Abstract
In order to improve the mechanical and tribological properties of epoxy composites, the hyperbranched polyesters with terminal carboxyl (HBP) were used for covalent functionalization of multi-walled carbon nanotubes (MWCNTs). The dispersibility and wettability of functionalized MWCNTs (HBP-NH2-MWCNTs) in epoxy resin (EP) were analyzed. The HBP-NH2-MWCNTs reinforced EP composites (HBP-NH2-MWCNTs/EP) were prepared. The microhardness and fracture toughness of HBP-NH2-MWCNTs/EP were tested, and the tribological behavior and mechanism under dry friction conditions were investigated. The results show that the hyperbranched polyesters coated on the surface of MWCNTs forms a physical barrier layer, blocking the van der Waals force between MWCNTs, effectively reducing the agglomeration phenomenon and significantly improving its dispersibility and wettability. Meanwhile, The flexible hyperbranched polyester on the surface of MWCNTs alleviates the stress concentration of the interface and improves the load transfer efficiency, which greatly improves the microhardness and fracture toughness. Under dry friction conditions, the friction coefficient and wear loss of HBP-NH2-MWCNTs/EP composites showed a trend of decreasing first and then increasing as the content of HBP-NH2-MWCNTs increased from 0.3 phr to 1.2 phr. When the content of HBP-NH2-MWCNT is 0.6 phr, the composites has the lowest friction coefficient and the wear loss, and the wear mechanism is mainly characterized by slight fatigue wear.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Epoxy resin (EP) is frequently used as a composite infiltrating matrix in many important fields such as aerospace, weaponry and ship transportation due to its machinability, chemical corrosion resistance, thermal stability and electrical insulation [1–4]. However, the three-dimensional network structure formed by curing the epoxy resin has an excessively high crosslink density, which causes serious problems such as brittleness and cracking. It is difficult to meet the operational requirements in some extreme environments, which limits its further application and development [5–9]. Carbon nanotubes (CNTs) are widely used as ideal reinforcing fillers in the preparation of high performance polymer matrix composites due to their excellent mechanical properties, good chemical and thermal stability. These years, there have been many investigations shown that CNTs can effectively ameliorate the mechanical and tribological properties of resin matrix [10–14]. However, because of the van der Waals force interaction between CNTs, they are usually entangled with each other, and the existing blending technology is difficult to achieve uniform dispersion of CNTs in the organic phase. Moreover, the compatibility of CNTs with polymers and organic phases is poor, resulting in weak interfacial adhesion and low load transfer efficiency between CNTs and polymer matrix, which severely limits the application field and reinforcement effect of the composites. Therefore, how to solve the uniform distribution of CNTs in the polymer matrix and enhance the interfacial adhesion between the CNTs and the resin to fully exert its reinforcing effect is an important issue to be solved.
The functionalization of CNTs has provided a broad prospect for its application in resin matrix composites. Recently, the reports on the study of functionalization of CNTs have been increasing [15–18]. According to the bonding type between the modified molecule and CNTs, the functionalization methods are divided into covalent functionalization and non-covalent functionalization. Covalent functionalization refers to the introduction of reactive groups such as carboxyl groups and hydroxyl groups at the sidewalls and end caps of CNTs by redox reaction. Further, these functional groups are used as reaction sites for further modification [19–21]. Non-covalent functionalization means that the modified molecular chains are adsorbed onto the CNTs by π–π stacking, hydrogen bonding or electrostatic interaction to improve the dispersibility and compatibility of the CNTs in the matrix. However, due to the weak force of the non-covalent bond, the composites usually have a low transfer load efficiency, which limits the reinforcement effect of the composites [22, 23].
Hyperbranched polymers show great potential for application in functionalized CNTs because of their unique highly branched three-dimensional molecular structure. Compared with linear polymers, hyperbranched polymers with three-dimensional structure have lower solution viscosity, better solubility and higher functional group density [24]. However, the current researches on hyperbranched polymer functionalized CNTs are usually limited to investigate their solubility and dispersibility only in organic solvents. There is few report on their dispersion behavior in epoxy resin, and the researches on the interfacial properties of hyperbranched polymer functionalized CNTs modified polymer matrix composites are still not deep enough. Moreover, the study on the tribological properties and mechanisms of hyperbranched polymer functionalized CNTs in the epoxy resin matrix has not been reported.
In this paper, in order to prepare epoxy composites with excellent mechanical and tribological properties, the hyperbranched polyesters with terminal carboxyl (HBP) were used for covalent functionalization of multi-walled carbon nanotubes (MWCNTs) and the HBP-NH2-MWCNTs were prepared successfully. The dispersibility, interfacial energy and adhesion work of HBP-NH2-MWCNTs in EP were investigated. Then HBP-NH2-MWCNTs reinforced EP composites (HBP-NH2-MWCNTs/EP) were prepared. The microhardness and fracture toughness of HBP-NH2-MWCNTs/EP were tested, and the tribological properties were systematically analyzed, which provided a theoretical basis for the application of HBP-NH2-MWCNTs/EP composites.
