussion
3.1. Oxidation dynamic curves
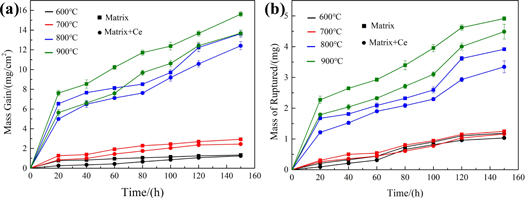
The curves of oxide mass gain and mass of ruptured oxide samples with error analysis under different oxidation conditions, as shown in figure 1. As illustrated in the oxidation curves, the oxidation of the experimental steels are not significant at 600 °C and 700 °C, so the error is relatively small. The oxidation mass gain and oxide shedding show a parabolic tendency with the prolongation of oxidation time. The higher temperature results in the greater mass gain and mass ruptured specimens at the same oxidation time. The mass gain and oxide shedding of the samples increase rapidly during the initial stage of oxidation (0–20 h). The mass increase becomes slower and tends to be stable when the oxidation time increases. After continuous oxidation for 150 h at 600 °C–900 °C, the oxide mass gains of the matrix steel specimens are 1.31 mg cm−2, 2.94 mg cm−2, 13.98 mg cm−2 and 15.63 mg cm−2, respectively. The oxide mass gains of the rare earth steel are 1.25 mg cm−2, 2.41 mg cm−2, 12.34 mg cm−2, 13.37 mg cm−2, respectively. It is worth mentioning that the oxide mass gain of Ce-containing steel is smaller than that of matrix steel under the same oxidation conditions, and the phenomenon is more pronounced at 800 °C and 900 °C. This phenomenon indicates that the rare earth steel has a better antioxidant effect with the increasing temperature. The amount of oxides shedding also satisfies the same law. Compared with 600 °C and 700 °C, it is attracted that the mass gain and the amount of oxide shedding have a leap forward growth over the entire oxidation when the oxidation temperature is 800 °C and 900 °C.
Figure 1. Oxidation dynamic curves (a) oxide mass gain and (b) mass of ruptured oxide.
Download figure:
Standard image High-resolution imageThe oxidation rate refers to the mass gain of the samples at unit area and unit time, which can be used to characterize the oxidation speed of the materials [19]. Oxidation is a dynamic gradual process and can be characterized by calculating the average oxidation rate:
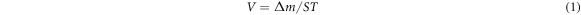
where, V is average oxidation rate, g/(m2 · h). Δm is oxidation weight gain quality, g. S is the contact area, m2. T is the oxidation time, h. The oxidation rate of specimens under different condition can be calculated by equation (1), as shown in figure 2. It is obviously that the oxidation rates of samples at different temperatures illustrate a similar change. Oxidation rate rapidly increases in the early stage, and gradually decreases when the oxidation time exceeds 20 h. The oxidation rate eventually tends to be stable with the extension of oxidation time. The oxidation process is roughly divided into three stages [20–22]: rapid oxidation stage, oxidation transition stage and oxidation equilibrium stage.
Figure 2. Oxidation rate curves of experimental materials at different temperatures.
Download figure:
Standard image High-resolution imageThe oxidation rate increases gradually with the rises of oxidation temperature. It is interesting to note that the early rapid oxidation time period is the same, even if the experimental steels are oxidized at different temperatures. They are both in the first 20 h of oxidation. However, the materials will be significantly different in the late oxidation transition stage and the oxidation equilibrium stage when they are oxidized at different temperatures. The experimental steels reach an oxidation equilibrium stage after oxidizing at 600 °C and 700 °C for 80 h. In comparison, the oxidation equilibrium stage is reached after oxidation for over 120 h at 800 °C and 900 °C. It is also clear that the oxidation rate of Ce-containing steel is always smaller than that of the matrix steel under the same conditions.
