Abstract
High yield, low cost, environmentally friendly chemical bath synthesis process is used to produce submicron structures of ZnO and ZnO-carbon composites. Synthesis of ZnO rod structures (submicron sized) were confirmed using SEM images. To synthesis ZnO-carbon composites, well characterized carbon spheres produced using aerosol assisted CVD technique was used. Use of carbon spheres as a seed during synthesis of ZnO is observed to produce multipod structures of ZnO on to the surface of carbon spheres; the growth is explained by proposing suitable model. Room temperature photoluminescence spectra recorded for ZnO and ZnO-carbon composites synthesized show a systematic disappearance of a band-edge emission (sharp peak present at ∼380 nm observed for pure ZnO) with increased number of carbon spheres. Competent photo-catalytic activity of synthesized composite is confirmed by studying photo degradation investigation performed using a model dye molecule i.e. methyl orange. The work reveals possibility to use synthesized composites as a nontoxic and biocompatible catalyst for fragmentation of a methyl orange dye molecule. Photo-degradation mechanism of a methyl orange dye is proposed herewith considering the role of carbon spheres and ZnO in presence of UV photon.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Accessibility of clean and pure water has become a major issue due to the discharge of organic pollutants/contaminants present into the rivers and the sea [1–3]. One of the major sources of water contamination is the often-uncontrolled growth of industrialization across the globe. Many industries/factories discharge their chemicals directly into the rivers without any due process. Such a discharge of toxic influents into the water has seriously affected the environment and biodiversity in our river systems [4, 5]. In several countries, about ∼17%–20% share of industrial water pollution comes from textile industries. At least 72 toxic chemicals related to textile dyeing have been detected in the river systems out of which ∼30 chemicals cannot be treated/removed completely, a serious threat to people dependent upon these river systems [6, 7]. Several efforts have been made to address issue of water contamination caused by the textile industries [8, 9]. Photodegradation of a dye molecule has been attempted as this route is reliable, simple, and the cost effective [10–12]. In this method, the fragmentation of a dye molecule can be achieved using a photocatalysts material which is stimulated/activated mainly by electromagnetic radiation. Researchers have already demonstrated various types of photocatalysts used for dye molecule degradation [13], with the intension that a photocatalyst material synthesized must not be another source of water contamination. In this regard ZnO, a wide band gap (∼3.37 eV) compound semiconductor has received special attention [14]. The attention is mainly due to the unique physical and chemical properties associated with ZnO [15]. Despite on many advantages for e.g. non-toxicity, biocompatibility as well as the ability to produce ZnO in bulk, its use as a photocatalyst material is restricted due to its photocatalytic efficiency [16, 17]. In order to increase the photocatalytic efficiency of ZnO researchers have tried various methodologies which include, doping ZnO with various transition metals [18], forming nanostructures of ZnO with diverse morphology [19, 20] as well as forming its hybrid composites [21–23]. Above all hybrid composites synthesized using various carbon allotropes are marked to be very promising and hence received special attention [24–26]. Apart from various carbon allotropes, carbon spheres (CS) have generated significant interest due to their various applications. They are made up of number of concentric carbon layers, where CS having diameter less than 1000 nm are observed to have similar properties such as graphene and/or fullerene [27]. The CS can be used as a supporting template for synthesizing various composite materials due to its unique properties which mainly include high surface area, high structural stability and many other properties [28]. Hence it is interesting to understand the role of CS while synthesizing various nano composites metal oxides and their related applications in various fields [29].
The aim of this work is thus two fold. First we wanted to demonstrate a cost effective and environmental friendly chemical bath route for photocatalyst materials [30]. In doing so, we wanted to use CS templating for the synthesis of a composite material to act as a potential candidate in order to degrade textile dyes. Second, we wanted to fully characterize the fabricated composite and demonstrate the photocatalytic activity of synthesized composites by recording UV–visible spectra of a mixture of Z-CS and methyl orange dye molecule dispersed in deionized (DI) water exposed to UV light. From observed results the role of CS in Z-CS is claimed to provide/serve as electron scavenging center which can effectively reduce the excitonic recombinations associated with pure ZnO, resulting to improve the photocatalytic activity of composite material synthesized.