2. Materials and methods
2.1. Materials
MWCNTs (purity>95%) with a mean diameter of 20–30 nm and length of 0.5–2 μm were purchased from Nanjing XFNANO Materials Tech Co., Ltd, China. The COOH-HBP used in this study was supplied by Hyperbranched Resin Tech Co., Ltd, China. The epoxy resin (epoxy equivalent: 185–192 g/eq) is from Jiangyin Shunsheng Composite Material Co., Ltd, China. The curing agent used in this work was QS-1622 supplied by Qingdaqishi Materials Co., Ltd, China. Sulfuric acid H2SO4 (98%), nitric acid HNO3 (68%), ethylenediamine (EDA), Tetrahydrofuran (THF), thionyl chloride (SOCl2), dicyclohexylcarbodiimide (DCC) and ethanol were purchased from Aladdin Biotechnology Co., Ltd, China.
2.2. Preparation of functionalized MWCNTs
The process of MWCNTs functionalization is shown in scheme
- 1.100 mg of p-MWCNTs was mixed with 120 ml of mixture solution of H2SO4 and HNO3 (H2SO4:HNO3 = 3:1). The reaction was carried out under an ultrasonic water bath at 50 °C for 3 h. After the reaction was completed, the mixture was allowed to stand for 30 min. Then the MWCNTs were filtered through a filter and centrifuged repeatedly with absolute ethanol. Finally, COOH-MWCNTs were obtained by vacuum drying.
- 2.COOH-MWCNTs were mixed with excess SOCl2 and sonicated in a water bath and heated at 70 °C for 24 h. Then the unreacted SOCl2 was removed by reduced pressure distillation. The resulting product was denoted as COCl-MWCNTs.
- 3.10 ml of EDA was added to a flask containing 50 ml of THF and sonicated for 10 min. Then COCl-MWCNTs was dispersed in 20 ml of THF and added dropwise to the flask in an ice bath. The solution was then mechanically stirred and sonicated for 24 h at room temperature. Then, the MWCNTs were filtered through a filter and centrifuged repeatedly with absolute ethanol. Finally, NH2-MWCNTs were obtained by vacuum drying.
- 4.100 mg of COOH-HBP and 20 mg of NH2-MWCNTs were added to 50 ml of THF. Then, an appropriate amount of DCC was added and sonicated for 2 h. After sonicating, the MWCNTs were filtered through a filter and centrifuged repeatedly with absolute ethanol. Finally, HBP-NH2-MWCNTs were obtained by vacuum drying.
Scheme 1. Synthetic Route of HBP-NH2-MWCNTs.
Download figure:
Standard image High-resolution image2.3. Preparation of MWCNTs/EP composites
The HBP-NH2-MWCNTs/EP were prepared by casting method. The steps are as follows:
- 1.100 mg of HBP-NH2-MWCNTs were dispersed in 100 ml of THF by sonication at 40 °C for 1 h. The product obtained was the dispersion of HBP-NH2-MWCNTs/THF.
- 2.Pour the appropriate amount of epoxy resin into a clean container and then add an appropriate amount of HBP-NH2-MWCNTs/THF dispersion. The reaction was carried out under an ultrasonic water bath condition of 60 °C for 2 h while stirring at a high speed of 2000 rpm with a stirrer for 3–4 h.
- 3.The dispersed mixture was placed in a vacuum desiccator and subjected to solvent removal treatment by a vacuum pump for 24 h. Then the curing agent were added and stirred for 5–10 min to fully react. Next, the mixture was evacuated at 70 °C for 1 h and then cast into a mold. Finally, it was placed in an oven at 80 °C for 2 h and cured at room temperature for 24 h to obtain the samples. In addition, the pure EP, p-MWCNTs/EP and NH2-MWCNTs/EP samples were also prepared as comparative, which is listed in table 1.
2.4. Characterization
The FITR spectra was measured by the PerkinElmer Spectrum Two, Waltham, MA, USA, and the wavenumber range was 400–4000 cm–1. Thermo gravimetric analysis (TGA) of the fillers and composites was conducted by Mettler Toledo SDTA851 with a heating rate of 10 °C min−1 under nitrogen atmosphere. The fractured surfaces of composites sprayed with gold was examined using a scanning electron microscope (SEM, Hitachi S4800, Japan). The dispersion state of MWCNTs was examined by a transmission electron microscopy (TEM, HITACHI H-7650). The contact angle of MWCNTs film with EP was measured by automatic contact angle measuring instrument (OCA20, Germany). The MWCNTs film was prepared by filtration of a MWCNTs/THF dispersion with a content of 1 mg ml−1, and the filter membrane was a polytetrafluoroethylene filter with a pore size of 0.45 μm.