In order to further investigate the oxidation feature, it should be pointed out that the oxidation kinetic curves are parabolic, which defined as following:
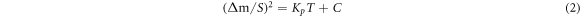
where Δm, S and T have the same meaning as in equation (1). Kp is the parabolic rate constant, and C is a constant [19]. The detailed oxidation kinetics of materials oxidized at different temperature is analyse by the square of the mass gain as a function of time. After fitting, we can get the Kp value of the experimental steels under different oxidation conditions. The specific data is shown in table 2. In addition, R2 is a certainty coefficient that indicates how well the statistical model fits a set of observations. It can be seen that the Kp values of the rare earth Ce steel are always smaller than the matrix steel under the same oxidation conditions from table 2, which further illustrates that the addition of the rare earth element Ce reduces the oxidation rate of the steel.
Table 2. Oxidation kinetic parameters of experimental steels (wt%).
| Parameters | ||||
|---|---|---|---|---|
| Materials | Temperature/°C | Kp | C | R2 |
| Matrix | 600 | 1.07 × 10−2 | 2.03 × 10−1 | 0.95 |
| 700 | 5.91 × 10−2 | 4.91 × 10−1 | 0.98 | |
| 800 | 1.17 | 1.76 | 0.89 | |
| 900 | 1.53 | 10.53 | 0.98 | |
| Matrix + Ce | 600 | 1.06 × 10−2 | −2.40 × 10−1 | 0.89 |
| 700 | 4.34 × 10−2 | −3.40 × 10−1 | 0.96 | |
| 800 | 0.94 | −2.39 | 0.95 | |
| 900 | 1.22 | −1.92 | 0.99 | |
3.2. Surface oxide analysis
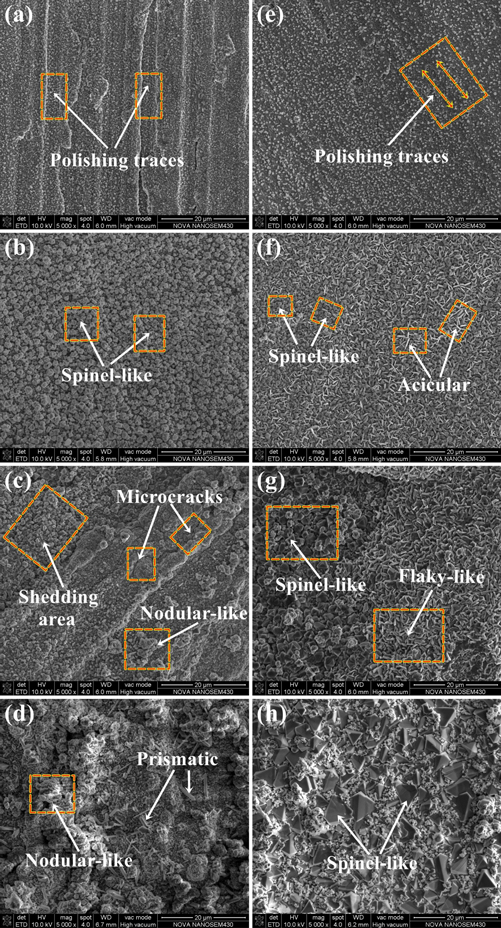
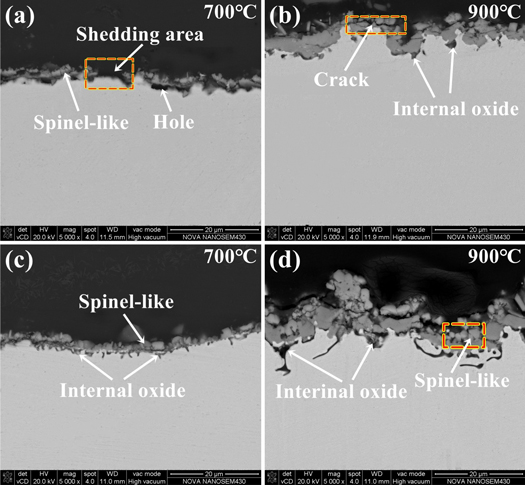
Figure 3 shows the oxidized surface of the experimental materials after continuous oxidation for 150 h at different temperatures. As illustrated in the figure, the surface morphology of the oxidized specimens varies greatly under different conditions. The oxides on the surface of the experimental steels are small at 600 °C, as shown in figures 3(a) and (e), and the polishing traces can still be seen. This indicates that the oxide layer of the experimental materials is very thin under 600 °C, and the oxide film is not complete and discontinuous. The oxides gradually grow up and the polishing traces on the surface of the samples are disappeared, when the oxidation temperature rises to 700 °C. As shown in figure 3(b), the surface of the matrix steel with a layer of uniform sized spinel-like oxide. Besides, some acicular-like oxide is evenly distribute on the surface of rare earth steel, as shown in figure 3(f). We have reason to believe that the addition of rare earth element Ce in the steel could promote the formation of acicular oxide at 700 °C. These acicular oxide fills the voids between the spinel oxide, and makes the surface oxides denser. The dense oxide film can inhibit the inward diffusion of oxygen ions and improve the antioxidant capacity of materials [10, 16].
