Abstract
A review about the use of fabricated mesoporous materials with emphasis on silica in water treatment is presented. The problem of water supply is a well-known global problem because many areas around the world are suffering due to the lack of water, and therefore, the treatment and reuse of wastewater is crucial. Mesoporous materials have some unique features, such as a high surface area, which is normally associated with wide pore volume in addition to good thermal and mechanical stability. These properties have attracted researchers to study the use of mesoporous materials in water treatment. The first section of the current review is focused on traditional materials that are used for water purification from different pollutants, in addition to the different techniques that are using for the preparation of the mesoporous materials. The second section discusses the several functionalization tactics of mesoporous materials and their role in the efficiency enhancement towards pollutant removal from water. Finally, the third section presents the use of mesoporous materials in two of the most popular applications in water treatment: adsorption and photocatalysis techniques.
Export citation and abstract BibTeX RIS
1. Introduction
The healthiness of humans and the environment is an important requirement for sustainable life. The availability of fresh and clean water is essential to protect a healthy life. Excesses in industrialization and civilization have resulted in unlimited water pollutants as organic and inorganic contaminants [1, 2]. Heavy metals, dyes, and aromatic hydrocarbons are considered the most common water pollutants, which have unlimited toxicity and hazards on the environment. Additionally, the presence of these pollutants in drinking water with high concentration causes serious problems in human health. Therefore, the removal of these impurities from the different water sources is a major focus of recent research [3].
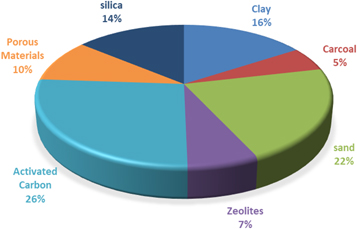
From the databases, in the last 20 years, scientists reported the use of several materials in water treatment process such as charcoal, activated carbon, clay, silica, zeolites and sand. In figure 1, the percent of use of different materials in the publications of water treatment in the last 20 years is plotted. These materials were used in different techniques such as ion-exchange, chemical treatment, and ultrafiltration [4–10]. However, amongst these techniques, adsorption technique was the most interesting one due to its unique advantages [11–14].
Figure 1. The % of the use of different materials in water treatment in the publications from 1998 to 2018.
Download figure:
Standard image High-resolution imageThe attention given to these materials was accompanied by a focus on its property's improvement, to be non-toxic, more effective, and low cost, which opens a huge door in the development of water treatment technology, where these materials play a critical role as promising an effective method used to remove environmental pollution and the advance of human life. Likewise, these enhanced materials will take a wide field in the developing of industry, agriculture, medicine, environment, aerospace, and information science due to the super specific benefits of these enhanced materials [15–17]. Among applications that used these materials, such as catalysis, separation, selective adsorption, drug delivery, and sensors [18–20], they have been used as catalyst supports, catalysts, and adsorbents in a bulky and/or a porous form and with minor modification in some cases [21]. Moreover, the focus on material porosity, control of surface area and structures, and pore architecture leads to advances in different applications [21–23]. Typically, in water purification, when the bulky material presents in a small size form or as a nanoparticle, these materials will have several advantages such as the large numbers of active sites and high surface area, which give high efficiency in the treatment process. However, the presence of some limitations such as aggregation and unsuitable growing can cause unstable situations and an increase of surface energy. In contrast, there is another type of material, porous materials in which the previous defects disappear and that have increasingly proved to show significant application values in removing pollutants. Porous materials are a type of material composed of a crosslinked network of pores, which plays a critical rule in water treatment by combining unique structure and physiochemical characteristics [24].
2. Porous materials in wastewater treatment
Porous materials can be used as a support for capturing the pollutants, where the pollutants form a thin layer on the walls or pore channels of the porous material [25, 26]. Moreover, the catalytic activity and selectivity for pollutant removal are affected by textural properties of the support. Sometimes, the support acts as an active component without precipitation in the catalysis process [27]. It is worth mentioning that porous materials perform well in removing pollutants because of qualities such as a large surface area of loading materials and the pores, whether ordered or disordered, of these materials may act as microreactors, increasing the contact between the catalyst active sites and the water contaminants.
On another hand, decreasing aggregation phenomenon caused the reduction of effective surface area and loss of active sites [28]. To suppress the aggregation of catalyst systems by using more than one modification style such as functionalization of the surface support with different efficient groups, the nanoparticles of the catalyst are immobilized by strongly binding to the surface of functionalized support. The encapsulation of catalyst nanoparticles into the channel and cavities of a support framework is another method to increase the particle catalyst dispersion and the stability of the catalytic process [29, 30]. Encapsulation by post-synthesis modifications such as impregnation or ion-exchange is quite difficult, but hydrothermal synthesis or chemical treatment may achieve excellent encapsulation.
Likewise, the porous materials have high catalytic performance due to their various species of surface active sites, so they can act directly as a catalyst with/without loading other catalysts. These materials usually have supplied the active sites that are needed to complete the catalytic reaction by various methods such as acidic/basic sites exhibited at the surface of the porous material, metal ions, clusters node, or terminal ligands [31, 32].
Generally, the International Union of Pure and Applied Chemists (IUPAC) has divided porous materials into three classes according to the pore diameter: microporous (pore size < 2 nm), mesoporous (2–50 nm), and macroporous (>50 nm) [33]. Furthermore, using the 'nano-size' concept expanded from 1 nm to 100 nm, so all the above materials may be called nanoporous, which is widespread in the literature as an index to micro- and/or meso-porous materials [34].
Since prehistoric times, humans used neutral porous materials or with some minor modification in a wide range of technological applications. The literature focused on the function of many kinds of porous materials as to whether they are natural or synthesized, such as porous carbon, alumina, metal oxides, and silicates [35]. Preferably, these materials should have high thermal and chemical stabilities with a high specific area and large volume-pore, and the distribution of narrow pore size plays a critical role in size-dependent applications, which allows more flexibility in host-guest interactions [36].