The D-type tester was used to measure the Shore hardness. The fracture properties of the epoxy composite were tested according to ASTM D5045-99 with a sample size of 50 × 10 × 5 mm3. The experimental loading rate was 3 mm min−1 with a temperature of 24 °C. Furthermore, at least 5 samples should be tested for the same component. The formula of fracture toughness (KIC) and critical energy release rate (GIC) is as follows [25]:
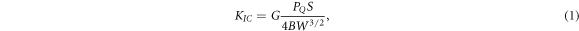
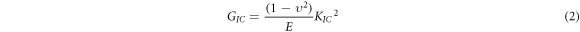
where, G is the crack shape factor, S is the span, and B, W, and E are the sample thickness, width, and elastic modulus, respectively. Both the EP and its composites have a Poisson's ratio of 0.35 [26].
The tribological properties of materials were measured by Tribological Testing Machine (HT-500). The rotating speed of the friction wear test was 200 r min−1, and the constant load was applied by 2.5, 5, 7.5 and 10 N. In addition, the test duration was 30 min with the 3 mm rotation radius. The upper sample was a Φ4 mm GCr15 steel ball with a surface roughness Ra = 0.02∼0.04 μm. The lower samples were EP, p-MWCNTs/EP, NH2-MWCNTs/EP and HBP-NH2-MWCNTs/EP composites. The friction coefficient is measured by the Tribological Testing Machine with its own software. The wear loss was measured using an analytical balance (ABT120-4M, KERN, Germany) with an accuracy of 0.1 mg, at least 5 samples should be tested for the same component. The three-dimensional profile was determined by an optical profiler Bruker Contour GT-K0.
3. Results and discussion
3.1. Characterization of the functionalized MWCNTs
The FITR spectra of HBP and functionalized MWCNTs are shown in figure 1. Figure 1(a) shows the FTIR spectra of HBP. It can be found that the stretching vibration peak of methylene is in the range of 2853–2955 cm−1. The peak in the range of 1495–1611 cm−1 is the stretching vibration of C=C in the benzene ring. The peak at 1639 cm−1 is the C=O stretching vibration peak from the carboxylic acid, and the peak at the range of 2520–2640 cm−1 are ascribed to the stretching vibration of O-H. The FTIR spectra of p-MWCNTs, NH2-MWCNTs and HBP-NH2-MWCNTs are shown in figure 1(b). It can be found that the infrared spectrum of p-MWCNTs shows a distinct peak around 1586 cm−1 due to the presence of graphite structure in the carbon nanotubes. At the same time, hydroxyl vibration peaks are observed in the range of 3200–3800 cm−1, which may be due to the adsorption peak of water molecules in the air or the introduction of hydroxyl groups during the purification of the original carbon nanotubes. Compared with the p-MWCNTs, the NH2-MWCNTs showed a C=O stretching vibration peak (secondary amide I) at 1707 cm−1. A mixed absorption peak of N–H bending vibration (secondary amide II) and C–N stretching vibration (secondary amide III) appeared at 1605 cm−1. At the same time, there is a distinct methylene peak at 2853–2926 cm−1. In addition, the N–H peak of the primary amide at 3280 cm−1 overlaps with the O–H peak, resulting in a larger peak. This result preliminarily showed that the surface of MWCNTs was successfully grafted with ethylenediamine. For the FITR spectrum of HBP-NH2-MWCNTs, the peak at 3280 cm−1 decreases compared to NH2-MWCNTs. This is because the carboxyl function of HBP reacts with the primary amine and the stretching peak of the primary amine disappears. Meanwhile, the stretching vibration peak of C=O on the HBP carboxylic acid group was produced at 1639 cm−1. The new absorption peaks indicated that HBP was grafted and adsorbed on NH2-MWCNTs, and HBP-NH2-MWCNTs were successfully prepared.
Figure 1. FITR spectra of HBP and functionalized MWCNTs. (a) HBP; (b) MWCNTs.
Download figure:
Standard image High-resolution imageTable 1. Composition of the epoxy composites.
| Series | Matrix (phr) | p-MWCNTs (phr) | NH2-MWCNTs (phr) | HBP-NH2-MWCNTs (phr) |
|---|---|---|---|---|
| Neat epoxy | 100 | — | — | — |
| p-MWCNTs/EP | 100 | 0.6 | — | — |
| NH2-MWCNTs/EP | 100 | — | 0.6 | — |
| HBP-NH2-MWCNTs/EP | 100 | — | — | 0.3 |
| 100 | — | — | 0.6 | |
| 100 | — | — | 0.9 | |
| 100 | — | — | 1.2 |
The TGA and DTG curves of HBP are shown in figure 2. It can be seen that HBP produces a sharp decomposition near 450 °C (the DTG curve showed a peak at 450 °C) and stabilizes at 9.59 wt% in the range of 500 °C–700 °C. There is about 90.41 wt% of HBP decomposed. Figure 3 shows the TGA and DTG curve of MWCNTs before and after functionalized modification. As can be seen from figure 3(a), the thermal weight loss of p-MWCNTs is only 2.05 wt%, which shows good thermal stability when the temperature was raised from 24 °C to 700 °C. In contrast, the weight loss of NH2-MWCNTs in the range of 24 °C–700 °C is about 7.43 wt%, which is due to the decomposition of EDA grafted on the surface of MWCNTs. The TGA curve of HBP-NH2-MWCNTs shows that the thermal weight loss in the range of 24 °C–700 °C is about 21.63%, and it also produced a sharp decomposition at 450 °C (the DTG curve showed a peak at 450 °C in figure 3(b)). This is mainly due to the decomposition of the surface grafted HBP.