2. Materials and methods
In order to synthesize Z-CS composite material, CS were first synthesized using aerosol assisted chemical vapor deposition (AACVD) technique. Toluene was used as a precursor for CS synthesis and the reaction was carried at 1050°C in presence of nitrogen atmosphere using tubular furnace. The detailed experimental procedure regarding synthesis of CS is reported elsewhere [31]. With prior structural and optical characterization CS synthesized were used for producing Z-CS composites by adopting ecofriendly chemical bath route [32]. For Z-CS synthesis, CS (varying weight as 10, 50, 100 and 300 mg respectively) were well dispersed in 50 ml DI water. Well dispersed CS solutions were added into 150 ml of zinc acetate solution prepared in DI water having 0.1 M under constant stirring conditions. The pH value of the mixture was then adjusted to 7.0 by adding appropriate quantity of 2 M NaOH solution. The reaction was allowed to continue for 2 h under constant stirring conditions, where temperature of the chemical bath was maintained at 80 °C. The final product was then rinsed repeatedly using DI water, methanol and finally dried at 100 °C for 2 h. The final product/precipitate was then characterized structurally, morphologically as well as optically using respective techniques which include x-ray Diffraction (Model: Bruker D8 Discover, Cu Kα radiation), Scanning Electron Microscopy (Model: FEI Quanta 250), Transmission Electron Microscopy (Model: FEI Tecnai g2), micro-Raman spectroscopy (Model: Renishaw spectrometer), UV-Visible spectroscopy (Model: Cary 5000 UV-Vis-NIR) and photoluminescence (PL) spectroscopy (Model: PC1 Spectrofluorimeter). In order to investigate the photocatalytic activity of synthesized composites towards dye molecule degradation experiments were planned using methyl orange dye. For this 10 mg of Z-CS (varying CS weight) composite each was added into 50 ml of aqueous solution methyl orange (10 ppm). The mixture was sonicated for 15 min and kept in dark for 30 min under steady stirring conditions to establish adsorption-desorption equilibrium between the photocatalyst and dye used. The mixture was then exposed to UV light (λ = 365 nm, Power 100 W; Light intensity at 10' ∼8.5 mW cm−2) placed at a distance of 10 inch from the source to initiate the fragmentation/degradation of a dye molecule. In order to investigate the percentage of dye molecule degradation, 4–5 ml of specimen was extracted after every 15 min from the parent solution which is under UV exposure. The specimen was then centrifuged to remove photocatalyst and optically investigated using UV-Visible spectrometer to confirm the percentage of dye molecule degradation if any.
3. Results and discussion
Figure 1 present the structural and morphological investigations performed on CS using x-ray diffractogram (XRD) and Transmission electron microscope (TEM).
Figure 1. (a) x-ray diffractogram and (b) TEM image recorded for CS synthesized using AACVD technique.
Download figure:
Standard image High-resolution imageA strong peak positioned at ∼25° observed in figure 1(a) confirms the presence of graphitic carbon [33]. From TEM image it is clear that the graphitic carbon has spherical shape sized in between 500–700 nm. Similarly growth of pure ZnO using chemical bath route was confirmed structurally as well as morphologically. Figure 2 present structural and morphological investigations performed on pure ZnO using XRD and SEM techniques. Peaks positioned at 2θ = 31°, 34°, 36°, 47°, 56°, 62°, 67° and 69° in x-ray diffractogram (figure 2(a)) confirms formation of single phase wurtzite ZnO [34]. From figure 2(b) growth of well isolated ZnO rods having length ∼1–2 μm can be seen. The ZnO rods are observed to have width within the range of ∼200–300 nm at one end and ∼250–400 nm at the other end. During synthesis (using aqueous solution of  ) the
) the  ions (source aqueous NaOH) plays an important role to from
ions (source aqueous NaOH) plays an important role to from  Due to the alkaline nature of the solution,
Due to the alkaline nature of the solution,  gets dissolve to form a single molecule of ZnO and/or to form growth unit (i.e.
gets dissolve to form a single molecule of ZnO and/or to form growth unit (i.e.  ). A single molecule of ZnO formed within the chemical bath then acts as a seed crystal. Due to adsorption of a growth unit on its polar and non-polar faces, the crystal starts growing longer and larger. The growth mechanism responsible to produce ZnO rod type structures has been already discussed with details somewhere else [35].