Among the porous materials, crystals of zeotypes are widely applied in various environmental applications because of the extraordinarily robust structures and regular distribution of pore system [37, 38]. The increasing demand for this porous materials consumption is largely based on the growing concern for controlling water and air pollution and for increasing the quality of life, which these materials as heterogeneous catalysts play a key role in the removal of harmful wastes. The wide use of zeolites, with large pollutant molecules that involve the transfer and conversion of macromolecules such as heavy oil cracking, separation media, and organic dyes, present difficult limitations. Therefore, micropores of zeolites need to improve by broadening the zeolite pore sizes [39], decreasing the crystal size or by combining a mesopores size within the microporous materials [40, 41].
Consequently, the fabricating and improving this new family of 'mesoporous materials' have become important developments in materials science, especially in catalysis. In addition, using nanosized mesopores as a hard template for synthesizing new nanomaterials has the possibility for new applications [42].
Porous carbonaceous materials also have been attractive materials applied to water treatment; they are considered one of the most inorganic microporous materials and used as a support or direct catalyst because of their geometric structure, variety and rich active sites, thermal conductivity, and microporosity features. For decades, the adsorption method was the technique that has been most used for water pollutants removal with porous carbonaceous adsorbents [43, 44], removing the impurities of neutral organic materials from water supplies by activated carbon [45]. However, porous carbon is considered a good host material for various nanoparticle dopants via a multi-step procedure for removing arsenic from drinking water [46, 47], and it is used for the adsorption of many toxic minerals, such as Cu(II), Cr(VI), Zn(II), and CN− [48]. The limitation in surface porosity of the carbonaceous class caused defection in its applications and this required the researchers to compose them with other meso-materials with higher flexibility in surface characteristics and more chemical/thermal stability. Meso-silica was the class that was most used in the combined materials in order to increase the carbonaceous material performance.
In the last decade, scientists generated a new class from porous materials that was different from the pure inorganic porous material: metal-organic frameworks (MOFs). MOFs are a new inorganic-organic hybrid of porous materials, which are composed of metal-containing nodes and possess various and easily tailored structures because of a diverse ligand structure [49–53]. Additionally, MOFs have exceptional properties such as tuneable pore dimensions, large surface area, unique porosity, and simple functionalization via different modification methods [54, 55]. As result of these properties, MOFs have been considered a promising material in several applications such as catalysis [56, 57], separation [58, 59], gas storage [60, 61], and organic conversion [62, 63]. These properties of MOFs make it a rich field for water treatment and have been wildly used in reducing hazardous contaminants [64, 65]. The integration of advantages of adsorption of MOFs has been extended to remove heavy metals/dyes, such as the removal of selenium species from drinking water [66]. MOFs also are also a promising material for the degradation of organic macromolecules such as dyes [67]. Using MOFs as a host framework to capture inorganic nanoparticles such as noble metals is a rich field in photocatalysis degradation under UV/Vis irradiation [68, 69].
Porous silica is a typical porous material that was recognized a long time ago as inorganic catalysts/supported material [70, 71], which were considered an ideal material in different applications due to its superior surface properties, which involve thermal/chemical stability, high specific area, low casting, low density, and variety of pore volume and distribution [72]. A simple comparison with other porous materials such as carbonaceous and zeolite, morphology, pore dimensions or structure of porous silica can be designed for more flexibility for taking into account the requirements of several applications [73–75]. Otherwise, the pore size of porous silica is related to selectivity and catalytic activity and has shown the influence of pore structure on products. The researchers have focused on the meso-class of porous silica with nanoparticle size for the last two decades due to its spongy morphology and high thermal/chemical stability [66–78]. In general, the best feature in the mesoporous silicates, namely, the flexibility and the high selectivity of the internal and external surface, can be modified with multiple inorganic/organic functional groups [79, 80]. The discovery of the first mesoporous silica was made in 1992 by Japanese scientists in a laboratory of Mobil Corporation and later called molecular 41 sieves (M41S). For instance, MCM-41, MCM-48, and MCM-50 all have a regular pore shape with a diameter in the range of 2–50 nm and a specific surface area of approximately 700 to 1500 m2 g−1, and they are highly functionalized, making them proficient support materials in catalysis, separation, adsorption, solar cell, sensors, drug delivery, and other applications. Given the amount of attention and resources devoted to this research topic, in 1995 to 1998, new mesoporous silica families were developed, such as Santa Barbara Amorphous No. 15 (SBA-15) [81], No. 16 (SBA-16) [82], Fudan University Material (FDU) [83], (FSM) [84], (MSU) [85] and Korea Institute of Technology (KIT) [86], which have various porosity/structure characteristics. The study of these mesoporous silica characteristics has provided useful information on its morphology [87–91].
In 2001, a new mesoporous silicate was synthesized for the first time in Technische Universiteit Delft (TUD-1). Unlike earlier meso-silicates, TUD-1 has a sponge-like framework, undefined pore distribution, and three-dimensional pores. These unique characteristics make it an ideal research material for large applications such as catalysis, separation, optical applications and others [92–94].
In the current review, the recent developments of several types of mesoporous materials in the field of water treatment are summarized. In the discussion, special attention to the synthetic strategies, functionalization and surface properties of mesoporous materials is highlighted.
3. Mesoporous materials in water treatment
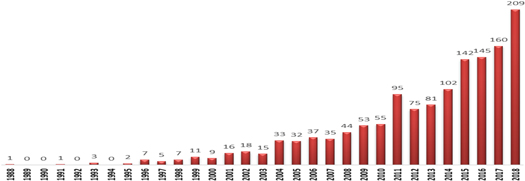
According to the database information on the use of mesoporous materials in water treatment from 1988 to 2018, a systematic increase in the number of publications can be readily observed. In 2018, the number of publications that provide a link between mesoporous materials and water treatment is almost twenty times higher than that in 1988 (figure 2). This simple statistic is an indication of the importance of the use of (functionalized-) mesoporous materials in the water treatment field.