Figure 2. TGA and DTG results of HBP.
Download figure:
Standard image High-resolution imageFigure 3. TGA and DTG results of MWCNTs. (a) TGA; (b) DTG.
Download figure:
Standard image High-resolution image3.2. The dispersibility of functionalized MWCNTs in organic solvent and EP matrix
The good dispersion of MWCNTs in organic solvents (THF) is essential to further achieve uniform dispersion of MWCNTs in the EP matrix. Therefore, the dispersibility of p-MWCNTs, NH2-MWCNTs and HBP-NH2-MWCNTs in the organic solvent (THF) was investigated by TEM image, as shown in figure 4. According to the TEM image, a large number of p-MWCNTs held together forming agglomerations (figure 4(a)). In contrast, the dispersibility of NH2-MWCNTs and HBP-NH2-MWCNTs was significantly improved. In particular, HBP-NH2-MWCNTs coated with hyperbranched polyester were uniformly distributed in an organic solvent without agglomerations. This is because carbon nanotubes modified by hyperbranched polyesters have a remarkable core–shell structure, in which the hyperbranched polyesters were used as a shell to form a physical barrier layer. The van der Waals force between the MWCNTs is blocked, and the agglomeration phenomenon between the carbon nanotubes is greatly reduced [27]. Hence, the dispersion of HBP-NH2-MWCNTs have been significantly improved.
Figure 4. TEM images of MWCNTs dispersed in THF. (a) p-MWCNTs in THF; (b) NH2-MWCNTs in THF; (c) HBP-NH2-MWCNTs in THF.
Download figure:
Standard image High-resolution imageIn order to further study the dispersion state of MWCNTs in EP matrix, ultrathin sections of epoxy composites were observed by TEM, and the results are shown in figure 5. It can be seen that in p-MWCNTs/EP, many p-MWCNTs are entangled and a serious agglomeration occurs (figure 5(a)). In contrast, the dispersibility of NH2-MWCNTs and HBP-NH2-MWCNTs in EP matrix is improved. In particular, HBP-NH2-MWCNTs exhibits more excellent dispersibility in the EP matrix without agglomeration and entanglement (figure 5(c)). This is because the functionalization makes the hyperbranched polyesters (HBP) grafted and coated on the surface of MWCNTs (HBP-NH2-MWCNTs), blocking the van der Waals force between MWCNTs, and realizing the uniform dispersion of MWCNTs in EP matrix, which will help to give full play to the reinforcing and toughening effect of epoxy resin.
Figure 5. TEM image of the dispersed state of functionalized MWCNTs in EP matrix. (a) 0.6 phr p-MWCNTs/EP; (b) 0.6 phr NH2-MWCNTs/EP; (c) 0.6 phr HBP-NH2-MWCNTs/EP.
Download figure:
Standard image High-resolution image3.3. The wettability and interfacial properties between HBP-NH2-MWCNTs and EP
The contact angle is an important index to characterize the wetting properties of MWCNTs and EP matrix. Therefore, the wettability between MWCNTs and EP was investigated by contact angle test of p-MWCNTs, NH2-MWCNTs and HBP-NH2-MWCNTs. The measurement results are shown in table 2. It can be seen that the contact angle of p-MWCNTs with EP is 69.2°. After functionalization, the contact angles of NH2-MWCNTs and HBP-NH2-MWCNTs with EP are reduced to 60.7° and 41.5°, respectively. In particular, the wettability of HBP-NH2-MWCNTs are significantly improved. Therefore, the functionalization makes the hyperbranched polyester (HBP) grafted and coated on the surface of MWCNTs (HBP-NH2-MWCNTs), which can greatly improve the wettability. This will be beneficial to improve the mechanical properties of epoxy composites.
Table 2. Contact angles of EP droplets on MWCNTs.
| p-MWCNTs | NH2-MWCNTs | HBP-NH2-MWCNTs | |
|---|---|---|---|
| Contact angle (°) |

|

|

|
Adhesion work is used to measure the energy required for interface separation. It can indicate the bond strength of the solid-liquid interface. In order to further study the interfacial properties of MWCNTs and EP matrix, the adhesion work was calculated based on the Young-Dupre equation [28]:
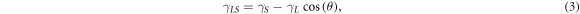
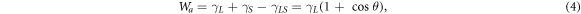
where, 
 and
and  are the surface energy of the solid, liquid and of the liquid-solid interface. Wa is the adhesion work of the liquid-solid interface.
are the surface energy of the solid, liquid and of the liquid-solid interface. Wa is the adhesion work of the liquid-solid interface.