). A single molecule of ZnO formed within the chemical bath then acts as a seed crystal. Due to adsorption of a growth unit on its polar and non-polar faces, the crystal starts growing longer and larger. The growth mechanism responsible to produce ZnO rod type structures has been already discussed with details somewhere else [35].
Figure 2. (a) x-ray diffractogram and (b) SEM image of pure ZnO synthesized using chemical bath route.
Download figure:
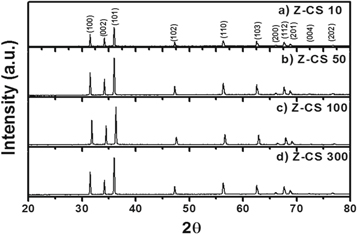
Standard image High-resolution imageFigures 3(a)–(d) show x-ray diffractogram recorded for Z-CS composites, where weight of CS was varied while synthesizing Z-CS composites. It is also clear from the diffractogram that formation of wurtzite ZnO is not getting hampered even though excess of CS are used while synthesizing Z-CS. To understand the exact role of CS while synthesizing Z-CS composites microscopic images were recorded using SEM and TEM techniques which are presented as figure 4. From SEM and TEM images multipod growth of ZnO is clear, most likely because of CS present during synthesis. It also reflect from figure 4 that formation of multipode structures of ZnO increases with increasing amount of CS used during Z-CS synthesis.
Figure 3. X-ray diffractogram for Z-CS composites where CS composition was varied a.s. (a) 10, (b) 50, (c) 100 and (d) 300 mg.
Download figure:
Standard image High-resolution imageFigure 4. SEM and TEM images (respective SAD patterns are shown as inset) of Z-CS composites synthesized by varying CS amount as (a) and (b) 10, (c and d) 50, (e) and (f ) 100 and (g) and (h) 300 mg.
Download figure:
Standard image High-resolution imageFrom figures 4(a)–(h) it is clear that CS is observed to be responsible in producing the multipod shaped Z-CS composite. We believe that CS used during synthesis may act as a seed. Evidences about excess of CS present in Z-CS for composite prepared with higher concentration CS (300 mg) can be seen in figures 4(g) and (h). Since we have proposed that CS play a vital role for the growth of Z-CS composite, herewith we intend to suggest a following model for observed the growth of multipod structures.
It is well accepted that the surface defects as well as nano pores present on any form of a graphitic carbon surface, may acts as a nucleation and/or active sites for subsequent growth [36, 37]. Presence of –OH and C=O groups on the surface of CS make it hydrophilic in nature [38]. Herewith we propose that Zn2+ produced during synthesis of ZnO using an aqueous solution of Zn(CH3COO)2 can get coupled with such active sites especially where –OH group is present due to the electrostatic interactions [32, 35, 39–41]. The Zn2+ ions coupled at the surface of CS further reacts with the OH− ion present in the reaction solution to produce Zn(OH)2 at the CS surface. Since reaction solution is weakly basic in nature: Zn(OH)2 produced at the surface of CS start dissolving to harvest a growth unit i.e. [Zn(OH)4]2− and/or single molecule of ZnO (nuclei) at the CS surface. The ZnO nuclei thus formed at the CS surface then acts as a seed crystal to which the growth units incorporate at the polar face of the ZnO crystal via dipole interaction resulting to produce observed multipod geometry at the CS surface [42]. The model has been presented as schematic
Schematic 1. Model presenting growth of Z-CS composite.
Download figure:
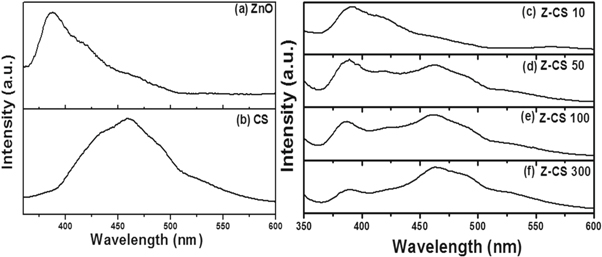
Standard image High-resolution imageOptical properties of pure ZnO, CS as well as Z-CS composites were investigated by recording PL and UV–vis spectra at room temperature. Figure 5 presents room temperature PL spectra recorded for each specimen.