Figure 2. The number of publications of water treatment by using mesoporous materials in the last 20 years.
Download figure:
Standard image High-resolution imageMore importantly, this attention of using mesoporous materials in water treatment was not limited to a particular region, country, or area, but it was extended to all over the world. The distribution of published research by location is plotted in figure 3.
Figure 3. The % of using mesoporous materials in different countries around the world, which the total published papers were 1485 documents.
Download figure:
Standard image High-resolution image4. The synthesis of mesoporous materials
The initial work of these studies was a parallel outgrowth with Mobil Oil mesoporous materials generated in 1992. There are several methods for preparation of mesoporous materials.
4.1. Sol-gel method
The sol-gel technique is considered a wet chemical method. It has another name, chemical solution deposition technique, used widely in the field of materials science. Recently, this technique has been widely used in order to synthesize mesoporous materials with different morphologies. Initially, a colloidal suspension is formed (called a sol) in which the inorganic network of material starts to grow. After that, the solution converts to a gel in a gelation process and the pore size/shape is controlled in the sol/gel transition. The more important feature in this technique is the possibility of adding more than one metal precursor in the colloidal solution. The adding of water allows dispersion of the oxide in dilute solution. Finally, the solid oxide is formed by a calcination process.
The sol-gel chemistry depends on the hydrolysis followed by condensation of precursor alkoxides with various stoichiometries [95, 96]. Various templates can be used in the sol-gel method, such as copolymers, surfactants, and small organic molecules [97].
4.2. Template assisted technique
In this technique, the template is responsible for the mesoporous synthesis. The classification of the template is divided into two categories. The first category is called hard-matter templating, which also called the exotemplate or nanocasting method. The porous material acts as a template in which the hollow pores are filled with inorganic precursor and then treated under suitable conditions to provide the framework of the exotemplate. The second category is called soft-matter templating, which is also called the endotemplate method. A surfactant is used as the template in order to synthesize ordered mesoporous material. In general, this technique is widely used and inexpensive [94–98].
This route was used for the first time in 1992 to prepare MCM-41 mesoporous silica. At that time, there were two pathways suggested for the synthesis of the mesoporous framework. In the first method, a surfactant generated the hexagonal structure followed by the addition of a silica precursor. After that, the silica source precipitated around this template, forming the final structure. In the second method, after the silica precursor was added, the hexagonal structure was formed. Finally, the surfactant was removed by calcination. SBA-15 mesoporous silica was synthesized by this pathway, which is a known liquid crystal template approach (LCTA) [99, 100].
5. Modification and control morphology
The possibility of modifying the mesoporous solid is one of unique features of them. However, the lack of constraint in pore volume of the mesoporous material in a simple comparison with microporous material such as zeotypes, allow more and more facility in the diffusion of bulky matter. Unrestriction of diffusion of products and reactants for mesoporous solid was shown even after incorporation is a strong index of a mesopore catalytically active sites system [101, 102]. Therefore, the mass transfer will be largely affected by the morphology and pore size of the catalyst during the target reaction. The effect of a material surface morphology was tested by comparing different silica meso-microporous activity by using the turnover frequency factor (TOF) between different functionalized samples, Fe-MCM-41 [103], Fe-HMC [104], Fe-MFI [105], and Fe-TUD-1. The catalytic performance towards the water treatment of Fe-TUD-1 was excellent compared to other methods [106]. A similar trend has recently been published in the catalytic hydrogenation of methyl isobutyl ketone (MIBK) over Al-TUD-1 and neat TUD-1, effected by improving of active site type, where the activity and selectivity were better with Al-TUD-1 due to increasing porosity [107].
There are three important factors that influence catalyst stability. The hydrothermal, the thermal, and mechanical stability of different mesoporous silicas such as MCM-41, MCM-48 [108], SiO2 [109], SBA-15 [110], and the new one, TUD-1 [111], have been discussed. It was shown that hydrothermal stability is highly dependent on pore wall thickness and the degree of polymerization in the silica type, where the best possible application of mesoporous such as catalysis is influenced by the degree of hydrothermal stability. There are several strategies that were used to improve this property, including the modification of the meso-material surface by sialylation [112], addition of salt [113], or by post-synthesis grafting of inorganic species such as Al, V, and noble metals, to post-chemical stability of wall thickness and to decrease the number of structural defect sites [114]. The direct incorporation of inorganic species will happen by appending on the pore wall or by bridging in the wall with silicon atoms such as adding alkylalkoxysilanes as a source of silica [115, 116].
Likewise, thermal stability is mostly influenced by the wall thickness as well, and the silica precursor used. On other hand, the mechanical stability is weakly influenced by the nature of the mesoporous silica and is more oriented to the application scope (table 1).
Table 1. The effect of thermal, hydrothermal, and mechanical treatment on the textural properties of mesoporous silica [117, 118].