Figure 3. SEM morphology of surface layer of specimens oxidized at 600 °C, 700 °C, 800 °C and 900 °C for 150 h (a) matrix and (b) matrix+Ce.
Download figure:
Standard image High-resolution imageIt is clearly observe that a shedding area on the surface of samples when the oxidation temperature reach 800 °C. Additionally, the surface oxides of the matrix steel are nodular-like and there are some microscopic oxidation cracks, as shown in figures 3(c) and (g), and the acicular oxides on the surface of rare earth steel gradually grow into a flaky-like with the increasing oxidation temperature. The morphologies of oxides on the materials at 900 °C are quite different. The surface oxides of matrix steel are loose and porous. Moreover, the oxides are severely detached and prismatic oxides can be observed in the shedding area, as shown in figure 3(d). It is believe that these columnar oxides may be the pre-formed spinel oxides. This is because the internal material exposure time is short when the oxide is detached, and the prismatic oxides cannot form a spinel-like. The surfaces of rare earth steel are mainly spinel-like, and a large number of fine granular oxides are dispersed around the spinel oxide, as shown in figure 3(h). These fine granular oxides compensate for the gap between the spinel oxides, which increase the compactness of the oxidized surface of the steel. This is because that the addition of rare earth element in the steel can promote the formation of surface spinel oxides at high-temperature [23, 24]. The presence of these spinel oxides can better prevent the interior of steel from further oxidation [9, 16, 23].
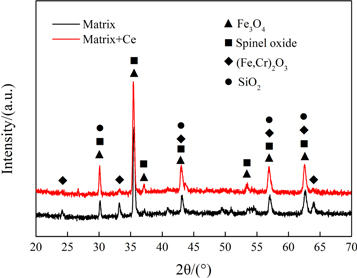
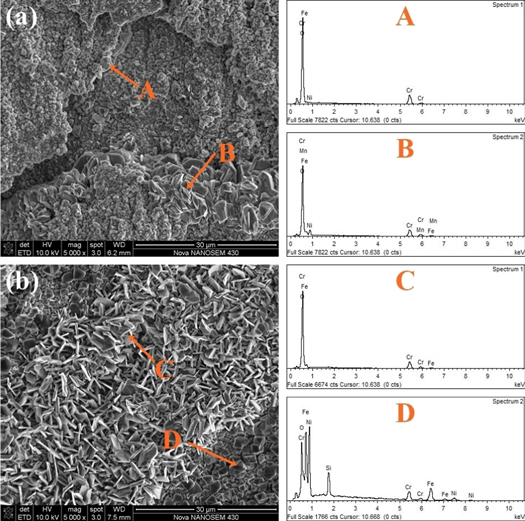
The XRD patterns of the oxide scales formed on the specimens at 900 °C in air for 150 h is shown in figure 4. It is found that the surface oxides of the experimental materials are mainly composed of Fe3O4, spinel-like, (Fe, Cr)2O3 and SiO2. The spinel oxides may include (Fe, Cr, Ni, Mn)3O4. Figure 5 also shows the EDS energy spectrum of the experimental steel oxide surface. The main elements on the surface of the tested samples are Fe, Cr, Ni, Mn, O, and Si. This result is consistent with the XRD data. Since the ionic radii and external electronic layout of Ni, Cr, Mn, and Fe are very similar [25, 26], it is difficult to determine the exact compositions of the oxide product by the position of the peak in the XRD pattern [25, 27]. Additionally, the peak of spinel-like oxide strength of the rare earth steel is relatively higher. During the oxidation process, the rare earth element Ce promotes the formation of spinel in the oxide [28], thereby better prevent the volatilization of the internal chromium-rich oxide at high temperature [29, 30].