According to the surface energy component method of Owens and Wendt [29], the liquid-solid interfacial energy can be expressed in the equation (5):
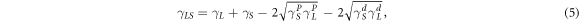
where,  and
and  are the polar components and nonpolar component of the surface energy of solid, respectively.
are the polar components and nonpolar component of the surface energy of solid, respectively.  and
and  are the polar components and nonpolar component of the surface energy of liquid, respectively. In which:
are the polar components and nonpolar component of the surface energy of liquid, respectively. In which:
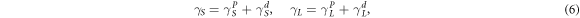
Based on the equations (3), (5) and (7) can be obtained:
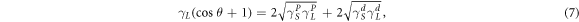
In this study, water and diiodomethane can be used to calculate the solid surface energy of MWCNTs due to the large difference in surface energy composition. Previous studies have shown that the surface energy of EP is 46 mJ m−2 [30]. The  and
and  of water are 21.8 mJ m−2 and 51 mJ m−2 and those of diiodomethane are 50.8 mJ m−2 and 0 mJ m−2 [31]. The calculation results are shown in table 3. It can be seen that HBP-NH2-MWCNTs have the largest surface energy, which mainly benefits from the increase of the polar component. The polar component of surface energy is caused by hydrogen bonding and dipole-dipole interaction [32]. After functionalization, the secondary amide group and the primary amine group on the surface of HBP-NH2-MWCNTs can enhance hydrogen bonding and dipole-dipole interaction, resulting in a significant increase in surface energy.
of water are 21.8 mJ m−2 and 51 mJ m−2 and those of diiodomethane are 50.8 mJ m−2 and 0 mJ m−2 [31]. The calculation results are shown in table 3. It can be seen that HBP-NH2-MWCNTs have the largest surface energy, which mainly benefits from the increase of the polar component. The polar component of surface energy is caused by hydrogen bonding and dipole-dipole interaction [32]. After functionalization, the secondary amide group and the primary amine group on the surface of HBP-NH2-MWCNTs can enhance hydrogen bonding and dipole-dipole interaction, resulting in a significant increase in surface energy.
Table 3. Contact angles of water and diiodomethane on MWCNTs and surface energy of MWCNTs.
| Contact angle (°) | Surface energy (mJ m−2) | ||||
|---|---|---|---|---|---|
| Water | Diiodomethane |
|
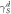
|
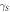
|
|
| p-MWCNTs | 72.5 | 38.6 | 6.14 | 40.30 | 46.44 |
| NH2-MWCNTs | 66.7 | 34.2 | 8.16 | 42.40 | 50.56 |
| HBP-NH2-MWCNTs | 31.5 | 26.1 | 5.21 | 45.75 | 70.96 |
The surface energy of the MWCNTs obtained in table 3 is substituted into the equations (3) and (4) to calculate the interfacial energy and adhesion work of the MWCNTs and the EP matrix, respectively. The results are shown in table 4. It can be seen that the adhesion work of p-MWCNTs and EP is 63.08 mJ m−2. After functionalization, the adhesion work of NH2-MWCNTs and HBP-NH2-MWCNTs with EP is significantly higher than that of p-MWCNTs. In particular, the adhesion work of HBP-NH2-MWCNTs and EP is up to 80.51 mJ m−2, which is about 27.63% higher than that of p-MWCNTs. In addition, the interfacial energy of HBP-NH2-MWCNTs and EP (37.32 mJ m−2) is 27.1% higher than that of p-MWCNTs. This indicates that the strongest bonding interface between HBP-NH2-MWCNT and EP is formed. This is because the functionalized carbon nanotubes (HBP-NH2-MWCNTs) have good wettability, which can effectively reduce the interface defects caused by poor wettability and improve the interfacial bonding strength.
Table 4. Adhesion work and interfacial energy of EP droplets on MWCNTs.
| p-MWCNTs | NH2-MWCNTs | HBP-NH2-MWCNTs | |
|---|---|---|---|
| Adhesion work (mJ m−2) | 63.08 | 66.38 | 80.51 |
| Interfacial energy (mJ m−2) | 29.36 | 30.20 | 37.32 |
3.4. Mechanical properties of functionalized MWCNTs reinforced epoxy composites
3.4.1. Microhardness
Hardness is the ability of a material to resist local plastic deformation or damage and is a comprehensive index of the mechanical properties for a material. The Shore hardness of EP and MWCNTs/EP are shown in figure 6. It can be seen that HBP-NH2-MWCNTs have the best enhancement effect on epoxy composites (HBP-NH2-MWCNTs/EP). Moreover, the Shore hardness of HBP-NH2-MWCNTs/EP showed a trend of increasing first and then decreasing with the increase of HBP-NH2-MWCNTs content. When the HBP-NH2-MWCNTs content is 0.6 phr, the Shore hardness of the HBP-NH2-MWCNTs/EP composite reaches the maximum value of 83.4 HD, which is increased by 38.07%, 3.73% and 2.58% than that of pure EP (60.4HD), 0.6 phr p-MWCNTs/EP (80.4HD) and 0.6 phr NH2-MWCNTs/EP (81.3HD), respectively. On the one hand, the strong interfacial adhesion between HBP-NH2-MWCNTs and EP (table 4) is beneficial to synergistically improve the strength of EP cross-linking system [33]. On the other hand, 0.6 phr HBP-NH2-MWCNTs was uniformly dispersed in EP without agglomeration and entanglement, which greatly improved the load transfer efficiency, so the Shore hardness value of 0.6 phr HBP-NH2-MWCNTs/EP is the biggest.