| Conditions | MCM-41(T) | MCM-41(FS) | SBA-15 | FSM-16 | MCM-48(T) | MCM-48(FS) | SiO2 | TUD-1 |
|---|---|---|---|---|---|---|---|---|
| BET SA (cm3/g) s | ||||||||
| 550 °C | 1128 | 1027 | 632 | 1172 | 1433 | 1319 | 0 | 0 |
| 650 °C | 1114 | 970 | 561 | 1112 | 1248 | 1312 | 0 | 0 |
| 750 °C | 403 | 879 | 446 | 915 | 108 | 1287 | 0 | 0 |
| 400 °C' 30% water vapor'48 h | 1048 | 892 | 533 | 995 | 1357 | 1176 | 0 | 0 |
| 400 °C' 30% water vapor'120 h | 1019 | 864 | 500 | 789 | 1318 | 1130 | 0 | 0 |
| 100 °C' 100% water vapor'16 h | 145 | 106 | 281 | 67.0 | 197 | 168 | 0 | 0 |
| PV (cm3/g) b | ||||||||
| 550 °C | 0.95 | 0.92 | 0.63 | 0.78 | 1.14 | 1.22 | 0 | 0 |
| 650 °C | 0.87 | 0.76 | 0.56 | 0.63 | 0.73 | 1.13 | 0 | 0 |
| 750 °C | 0.26 | 0.68 | 0.48 | 0.44 | n | 0.94 | 0 | 0 |
| 400 °C' 30% water vapor'48 h | 0.72 | 0.66 | 0.57 | 0.52 | 1.00 | 1.00 | 0 | 0 |
| 400 °C' 30% water vapor'120 h | 0.47 | 0.58 | 0.55 | 0.36 | 0.93 | 0.94 | 0 | 0 |
| 100 °C' 100% water vapor'16 h | N | n | 0.47 | n | n | n | 0 | 0 |
| BJH PD (Å) d | ||||||||
| 0 h | 28.0 | 31.7 | 55.7 | 23.8 | 26.1 | 29.7 | 0 | 0 |
| 48 h | 25.2 | 26.6 | 54.0 | 18.9 | 25.4 | 28.0 | 0 | 0 |
| 120 h | 18.5 | 24.7 | 53.9 | 16.6 | 25.4 | 28.0 | 0 | 0 |
| wall thickness (Å) | ||||||||
| 9.70 | 11.0 | 29.7 | 9.90 | 9.30 | 9.40 | |||
(T) silica source is TEOS, (FS) silica source is fumed silica.(n) No capillary condensation step was observed in N2 isotherm.(s)BET surface area. (b) pore volume. (d) BJH pore diameter.
The activity of meso-material is sometimes affected by side interactions between support and substrate such as enhancement of catalytic activity in the styrene epoxidation reaction that increased when the surface of TUD-1 was modified with different loadings of gallium and optimizing the solvent system [119]. The DRIFT and TEM measurements refer to high activity of epoxidation reaction after addition of gallium in an even dispersion on the mesoporous TUD-1 framework, resulting in maximum availability of active sites, giving an increase in turnover frequency compared to neat TUD-1 catalyst. The activity for the synthesis of 1,4-dihydropyridine and  -amino carbonyl compounds through Hantzsch and Mannich reactions was largely increased by using Al-Fe-TUD-1 instead of siliceous Al-TUD-1 and Fe-TUD-1. The bimetallic sample satisfied both the Lewis and Brønsted acid sites and had an even distribution of Al+3 and Fe+3. However, the observations indicated the acidity of Al-TUD-1 over that of Fe-TUD-1 and Al-Fe-TUD-1, which enhanced the interactions with bases, resulting in an increase in activity of Al-TUD-1 catalyst over the other [120]. Another example related to the role of the surface environment of the catalyst and reaction conditions on its activity was by reaction of oxidative self-coupling of benzyl amine. This oxidation was carried out on MCM-41 and Al grafted MCM-41 and the optimum activity was in a ratio (Si/Al = 20), in which the activity was 53% instead 39% in MCM-41. Additionally, because of the acidity of the catalyst, the activity was influenced by the polarity of solvent, the nature of the amine, and the presence of air [121].
-amino carbonyl compounds through Hantzsch and Mannich reactions was largely increased by using Al-Fe-TUD-1 instead of siliceous Al-TUD-1 and Fe-TUD-1. The bimetallic sample satisfied both the Lewis and Brønsted acid sites and had an even distribution of Al+3 and Fe+3. However, the observations indicated the acidity of Al-TUD-1 over that of Fe-TUD-1 and Al-Fe-TUD-1, which enhanced the interactions with bases, resulting in an increase in activity of Al-TUD-1 catalyst over the other [120]. Another example related to the role of the surface environment of the catalyst and reaction conditions on its activity was by reaction of oxidative self-coupling of benzyl amine. This oxidation was carried out on MCM-41 and Al grafted MCM-41 and the optimum activity was in a ratio (Si/Al = 20), in which the activity was 53% instead 39% in MCM-41. Additionally, because of the acidity of the catalyst, the activity was influenced by the polarity of solvent, the nature of the amine, and the presence of air [121].
The hydrophilic and hydrophobic properties of the surface also have a large effect on the activity of the catalyst. In the removal of a typical cationic dye (Methylene Blue) from an aqueous solution on serious of Al-MCM-41 (with different loading of Al), the activity of catalyst was affected by a hydrophobic interaction as well as the number of active sites. In this reaction, the adsorption performance increased with increasing Si/Al ratio. The removal efficiency was reached at 80% with Al-MCM-41-10 after regeneration five times [122]. The epoxidation of alkenes over titanium-grafted mesoporous silica; e.g. silylated Ti-SiO2, is considered the most hydrophobic catalyst compared to neat SiO2, which increased the alkene epoxidation [123–125]. However, using the organic hydroperoxides as oxidants in liquid phase reactions with hydrophobic Ti-MCM-41 as a catalyst showed high activity and selectivity in the epoxidation of olefins. Moreover, epoxide yields exhibited important enhancements with increased silylation of the Ti-MCM-41 samples. Additionally, with the Ti-mesoporous with larger pores compered to microporous, the oxidant can easily penetrate the meso-system [126].
As started above, the functionalization can be applied to control surface functionality, to incorporate function groups, or to change textural properties. There are many possible strategies for the modifications that are useful for the application of these materials as catalysts, which will be discussed in the following.
6. Functionalization of mesoporous materials
In most cases, the mesoporous silica does not act as a catalyst for water treatment due to its inert nature. Therefore, it is very important to improve the mesoporous silica functions by applying various functionalization techniques. According to the Scopus© site, there are attractive considerations towards fabricating mesoporous material in order to enhance its performance in water purification; see figure 4.