Figure 4. XRD patterns of experimental steels after oxidation at 900 °C for 150 h.
Download figure:
Standard image High-resolution imageFigure 5. SEM morphology and corresponding EDS spectroscopy of the oxidation surface of experimental steels (a) matrix and (b) Matrix + Ce.
Download figure:
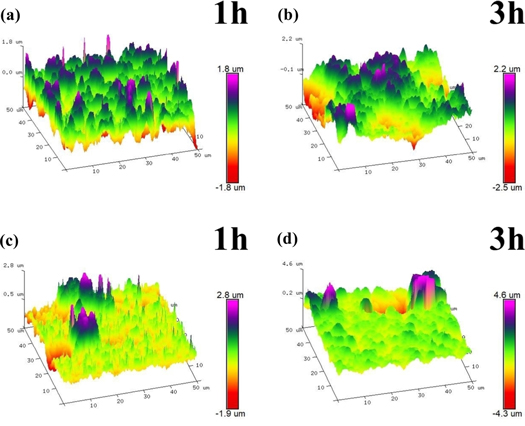
Standard image High-resolution imageFigure 6 illustrates typical three-dimensional AFM features of the experimental specimens after exposure to air at 900 °C for different time. The atomic force microscope is a three-dimensional characterization of the microscopic surface of the materials, the area of the oxide film studied is only 50 × 50 μm2, and the different heights of the oxide are described by color changes [20, 31]. It can be clearly seen from the results that the three-dimensional morphologies of specimens after oxidation under different time are very different. When the oxidation time increases from 1 h to 3 h, the oxide gradually grows and the surface of the specimen is rougher. Compared with the matrix steel, the number of high peaks on the surface of the Ce containing steel is less under the same initial oxidation conditions. This phenomenon indicates that the rare earth element Ce inhibits the growth rate of early oxides, which is consistent with other studies [10, 13].
Figure 6. AFM oxide film surfaces topographies of the experimental samples exposed to air at 900 °C for 1 h and 3 h (a), (b) matrix and (c), (d) matrix + Ce.
Download figure:
Standard image High-resolution imageThe surface morphology of the oxide film is further analyze by the roughness parameters Ra and Rq obtained by AFM. Where Ra is the arithmetic mean deviation and Rq is the root-meansquare deviation. It is known that parameters Ra and Rq can reflect the surface properties of the oxide layer from different aspects. These specific roughness parameters are shown in table 3. It is worth mentioning that the values of Ra and Rq of the matrix steel at 1 h are greater than those of Ce containing steel. However, the values of rare earth steel Ra and Rq are greater than those of the matrix at 3 h. The results show that the addition of rare earth element Ce into steel does not only reduces the growth rate of the experimental steel oxide, but also promotes the preferential growth of the spinel-like oxide in the early stage of oxidation [9–11, 24].