Figure 6. Shore hardness of functionalized MWCNTs reinforced epoxy composites.
Download figure:
Standard image High-resolution image3.4.2. Fracture toughness
The fracture toughness is an important index to characterize the ability of materials to withstand crack generation and propagation. The fracture toughness results of the different types of MWCNTs/EP samples are shown in figure 7. It can be seen that all types of MWCNTs can increase the KIC and GIC of the EP matrix. Particularly, epoxy composites reinforced by HBP-NH2-MWCNTs have better toughening effects than the others. the KIC and GIC of 0.6 phr HBP-NH2-MWCNTs/EP composites reach 1.23 MPa·m1/2 and 0.35 kJ m−2, respectively, which is 89.2% and 169.2% higher than pure EP. The reasons may be illustrated by the following two aspects. Firstly, HBP-NH2-MWCNTs/EP can achieve effective stress transfer due to the good dispersion and strong interfacial properties between HBP-NH2-MWCNTs and EP (Adhesive work: 80.51 mJ m−2, interface energy: 37.32 mJ m−2). Secondly, the flexible hyperbranched polyester layer on the surface of the HBP-NH2-MWCNTs may mitigate the stress concentration at the interface by deformation, making HBP-NH2-MWCNTs bear load more uniformly. So the 0.6 phr HBP-NH2-MWCNTs composites exhibit the best toughening effect.
Figure 7. Fracture toughness of EP and MWCNTs/EP. (a) 0.6 phr MWCNTs/EP; (b) HBP-NH2-MWCNTs/EP.
Download figure:
Standard image High-resolution image3.5. Tribological behavior and mechanism of MWCNTs/EP
3.5.1. Tribological behavior
Figure 8 shows the variation of friction coefficient and wear loss of functionalized MWCNTs reinforced epoxy composites. The upper sample was a Φ4 mm GCr15 steel ball with a surface roughness Ra = 0.02∼0.04 μm. The lower samples were EP, p-MWCNTs/EP, NH2-MWCNTs/EP and HBP-NH2-MWCNTs/EP composites. Under dry friction conditions, the friction coefficient of HBP-NH2-MWCNTs/EP composites varied from 0.255 to 0.605 (figure 8(a)). In particular, the 0.6 phr HBP-NH2-MWCNTs/EP composite has the lowest friction coefficient of 0.255, which is 64.8%, 40% and 36.23% lower than that of pure EP (0.725), 0.6 phr p-MWCNTs/EP (0.425) and 0.6 phr NH2-MWCNTs/EP (0.4), respectively. The trend of wear loss is the same as that of friction coefficient (figure 8(b)). As the HBP-NH2-MWCNTs content increased from 0.3 phr to 0.6 phr, the wear loss of the HBP-NH2-MWCNTs/EP composite decreased from 1.3 mg to 0.7 mg. However, when the content of HBP-NH2-MWCNTs increased to 1.2 phr, the wear loss of the composites increased to 1.7 mg. Similarly, the wear loss of 0.6 phr HBP-NH2-MWCNTs/EP composites are the lowest, which are 73.1%, 36.4% and 22.2% lower than that of pure EP (2.6 mg), 0.6 phr p-MWCNTs/EP (1.1 mg) and 0.6 phr NH2-MWCNTs/EP (0.9 mg), respectively.
Figure 8. Friction coefficient and wear loss of MWCNTs/EP composites. (a) 0.6 phr MWCNTs/EP; (b) HBP-NH2-MWCNTs/EP. (Load: 5 N, sliding speed: 200 r min−1, time: 30 min).
Download figure:
Standard image High-resolution imageFigure 9 shows the three-dimensional cross-section images of wear tracks of EP and MWCNTs/EP composites and the upper sample was a Φ4 mm GCr15 steel ball with a surface roughness Ra = 0.02∼0.04 μm. It can be seen that the wear surface of pure EP is uneven, and the width and depth of the wear scar are respectively 1645.65 μm and 234.76 μm, and the edges are irregular, accompanied by a large number of microcracks. In contrast, the wear surface of the composites reinforced by MWCNTs become smooth, the edges are flat, and the width and depth of the wear scars are significantly reduced. In particular, HBP-NH2-MWCNTs reinforced epoxy composites exhibit excellent tribological properties. It can be seen from figures 9(d)–(g) that the wear scar width and depth of HBP-NH2-MWCNTs/EP composites show a trend of decreasing first and then increasing with the content increasing (0.3 phr∼1.2 phr). In addition, the width and depth of wear scars of 0.6 phr HBP-NH2-MWCNTs/EP composites are the smallest, which are 83.72 μm and 9.50 μm, respectively. Compared with pure EP, they are reduced by 49.52% and 95.95%, respectively.