Figure 4. The % of various functionalized techniques of mesoporous material in order to water treatment.
Download figure:
Standard image High-resolution imageMost of these procedures dealing with incorporation of active species in the wall of the silica structure or via deposition of active sites in the inner channel of silica. Specifically, the large pore and high surface area and unique framework produce great capabilities of mass transfer and exhibit high active site concentration per mass. There are different possible pathways to functionalize mesoporous silica to enhance and/or create a new catalytic activity such as organic ligands, organometallic complexes, or inorganic species [127].
In the following, we will introduce a third type, which focuses on the incorporation of inorganic ions of metals or nanoparticles of a metal oxide/metal deposit in the mesopores of silica. Interestingly, the features of these active sites are varied and controlled by synthesis, whether direct synthesis or post-synthesis.
There are two possible pathways can be using for modification of the mesoporous framework:
6.1. Direct synthesis
This style has been making mixture involving both silicon source with heteroatoms to become totally incorporated. Clearly, the outcome almost always results in homogenous incorporation of the functionalized element.
6.1.1. Sol-gel technique
This method has another name, which is direct hydrothermal treatment (DHT). The unique feature of the sol-gel is dependent on a one-pot step and is dependent on the addition of inorganic precursors into the mixture at the same time. At this time, the metal ions will be ready to be introduced in the meso-framework. Consistently increasing the metal ion concentration to higher loading will form nanoparticles, which may be placed in the pore or exhibit a separate phase. However, the high loading may be a defect in the catalyst framework as a negative effect of loading. To prevent the formation of a separated phase, the loading percent should be kept low [128, 129].
In addition to the simplicity of the sol-gel method, control of the nanoparticle size can be achieved via thermal treatment and ageing time under moderate conditions [130]. The sizes of the silica nanoparticles produced at different thermal treatments and different ageing times varied. The nanosize increased with increasing temperature and time. The results showed that at 600 °C and 2 h aged time, the size of the particles was 79.68 nm, while the size of the particles became 87.35 nm at 700 °C and 2 h. On other hand, by increasing the aging time to 4 h, the size also increased from 79.68 nm to 147.6 nm [131]. Otherwise, the presence of the nanoparticles within the framework sometimes decreased the influence of adsorption, but it can work well without nanoparticles [132].
6.2. Post-treatment
This technique will happen via more than one step. Initially, fabrication of mesoporous silica is done. After that, the heteroatoms are grafted into meso-materials as a modification reaction, allowing the heteroatoms to connect and be inserted into the support framework:
6.2.1. Impregnation technique
This technique is considered as a conventional method. Impregnation is widely used for functionalizing the metal ions in the form of isolated sites or various nanosizes in the pores on the external support surface. Simply, this strategy involves immediate impregnation of the metal solution with the mesoporous precursor, following many processes such as thermal decomposition and thermal/ultrasonic treatment. The dispersion of additive active sites results in non-homogeneous distribution over the mesoporous surface and may be present inside the pores without a true link. Additionally, sequential impregnation is a requirement for good impregnation in order to ensure a totally metal-filled mesoporous solid. The yield of nanoparticles of different size depends on the metal precursor amount and sometimes the pore size of the solid [133]. Mixed-amine was adopted with MCM-41 by wet impregnation via placing a well-dispersed suspension mixture for 4 h and 16 h in an oven to obtain the dry product [134–137].
6.2.2. Chemical—grafting approach (CG)
The grafting technique is realized by a chemical reaction to graft various functional groups onto the silica surface. Therefore, this method involves two steps. The first step is synthesis of mesoporous silica. This process should involve removing water that is adsorbed in a silica framework by a vacuum system or applying a calcination process as a last step. Then, the silica framework is dipped into a metal complex solution in order to form strong covalent bonds via a chemical reaction between support functional groups (e.g., OH, Si–O) and precursor complex. Finally, the heteroatom-functionalized mesoporous silicate is treated by washing, filtering, and drying. The tuning of the pore size in the functionalized mesostructured may be accomplished by different ways. Controlling the grafting amount is by the degree of functional species stability, dihydroxylation, and multiple grafting. Most importantly, high dispersion, low sintering, low agglomeration, and easy linking with internal pores make the grafting treatment superior to impregnation [138–140].
6.2.3. Deposition-precipitation approach (DP)
Obtaining functionalized mesoporous material with high loading/dispersion of metal is a great interest in the field of catalysis. The deposition-precipitation technique is based on high loading/dispersion, which is dependent on the straightforward basification of the dipping solution by controlling the temperature, pH, and hydrolysis conditions. Comparing the deposition-precipitation technique with ion exchange or impregnation, which are usually used, shows that ion exchange can obtain high metal dispersions but has loading limitations. On the other hand, impregnation can obtain high metal loading but has limited dispersion [141, 142]. The synthesis of guaiacol via dehydrogenation of 2-methoxycyclohexanol via SiO2 over a Ni-Rh catalyst was prepared with the DP technique [143].
6.2.4. Template ion-exchange with transition metals (TIE)
This approach is based on implantation of the transition metal ions into the as-synthesized mesoporous silicate. The template ion-exchange (TIE) depends on the replacement of a cationic template, which is embedded in the channels of silica framework, by metal cations. The TIE approach provides highly dispersed metal ions on the wall surface of the mesostructure inside the pores, which is counter to the direct hydrothermal treatment (DHT), which tends to incorporate metal ions inside the mesoporous structure. A good example of this case is the synthesis of V–, Fe−, Cu−, Cr− and Mn-MCM-41 by two methods, template ion-exchange (TIE) and direct hydrothermal treatment (DHT). The resulting different coordination environments and locations of the implanted metal cations exhibit remarkably different catalytic activity [144–147]. Otherwise, TIE is still superior to impregnation or chemical vapour deposition (CVD) approaches, since impregnation and CVD are based on precipitation onto an outer support, but if the outer support is capped, its use becomes limited [148, 149].