Figure 5. Room temperature PL spectra of (a) ZnO, (b) CS and (c)–(f) for Z-CS composites varying the composition of CS.
Download figure:
Standard image High-resolution imageTypical characteristic spectra i.e. presence of sharp emission in UV region (∼380 nm) with a broad shoulder towards visible region is observed for pure ZnO synthesized [43]. The observed UV emission can be attributed to band-edge emission, whereas the emissions observed in visible range are due to deep-level defects present in ZnO rods synthesized [44]. PL spectra recorded using CS (figure 5(b)) show presence of a board band centered at ∼460 nm. Presence of typical emission observed confirm the presence of defects on the surface of CS as assumed. These surface defect actually acts as energy traps, contributing radiative recombination of excitons [45–47]. The PL spectra recorded for Z-CS composites (figures 5(c)–(f)) varying CS concentration show presence of UV emission (i.e. ∼380 nm) along with broad band present in the visible region (i.e. ∼460 nm). Visible broad band is observed to increase with increase in concentration of CS in the Z-CS composite. The Z-CS composite with high concentration CS show prominent decrease in the relative intensity ratio of UV and visible peak emission. Considering Z-CS system as heterojunction system, formation of space charge region became obvious phenomenon. Formation of such region are responsible to suppress the recombination rate of photogenerated electron-hole pair, resulting in decrease of intensity ratio of UV and visible peak emission as observed [48]. Figure 6 presents Arrhenius plot extracted using room temperature UV–vis spectra recorded for each specimen. It is clear from the plot that ZnO and Z-CS composites are having strong absorption in the UV region.
Figure 6. Arrhenius plot for ZnO and Z-CS composites.
Download figure:
Standard image High-resolution imageIn order to extract the structural information from ZnO, CS and Z-CS composite materials synthesized at microscopic level, micro-Raman measurements are recorded at room temperature and presented as figure 7. Intense peaks positioned at 439.26 and 574 cm−1 as observed in figure 7(a) can be attributed to  phonon modes of ZnO respectively [49, 50]. The
phonon modes of ZnO respectively [49, 50]. The  frequency mode is sensitive to the stress produced in the crystal structure and can be used to comment on oxygen vacancies produced in ZnO if any [51, 52]. Hence shift and width associated with peak position is correlated either with change of isotopic masses of atoms constituting ZnO or with the presence of homogeneously distributed impurities and the quality of ZnO crystal respectively [53, 54].
frequency mode is sensitive to the stress produced in the crystal structure and can be used to comment on oxygen vacancies produced in ZnO if any [51, 52]. Hence shift and width associated with peak position is correlated either with change of isotopic masses of atoms constituting ZnO or with the presence of homogeneously distributed impurities and the quality of ZnO crystal respectively [53, 54].
Figure 7. Raman Spectra recorded for (a) ZnO, (b) CS and (c)–(f) for composites (Z-CS) having various concentration of CS.
Download figure:
Standard image High-resolution imageFigure 7(b) present micro Raman spectra recorded for CS which confirms presence of D (∼1320 cm−1) and G band (∼1580 cm−1) as expected. Presence of these bands can be attributed to distortions and tangential carbon stretching induced with CS [55]. The relative intensity ratio (i.e.  ) calculated form intensity of respective band confirm defects associated with CS structures [56]. Figures 7(c)–(f) present Raman spectra recorded of Z-CS composites prepared by varying CS concentration. With increasing CS concentration in Z-Cs composites the intensity of
) calculated form intensity of respective band confirm defects associated with CS structures [56]. Figures 7(c)–(f) present Raman spectra recorded of Z-CS composites prepared by varying CS concentration. With increasing CS concentration in Z-Cs composites the intensity of  mode is observed to decrease while, the intensity of G band is observed to increase. These result thus support the possible interaction of ZnO with CS forming relevant composite as mentioned earlier.
mode is observed to decrease while, the intensity of G band is observed to increase. These result thus support the possible interaction of ZnO with CS forming relevant composite as mentioned earlier.