Figure 9. Three-dimensional cross-section images of wear tracks. (a) EP; (b) 0.6 phr p-MWCNTs/EP; (c) 0.6 phr NH2-MWCNTs/EP (d) 0.3 phr HBP-NH2-MWCNTs/EP (e) 0.6 phr HBP-NH2-MWCNTs/EP (f) 0.9 phr HBP-NH2-MWCNTs/EP (g) 1.2 phr HBP-NH2-MWCNTs/EP. (Load: 5 N, sliding speed: 200 r min−1, time: 30 min).
Download figure:
Standard image High-resolution image3.5.2. Tribological mechanism
The wear surface of EP was observed by SEM, as shown in figure 10, in which the upper sample was a Φ4 mm GCr15 steel ball with a surface roughness Ra = 0.02∼0.04 μm. It can be seen that a large number of brittle cracks and micro-tears appear on the surface of the pure EP. In addition, the wear surface has formed obvious layered structure and produced a large number of flake-like shedding (figure 11), which shows severe micro-brittle fracture wear. This is because pure epoxy resin is brittle and has poor tribological properties under the action of periodic friction.
Figure 10. SEM image of the wear surface of EP.
Download figure:
Standard image High-resolution imageFigure 11. SEM image of the wear surface of p-MWCNTs/EP.
Download figure:
Standard image High-resolution imageFor the p-MWCNTs reinforced epoxy composites, the wear surface morphology with the Φ4 mm GCr15 steel ball is shown in figure 11. It can be seen that the wear surface of p-MWCNTs/EP is relatively flat compared with pure EP. There are a large number of small scale-like structures along the sliding direction, accompanied by some microcracks. In addition, there are some traces of deformation and adhesion distributed on the wear surface. The brittle fracture wear condition is significantly improved. The wear mechanism is characterized by adhesive wear, accompanied by fatigue wear. Firstly, this is due to the high hardness of MWCNTs, which plays the role of preferential load-bearing in the resin matrix. Secondly, under the action of external loads, the thin transfer film formed between the steel ball and the resin matrix due to the exfoliated of carbon nanotubes with self-lubrication characteristics, resulting in a smaller friction coefficient between the friction pairs and playing a good lubrication role. Thirdly, in the process of friction, the microhardness and fracture toughness of composites with MWCNTs are higher than those of pure EP (figures 6, 7), which can resist the extrusion and friction of steel balls and show better tribological properties. However, p-MWCNT has a serious agglomeration in the resin matrix (figure 5(a)), resulting in weak interfacial properties (table 4), which severely limits the enhancement on the tribological properties of epoxy resin.
Figure 12 shows the wear surface morphology of the HBP-NH2-MWCNTs/EP composites, in which the upper sample was a Φ4 mm GCr15 steel ball with a surface roughness Ra = 0.02∼0.04 μm. It can be seen that the 0.6 phr HBP-NH2-MWCNTs reinforced composites have a flat wear surface with only a small amount of slight fatigue cracks (figure 12(b)). The wear mechanism is mainly characterized by slight fatigue wear. This is mainly because the flexible hyperbranched polyester layer is firmly coated on MWCNTs and forms strong interfacial adhesion with EP matrix. When subjected to concentrated stress, the flexible hyperbranched polyester layer can effectively withstand partial stress, prevent MWCNTs from being destroyed and pulled out, and greatly improve the tribological properties of the composites.
Figure 12. SEM image of the wear surface of HBP-NH2-MWCNTs/EP composites (a) 0.3 phr HBP-NH2-MWCNTs/EP (b) 0.6 phr HBP-NH2-MWCNTs/EP (c) 0.9 phr HBP-NH2-MWCNTs/EP (d) 1.2 phr HBP-NH2-MWCNTs/EP (Load: 5 N, sliding speed: 200 r min−1, time: 30 min).
Download figure:
Standard image High-resolution imageThe content of HBP-NH2-MWCNTs has an important influence on the tribological properties of HBP-NH2-MWCNTs/EP composites. When the content of HBP-NH2-MWCNTs is less than 0.6 phr, the tribological properties of HBP-NH2-MWCNTs/EP composites are decreased. Observed from the wear surface of 0.3 phr HBP-NH2-MWCNTs/EP composites (figure 12(a)), there are a large number of microcracks, and some pits formed by flaking. This is because the content of HBP-NH2-MWCNTs dispersed in the resin matrix is too low (figure 12(a), the TEM of HBP-NH2-MWCNTs/EP slice), resulting in weak load-bearing effect. Meanwhile, the enhancement effect on the microhardness and fracture toughness of composite materials is not obvious. The wear mechanism is mainly characterized by microscopic brittle fracture wear, accompanied by certain fatigue wear.