7. Adsorption in water treatment
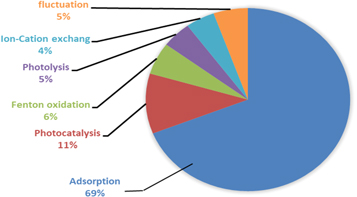
Generally, adsorption is considered one of the most attractive techniques applied to remove inorganic and organic pollutants in water; see figure 5.
Figure 5. The % of the most techniques which have been used in water treatment.
Download figure:
Standard image High-resolution imageAdsorption process is accumulation of pollutant molecules at interface between two phases, whether were gas-solid, gas-liquid, liquid-liquid system. Recently, the adsorption considered a super spread technique for several reasons, while it is considered the most economic method for the removal of toxic contaminants, in addition to the simplicity in operation and designing of adsorbents. Moreover, in most cases, the adsorption considered a reversible operation and the reusing of adsorbents several times more possibility. Many factors affect the adsorption operation such as the nature of adsorbent and adsorbate, the degree of temperature, the PH of solution, the initial concentration of contaminants, the contact times and the speed of stirring. More importantly, the presence of strong adsorption interaction between adsorbent material and target pollutants. Adsorption kinetics and isotherms are a necessary process for studying the adsorption mechanism. A number of models are used for describing the relation between the concentration of adsorbent and adsorbate at certain temperature, for example, Langmuir, Freundlich, Dubinin-Radushkevich, Redlich-Paterson, Halsey and Sips. One of important evidence to determine the adsorption efficiency is calculating the amount of pollutant that can be uptake on the adsorbent at certain pollutant concentration and temperature, which can be described via uptake relation:
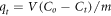
Where C0 and Ct indicated to initial concentration and concentration at (t) time of pollutant respectively, (V) the volume of solution in (L), and (m) the weight of adsorbent in (g) [150]. There are two categories of adsorption; in one of them, the adsorption is concerned with the van der Waals attraction force between adsorbate and adsorbent, which called physisorption, while in the other one, chemisorption occurs via a chemical bond formed between the concerned pollutants and surface of the adsorbent [151]. The percentage of pollutants removed from water is dominated by the adsorption performance. Furthermore, the adsorption capacity is closely related to the capacity of the adsorbent used. Likewise, the adsorbent capacity depends on its surface properties, such as specific surface area, availability of active sites, the geometry of the surface, and affinity towards the adsorbate. Hence, the porosity of the surface provides an excellent chance with active sites in order to be a super adsorbent [152, 153].
7.1. Heavy metals adsorption by mesoporous silica
Heavy metal ions such as Zn, Cd, Pb, and Cr are known as some of the dangerous pollutants present in a water resource. The direct or indirect discharge of these ions as a side product from various industries into water is an illustration of the wide contributions for water pollution. Adsorption is recognized as one of the versatile techniques because of its low price, effectiveness and flexibility.
Various heavy metal ions were removed from wastewater [154]. Imprinted nickel ions in SBA-15 and MCM-41 through co-condensation were used as a mesoporous adsorbent to high recovery to remove Ni+2 cations from wastewater. The adsorption rate and capacity were up 95% [155]. Determination of trace amounts of Pb+2 in aqueous solution was made using mesoporous silica materials functionalized with Pb(II). Comparing ion-imprinted catalysts with non-imprinted catalysts provides clear evidence of the efficiency of modified mesoporous catalysts [156, 157]. Synthesis of self-adsorbing mesoporous silica was performed under different conditions. The adsorption selectivity of Pd+2, Ni+2, and Cu+2 that affected the surface area and pore size of the adsorbent has been studied [158]. The removal of phosphate-polluted water by pure MCM-41 and functionalized with amino groups using grafting and co-condensed method was studied. Amino-functionalized MCM-41 via the grafting method was the most effective for removal of the pollutant [159]. On other hand, the bifunctional modified MCM-41 Al+3, Ti+4 was used to effectively remove Cd+2 ions from wastewater. The preparation of the catalysts was by incorporating ions as inducements into MCM-41[160]. Similarly, high photoactivity resulted from the combined photocatalysts of TiO2/Al-MCM-41 and TiO2/Al-SBA-15 compared to the original catalysts with the grafting method for treatment of phenolic compounds in wastewater [161].
Separation of uranium U(VI) from aqueous solution on SBA-15 and dihydroimidazole functionalized SBA-15 (DIMS) that was synthesized by the post-grafting method with high surface area and uniform pore structure was studied. Therefore, DIMS removed high selectivity towards U(VI) in solutions containing many competing metal ions [162]. Additionally, the M41S and SBA series, due to their unique mesoporous pore structure, were preferred for removal of Cr(VI) from wastewater [163]. Modifying SBA-15 and MCM-41 with amino groups results in effectively removing heavy metals such as Ni+2, Pb+2, Cu+2, Cd+2, Cr+2, and Hg+2 and showed a high adsorption rate, almost reaching 99% with a single-metal solution, which is more than for the multi-metal ones [164]. Moreover, SBA-15 functionalized with two types of functional groups as adsorbents, propyl-ammonium functionalized SBA-15 and propyl-N, N, N-tri methyl ammonium functionalized SBA-15 for removing nitrates anion from aqueous solution were studied. The strategies of synthesis were post-synthesis grafting and co-condensation. The adsorption performance showed influence by the nature of the functional group and the method of synthesis [165]. Decontamination of As(V), Cr(VI), and Hg(II) ions from tap water by SBA-15 functionalized with aminopropyl and N-propyl salicylaldimine groups significantly promotes the adsorption capacity. The selectivity of adsorption is affected by parameters such as pH [166]. The adsorption of toxic metals from wastewater was also done by TUD-1 mesoporous material functionalized with -COOH using a post-synthesized pathway [167]. Therefore, according to earlier examples, the retention of heavy metal pollutants is primarily dependent on the features of the mesoporous silica and the nature of the functional group selected. The amino-functional group is considered the one that has the most attractive ability to capture heavy metals from water media; see table 2.