Table 3. Roughness of oxide film on the surface of experimental samples.
| Samples | 1 h | 3 h | ||
|---|---|---|---|---|
| Ra/μm | Rq/μm | Ra/μm | Rq/μm | |
| Matrix | 0.443 | 0.542 | 0.576 | 0.707 |
| Matrix + Ce | 0.356 | 0.532 | 0.618 | 0.949 |
3.3. Cross-sectional analysis of the oxide
The vertical cross section of the specimens after the oxidation at 700 °C and 900 °C for 150 h are also observed, and the results are shown in figure 7. It is shown that the oxidation of material is not significant at 700 °C, and the oxide layers on both samples are relatively thin. At the same time, the oxide layer of matrix steel is discontinuous, and oxidation cracks, holes and shedding area are clearly observed, as shown in figure 7(a). But the oxide layer of the rare earth steel in figure 7(c) is more uniform and continuous. The outer layer of oxide film is spinel-like and forms a large amount of internal oxides in the vicinity of substrate in figure 7(c). When the oxidation temperature rises to 900 °C, the oxides are more likely to grow, so the samples will have thicker oxide layer in the same oxidation time [28]. As can be seen from the comparison of in figures 7(b) and (d), the thickness of oxide layer on the matrix steel is about 5–10 μm on average, and the oxide film on the Ce-containing steel is approximately 10–15 μm. Additionally, the oxide film of matrix steel is uneven in thickness and has cracks. Only a small amount of internal oxide is formed at the bottom of the oxide layer. The oxide film of Ce containing steel is thicker, and a large amount of internal oxides which extend to the inside of the substrate appear. The oxide layer containing Ce steel is denser with fewer cracks and holes. Most scholars believe that rare earth elements can promote the formation of internal oxides in alloys [32, 33]. The presence of oxides between the oxide layer and substrate will provide a better pinning effect on the external oxide film, and reduce the shedding of the oxide [16, 32], thereby achieving better oxidation resistance.
Figure 7. Cross-sectional images of experimental steels after high temperature oxidation for 150 h (a), (b) matrix and (c), (d) matrix + Ce.
Download figure:
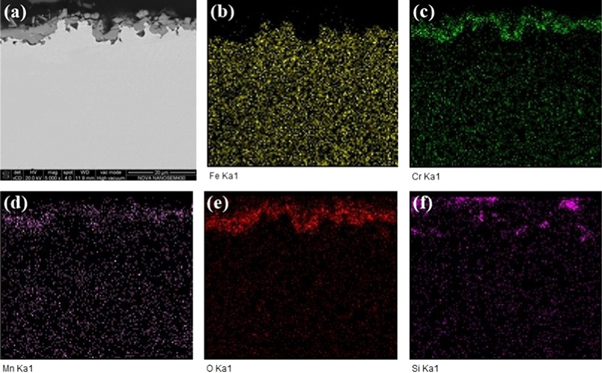
Standard image High-resolution imageTo further determine the compositions of the experimental steels oxide, we performed an EDS analysis of the cross section of oxidation. Figures 8 and 9 show the SEM and corresponding EDS maps of oxide layer cross section of matrix steel and rare earth steel, respectively. It is found that the oxides of experimental materials are mainly composed of elements such as Fe, Cr, Mn, O and Si. This result is consistent with the XRD analysis of oxide, is shown in figure 4. Compared with the matrix steel, the content of Cr and Mn are higher in the rare earth steel oxide layer. Combined with XRD pattern, we have reason to believe that the element Cr may mainly participates in the formation of Cr2O3, MnCr2O4. In addition, a small amount of FeCr2O4 and FeMn2O4 may be generated during the oxidation process. The element Mn may form spinel oxides MnCr2O4 and FeMn2O4, and the element Si mostly forms the interinal oxide SiO2 near the substrate.