However, when the content of HBP-NH2-MWCNTs is larger than 0.6 phr, the tribological properties of the composites also decreases. Figure 12(c) shows the wear surface morphology of 0.9 phr HBP-NH2-MWCNTs/EP composites. It can be seen that the wear scar of the composites is widened and there are a large number of scaly concave and convex structures on the surface. This is because 0.9 phr HBP-NH2-MWCNTs in the resin matrix have a agglomeration and entanglement phenomenon, causing local stress concentration. In particular, when the content of HBP-NH2-MWCNTs increase to 1.2 phr, the wear surface of the composites become more rough and there are a large number of microcracks and large-sized pits (figure 12(d)). This is because 1.2 phr HBP-NH2-MWCNTs are agglomerated seriously (figure 12(d): the TEM of HBP-NH2-MWCNTs/EP slice), resulting in easily detachment of MWCNTs from the matrix. So the tribological properties of the composites are degraded. The wear mechanism changes to severe fatigue wear, accompanied by certain microscopic brittle fracture wear.
In addition, 0.6 phr HBP-NH2-MWCNTs/EP composites were used as examples to study the wear surface morphology and mechanism under heavy load (10 N) conditions. It can be seen that the wear surface becomes rough than that under light load (5 N). The phenomenon of delamination was obvious, and there were signs of adhesion and tear (figure 13). The wear mechanism is mainly characterized by fatigue wear and adhesive wear. This is because the increase of load will lead to the rapid increase of contact stress on the wear surface. The contact radius and contact stress can be calculated by Hertz contact formula [34]:
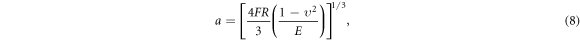
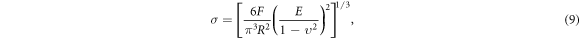
where F is the normal load, R is the radius of GCr15 steel ball, E is the elastic modulus of composites, and v is the Poisson's ratio of composites.
Figure 13. SEM image of the wear surface of 0.6 phr HBP-NH2-MWCNTs/EP composite under 10 N.
Download figure:
Standard image High-resolution imageThe contact stresses on the wear surfaces of HBP-NH2-MWCNTs/EP composites under different loads are listed in table 5. It can be seen that the calculated value of contact stresses reaches 185.32 MPa when the load is 10 N. Under high contact stress, the plastic deformation of composites is serious, which makes it easy to cause adhesion between composites and steel balls, resulting in adhesive wear of composites during relative sliding.
Table 5. Contact stress of wear surface under different load conditions.
| Load (N) | 2.5 | 5 | 7.5 | 10 |
|---|---|---|---|---|
| Contact radius (mm) | 0.10 | 0.21 | 0.32 | 0.41 |
| Contact stress (MPa) | 116.71 | 147.09 | 168.37 | 185.32 |
Combined with the wear surface morphology of the different MWCNTs/EP composites observed in figures 11–13, the wear mechanism of MWCNTs in the ideal dispersion state is disclosed. The schematic diagram is shown in figure 14. In the process of friction, the wear of EP matrix on the surface was first observed, and the uniformly dispersed MWCNTs in the matrix were exposed (figure 14(a)). Because of its high hardness, MWCNTs in the matrix play the role of preferential load bearing. In addition, the functionalized MWCNTs have strong interfacial adhesion with resin matrix and high load transfer efficiency. As the test progresses, part of the carbon nanotubes breaks and peels off under the action of periodic friction, forming a thin lubricating film between the steel ball and the resin matrix, which plays a good role in friction reduction (figure 14(b)).
Figure 14. Schematic diagram of MWCNTs/EP wear mechanism.
Download figure:
Standard image High-resolution image4. Conclusions
- (1)The carbon nanotubes (MWCNTs) were oxidized by sulfuric acid and nitric acid, and the amino reactive groups were introduced on the surface of MWCNTs by grafting ethylenediamine. Then the grafting and coating of hyperbranched polyesters (HBP) on the surface of carbon nanotubes (HBP-NH2-MWCNTs) was successfully achieved with the amino reactive group as a growth point. After functionalized treatment, the hyperbranched polyester (HBP) coated on the surface of MWCNTs forms a physical barrier layer, which effectively blocks the van der Waals force between MWCNTs, avoids agglomeration and entanglement, and significantly improves the dispersion performance. Meanwhile, the contact angle of HBP-NH2-MWCNTs with EP is reduced, the surface energy, adhesion work and interface energy are increased, and the wetting property is significantly improved.
- (2)Under dry friction conditions, HBP-NH2-MWCNTs/EP composites exhibited the most excellent tribological properties. As the content of HBP-NH2-MWCNTs increases from 0.3 phr to 1.2 phr, the friction coefficient and wear loss of the composites varies in 0.255∼0.605 and 0.7∼1.7 mg, the width and depth of the wear marks varies in 830.72∼1714.44 μm and 9.50∼243.50 μm. In particular, 0.6 phr HBP-NH2-MWCNTs/EP composites exhibit the best tribological properties. There are only a small amount of slight fatigue cracks and the wear mechanism is mainly characterized by slight fatigue wear.
Author contributions
Investigation, X W and T X; Project administration, Z Z; Supervision, B T; Writing–original draft, J T; Writing–review & editing, Y T.
Funding
This work was supported by the Natural Science Foundation of China, project no. 51708553. It was performed using the equipment at Nanjing University of Aeronautics and Astronautics (NUAA).
Conflicts of interest
The authors declare no conflict of interest.
