Table 2. Removal heavy metals onto various mesoporous silicates.
| Adsorbent mesoporous silica | Function group | Adsorbate | Adsorption capacity qm | References |
|---|---|---|---|---|
| MCM-41 | — | Ni (II) | 20.8 mg g−1 | [168] |
| amino- | Cd (II) | 64.73 mg g−1 | [169] | |
| NH2− | Cu(II) | 138.8 mg g−1 | [170] | |
| N-Hdhba | Cu(II) | 222.2 mg g−1 | ||
| Fe3O4/NH2− | Pb (II) | 46.08 mg g−1 | [171] | |
| MCM-48 | NH2− | Cd(II) | 0.65 mmol g−1 | [172] |
| Co(II) | 0.46 mmol g−1 | |||
| Cu(II) | 1.06 mmol g−1 | |||
| Pb(II) | 0.28 mmol g−1 | |||
| Cu(II) | 0.52 mmol g−1 | [173] | ||
| Mn(II) | 0.8 mmol g−1 | |||
| TUD-1 | COOH− | Cd(II) | — | [174] |
| SBA-15 | Ni(II) | 22.9 mg g−1 | [168] | |
| Aminopropyltriethoxysilane | As(V) | 20.6 mg g−1 | [175] | |
| Cr(VI) | 97.6 mg g−1 | |||
| Hg(II) | 7.6 mg/g | |||
| N-propylsalicylaldimine | As(V) | 16.3 mg g−1 | ||
| Hg(II) | 7 mg g−1 | |||
| Aminopropyltriethoxysilane | U(VI) | 90 mg g−1 | [176] | |
| Aminopropyltriethoxysilane/Carbon | U(VI) | 170 mg g−1 | ||
| Dopamine | U(VI) | 196 mg g−1 | [177] | |
| Mn | Cu(II) | 19.9 mg g−1 | [178] | |
| SiO2 | Fe3O4/−EDTA- | Pb (II) | 114.94 mg g−1 | [179] |
| Cu(II) | 37.59 mg g−1 | |||
| Ni (II) | 32.15 mg g−1 | |||
| Cd(II) | 50.25 mg g−1 | |||
| NH2−/Fe3O4 | Cu(II) | 0.69 mmol g−1 | [180] | |
| Pb (II) | 0.54 mmol g−1 | |||
| Cd(II) | o.33 mmol/g | |||
| Mg | Pb (II) | 557.9 mg g−1 | [181] | |
| HCMSSs | NH2− | Cd(II) | 190.5 mg g−1 | [182] |
| Pb(II) | 194.4 mg g−1 | |||
| Zn(II) | 193.0 mg g−1 |
7.2. Dyes and organic pollutants adsorption by mesoporous silica
The textile industry produces millions of various dyes, most of the by-products of which are discharged in water and destroy the environment. The nature of the dye's structure along with the chemical stability and low degradability of their huge aromatic structure are considered the major problems for removing these pollutants [183]. Adsorption is one of the most used techniques for dye separation due to facility of use, low cost, high selectivity, and its widespread application [25]. A special surface structure and the ability to modify the surface chemistry of mesoporous silica by incorporating various functional groups increase the adsorption capacity to a high level [184–186]. Removal of crystal violet dye from aqueous solution onto MCM-41 and sulfated-MCM-41 mesoporous was done. The modified catalysts were prepared by impregnation pathway and the degradation of dye increased with an increase of pH [187]. Therefore, the effect of using low-cost materials such as a silicon source to preparing MCM-41 mesoporous was studied. The efficiency of removing methylene blue dye from water refer towards adsorbent candidate to treat macromolecular from water [188]. Studying the effect of temperature on adsorption capacity of safranin dye throw prepared MCM-41 mesoporous. The result refers to the adsorption, which is exothermic in nature [189]. Further, the textural properties with different conditions of three mesoporous catalysts, silica gel, disordered mesoporous carbon, and SBA-15 samples towards methylene blue dye removal were investigated [190]. The backbone of the catalyst plays a vital role in enhancing dye degradation. Variant loading of metals Ti /Si on MCM-41 and MCM-48 appear to have varying efficiency to rhodamine B dye degradation from the water sample [191]. Moreover, the adsorption behaviour of many dyes such as Malachite green, Congo red, and Methyl violet was associated with electrostatic interactions and hydrogen bonding between sorbent surface and sorbate molecules. The modification of meso-material Si-MCM-41 was by incorporating aspartic acid, polyethyleneimine, and β-cyclodextrin via a controlled hydrolysis and condensation strategy [192]. Therefore, generally, the efficiency of mesoporous silicate in the adsorption method is greatly dependent on the nature of the adsorbent surface, where the modifying silica network with a suitable functional group causes a clear increase in adsorption capacity of a specific pollutant (table 3).