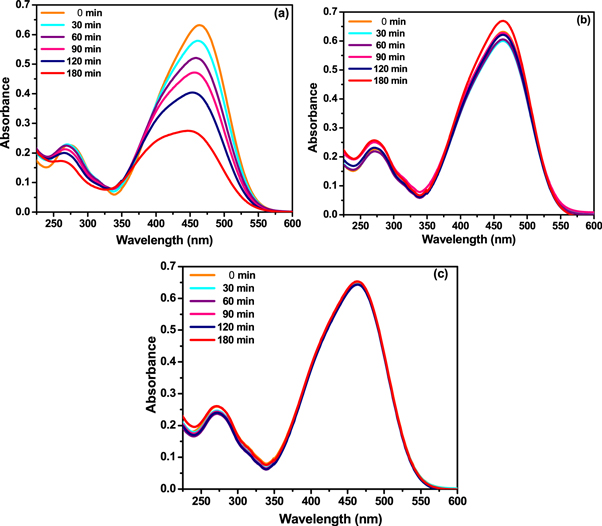
Photocatalytic activities associated with pristine ZnO and CS using methyl orange dye were investigated by recording UV–vis spectra in absorption mode. For this respective mixtures were exposed to constant UV light where changes linked with dye concentration were determined by recording UV–vis spectra at some time intervals (presented as figure 8). From figure 8(a) the photocatalytic activity of pristine ZnO is observed to be ∼56% while, no such activity is observed in the case of pristine CS (figure 8(b)). Figure 8(c) present blank test performed on 50 ml of aqueous solution of methyl orange (10 ppm), which confirms absence of dye molecule degradation due to UV source used.
Figure 8. Photocatalytic activity of (a) ZnO (b) CS and (c) Blank test for methyl orange dye molecule.
Download figure:
Standard image High-resolution imageSimilar investigation (figure 9) were performed using mixture of methyl orange and Z-CS composites in order to investigate the photocatalytic response. As compare with other Z-CS composites, it is observed that the composite synthesized using concentration of CS ∼100 mg (figure 9(c)) show the highest degradation efficiency (i.e. ∼87%). Only ∼25% degradation efficiency recorded in case of Z-CS composite synthesized using concentration of CS ∼300 mg is very surprising. This can be due to high amount of unreacted CS observed in the composite material, which are observed to embed ZnO rods blocking their active faces (i.e. polar surface), giving rise to scattering phenomenon [57].
Figure 9. Photocatalytic activity of Z-CS composites at different amount of CS. (a) 10, (b) 50, (c) 100 and (d) 300 mg.
Download figure:
Standard image High-resolution imageFigure 10 present the plot of  Vs time extracted from figures 8 and 9 i.e. photocatalytic activity recorded for ZnO and Z-CS composites at different amount. From figure 10 it is clear that the degradation studied follows the pseudo first-order kinetics [58, 59]. The reaction rate concentration extracted from each plots are listed as table 1.
Vs time extracted from figures 8 and 9 i.e. photocatalytic activity recorded for ZnO and Z-CS composites at different amount. From figure 10 it is clear that the degradation studied follows the pseudo first-order kinetics [58, 59]. The reaction rate concentration extracted from each plots are listed as table 1.
Figure 10. Plot of degradation rate for ZnO and Z-CS composite.
Download figure:
Standard image High-resolution imageTable 1. Photocatalyst degradation rate extracted for ZnO and Z-CS composite containing different amount of CS.
| Specimen | Regression Coefficient (R2) | Rate Constant (min−1) | (%) Efficiency |
|---|---|---|---|
| ZnO | 0.90516 | 0.00424 | 56 |
| ZCS 10 | 0.92472 | 0.00511 | 61 |
| ZCS 50 | 0.94845 | 0.0053 | 63 |
| ZCS 100 | 0.98417 | 0.01012 | 87 |
| ZCS 300 | 0.97401 | 0.0015 | 25 |
Figure 11 present the graph for Methyl Orange dye degradation using various ZnO based photocatalysts in comparison with our results (especially for Z-CS 100 composite). From the graph it is clear that the result obtained using environment friendly Z-CS composites are promising candidate for dye molecule degradation. The photocatalytic degradation of a dye molecule involves generation of free radicals followed by oxidization of a dye molecule leading towards its fragmentation [60]. Considering the mentioned fact herewith we propose a mechanism which can explain degradation of a dye molecule using Z-CS composite.