Figure 8. SEM and corresponding EDS maps of matrix steel cross-sectional oxidized at 900 °C for 150 h.
Download figure:
Standard image High-resolution imageFigure 9. SEM and corresponding EDS maps of Ce-containing steel cross-sectional oxidized at 900 °C for 150 h.
Download figure:
Standard image High-resolution imageThe presence of Cr-rich and Mn-rich oxides increases the compactness of the oxide film. These dense spinel-like oxides in the outer layer of rare earth steel can slow the infiltration of oxygen ions into the substrate. At the same time, they can effectively block the volatilization of the internal oxides [16, 34]. Rare earth element Ce can also promote the formation of SiO2 on the inner side of the oxide layer [10]. The SiO2 will extend longitudinally from the oxide layer into the substrate to a certain extent, as shown in figures 6(c) and (d), which serves as a pinning effect to increase the adhesion of surface oxides [16, 32].
The oxidation rate of the experimental steels are lower when oxidized at 600 °C and 700 °C. This is because that the oxides on the surface of the samples grow slowly at lower oxidation temperatures, and the oxidation is not obviously. As the oxidation temperature increase to 800 °C and 900 °C, the oxides growth accelerate, and a shedding area appear on the surface of the samples. The experimental steels have different oxidation behavior at 600 °C–900 °C, however, the oxidation resistance of rare earth steel is always better than the matrix steel under the same oxidation conditions. To combine with the oxidation kinetic curve, the surface and cross-section oxidation morphology, we seemingly have enough reasons to believe that adding Ce to the steel not only inhibits the growth of surface oxides, but also promotes the formation of spinel-like oxides and Cr-rich oxides on the surface of the specimens, which improves the compactness of the surface oxides, and reduces the oxidation rate of the experimental materials. Meanwhile, the rare earth element Ce promotes the formation of Si-rich oxide inside the experimental steels, and suppresses the shedding of the oxide. We conjecture that the appearance of acicular-like and flaky-like oxides in rare earth steel should be the early existence of spinel oxides. The dense oxide film can inhibit the diffusion of ions, and improve the oxidation resistance of the material [10, 16, 19].
4. Conclusions
This study investigated the high temperature oxidation behavior of rare earth steel at 600 °C–900 °C in air. The main conclusions are as follows:
- (1)The experimental materials have similar oxidation kinetics when oxidized at 600 °C–900 °C. The curves of mass gain and mass of ruptured oxide are similar to a parabolic shape throughout the oxidation process. The value of oxidation rate reach a maximum at 20 h, then gradually decrease and stabilize with the extension of oxidation time.
- (2)The oxidation of the experimental materials at 600 °C and 700 °C are not obvious. After continuous oxidation for 150 h, the oxidative weight gain and oxide shedding amount are only below 3 mg cm−2 and 1.5 mg cm−2, respectively. After continuous oxidation at 800 °C and 900 °C for 150 h, the oxidative weight gain and oxide shedding amount exceed 12 mg cm−2 and 2.5 mg cm−2, respectively.
- (3)The Ra and Rq values of the rare earth steel are smaller than that of the matrix steel oxidation at 900 °C for 1 h, but these are larger than that of the matrix steel when oxidize for 3 h. In the early stage of oxidation, the high peaks number of rare earth steel oxides are always less than that of the matrix steel.
- (4)The element of Ce can promote the formation of surface spinel oxide and improve its compactness, promote internal oxides Si-rich at the oxide/matrix interface. Internal oxide increases the contact area between the oxide and the substrate, improves the adhesion of the oxide film, and inhibits the shedding of oxides.
Acknowledgments
This work is funded by GDAS' Project of Science and Technology Development (Nos. 2019GDASYL-0501014 and 2019GDASYL-0104024), Guangdong Province Key Area R&D Program (2019B010942001), Science and Technology Planning Project of Guangzhou (No. 201907010026 and 201906040007).