Figure 11. Graph indicating methyl orange dye degradation using various ZnO based photocatalysts in comparison with our result [61–64].
Download figure:
Standard image High-resolution imageThe metastable plane (i.e. polar face) of ZnO where, presence of high concentration defects such as 
 free electrons as well as surface defects are predominant and are treated to be chemically active [65, 66]. When the dye solution (i.e. methyl orange in DI water) comes in contact with the metastable plane of ZnO, enough energy is provided via quasi-one dimensional dynamics to water molecule to repeatedly associate and dissociate the molecule producing OH− species [67–69].
free electrons as well as surface defects are predominant and are treated to be chemically active [65, 66]. When the dye solution (i.e. methyl orange in DI water) comes in contact with the metastable plane of ZnO, enough energy is provided via quasi-one dimensional dynamics to water molecule to repeatedly associate and dissociate the molecule producing OH− species [67–69].
Parallel to this under continuous UV expose, ZnO present in the mixture (Z-CS composite + methyl orange + DI water) undergoes photo emission process [70, 71]. As shown in the schematics (figure 12) during excitation and de-excitation process the transfer of electrons from the valance band to conduction band and from there to various defect levels becomes an obvious phenomenon. Further accepting formation of Z-CS composite; it is anticipated that electrons from conduction band of ZnO can migrate to CS to achieve Fermi level  equilibrium. Parallel to this when the mixture is exposed to constant UV radiations, migration of electrons from CS to various defect levels of ZnO are also expected. Under these continuous electron migration processes conversation of
equilibrium. Parallel to this when the mixture is exposed to constant UV radiations, migration of electrons from CS to various defect levels of ZnO are also expected. Under these continuous electron migration processes conversation of  (present at the polar face of ZnO) to superoxide (i.e.
(present at the polar face of ZnO) to superoxide (i.e.  ) is proposed. Similarly the holes produced at the valence band of ZnO during the excitation process may react with OH− species (origin as discussed before) to convert it into super-hydroxyl
) is proposed. Similarly the holes produced at the valence band of ZnO during the excitation process may react with OH− species (origin as discussed before) to convert it into super-hydroxyl  radical. Highly unstable species (i.e.
radical. Highly unstable species (i.e.  and
and  ) generated then react with methyl orange dye molecule and are solely responsible for fragmentation of the dye molecule.
) generated then react with methyl orange dye molecule and are solely responsible for fragmentation of the dye molecule.
Figure 12. (a) Quantitative electronic band structure for ZnO/CS system and (b) schematic presentation of a dye molecule degradation process.
Download figure:
Standard image High-resolution image4. Conclusion
Simple and environmental friendly chemical bath route is demonstrated for the synthesis ZnO-Carbon (Z-CS) composites. Structural, microscopic and optical properties of synthesized materials were investigated using respective techniques. Photocatalytic activity of said materials were evaluated in order to study dye molecule degradation under constant UV expose. The Z-CS composites synthesized using CS concentration as 100 mg is observed to exhibit higher photocatalytic activity (i.e. 87%) as compare with the other composites synthesized. Generation of highly unstable species i.e.  at the polar face of ZnO due to various migration process of electrons is proposed. The unstable species generated are then suggested to reacts with methyl orange dye molecule leading towards its degradation. This study may explore the synthesis of low cost photocatalyst and understanding their photocatalytic mechanism for effective energy and environmental application.
at the polar face of ZnO due to various migration process of electrons is proposed. The unstable species generated are then suggested to reacts with methyl orange dye molecule leading towards its degradation. This study may explore the synthesis of low cost photocatalyst and understanding their photocatalytic mechanism for effective energy and environmental application.