Table 3. The adsorption efficiency of a various dyes by mesoporous silicates.
| Dye | Mesoporous silicate | Removal Percentage | ||||||
|---|---|---|---|---|---|---|---|---|
| Dye Name | Class | MPSs | Function group | Surface area (m2/g) | Pore volume (Å) | Q e (mmol g−1) | R% | References |
| MCM-41 | — | 1647 | 38 | 0.14 | — | [193] | ||
| MCM-48 | — | 1149 | 30 | 0.04 | — | |||
| Methylene Blue (MB) | Cationic | MCM-50 | — | 1222 | 56 | 0.07 | — | |
| MCM-41 | Carboxylic | 754 | 25.5 | 0.3 | — | [194] | ||
| SBA-15 | — | 659 | 52 | 0.15 | 99.1 | [195] | ||
| SBA-15 | Hyperbranched-polyglycerol | 707 | 19 | <0.5 | — | [196] | ||
| SiO2 | Carboxylic | 906 | 112 | — | — | [197] | ||
| SBA-3 | — | 1423 | — | 0.88 | — | [198] | ||
| SBA-15 | — | 668.59 | — | 0.88 | 99 | [199] | ||
| MCM-41 | Al | 940 | 24.4 | 0.21 | 94 | [200] | ||
| Methylene Green (MG) | Cationic | MCM-14 | — | 1003.5 | 39.6 | 0.25 | — | [201] |
| Basic Violet | Cationic | MCM-41 | — | 1003 | — | 1.04 | — | [202] |
| 10(BV-10) | MCM-41 | Al | 1003.5 | 39.6 | 0.85 | — | [203] | |
| Yellow dye (YD) | Cationic | MCM-41 | Al | 1448 | — | — | 92 | [204] |
| Crystal violet (CV) | Cationic | MCM-41 | Sulfated | 938.62 | — | 0.34 | 98 | [205] |
| MCM-41 | — | 1003.5 | 39.6 | 0.5 | — | [201] | ||
| Janus Green B (JGB) | Cationic | SBA-15 | — | 659 | 52 | 0.13 | — | [195] |
| Safranine T (ST) | Cationic | SBA-15 | — | 768 | 89 | — | 83.6 | [206] |
| Malachite Green (MG) | Cationic | SBA-15 | — | 768 | 89 | — | 95.7 | |
| Rhodamine B (RB) | Cationic | MCM-41 | Al | 940 | 24.4 | 0.09 | 67 | [200] |
| Cationic | MCM-41 | — | 1003.5 | 39.6 | 0.8 | — | [201] | |
| Yellow 87 | Cationic | MCM-41 | — | 893 | 32.6 | 1.17–0.3 | 44 | [207] |
| Cationic | MCA | — | 634 | 156.6 | 1.17–0.3 | 44 | ||
| Acid Blue 25 (AB-25) | Anionic | MCM-41 | NH2- | 774 | 25 | 0.6 | — | [194] |
| SiO2 | NH2- | 313 | — | 78 | [208] | |||
| Acid Blue 113 (AB-113) | Anionic | SBA-3 | Amino- | 1005 | 163 | — | 83.6 | [209] |
| Acid Red 114 (AR-114) | Anionic | SBA-3 | — | 1435 | 192 | — | 85.5 | |
| Acid Red 14 (AR-14) | Anionic | SiO2 | NH2- | 313 | — | — | 75 | [208] |
| Acid Green 28 AG-28) | Anionic | SBA-3 | Amino- | 1290 | 192 | — | 95.9 | [209] |
| Acid Yellow 127 (AY-127) | Anionic | SBA-3 | Amino- | 1092 | 168 | — | 88.2 | |
| Acid Black 1 (AB-1) | Anionic | SiO2 | NH2- | 313 | — | — | 83 | [208] |
| Methyl Orange (MO) | Anionic | SBA-3 | — | 1435 | — | 0.52 | 82 | [210] |
| MCM-41 | NH3+ | 403 | 25.4 | 1.12 | — | [211] | ||
| Orange G (OG) | Anionic | SBA-3 | — | 1435 | — | 0.35 | 79 | [210] |
| Orange Iv (OIV) | Anionic | MCM-41 | NH3+ | 403 | 25.4 | 1.09 | — | [211] |
| Red Violet X-2R | Anionic | SiO2 | Dimethyl-decylamine | 516 | 46 | 0.96 | — | [212] |
| Remazol Red | Anionic | MCM-41 | NH2- | 215 | 18 | — | 99.1 | [213] |
| Dark Yellow GG | Anionic | SiO2 | Dimethyl-decylamine | 285 | 190 | 1.71 | — | [214] |
7.3. Organic pollutants adsorption by mesoporous silica
Depollution of water from organic pollutants is one of the most important challenges for our future due to their having highly damaging effects on health and the environment and having high toxicity coupled with poor bio-degradability. Bio-treatments are considered to be insufficient due to regeneration problems that might be take place. Using adsorption technologies to recover wastewater is a very useful technology, especially when using a series of meso-materials, including modifying the meso-material to satisfy required objectives in water depollution.
Synthesized pure silica SBA-15 may be functionalized with a series of organo-silane groups via the sol–gel method to remove phenols. The adsorption performance depends on the percentage of loading and the nature of the pollutant [215]. MCM-48, HMS and SBA-15 may be used as an adsorbent to removing large-molecule pesticides such as DDT from water samples. The adsorption capability increases with higher surface area and larger pore dimensions [216].
8. Photocatalysis in water treatment
In the past, photocatalysis was considered one of the most used strategies in water treatment, especially with industrialization development and a clear shortage of freshwater supplies. Since then, several practical techniques have been applied to develop wastewater treatment strategies. Conventional techniques that have been employed previously for removing contaminants may be accompanied by the production of secondary pollution. Therefore, the development of water treatment techniques has attracted great attention in research labs and focused on sustainable, non-destructive, low cost, and green methodologies.
Recently, the photocatalysis technique has used a unique feature, focused on using sunlight in order to increase the removal efficiency of harmful contaminants such as heavy metals, dyes, and other organic pollutants via a solid, namely, a photocatalyst. The photocatalyst is expected to play a key role in photocatalysis in reducing the concern over water resource pollution.
The photocatalysis method shows a variety of features such as moderate conditions in temperature, high oxidation power, and a green-method, which offer an easy pathway to overcoming challenges associated with energy and sustainability by consideration of the solar energy as a source [217, 218]. As is well known, the efficiency of photocatalysis is equally affected by the nature of the photocatalyst and the properties of the light source. Sunlight is considered to be an ideal irradiated light source in order to activate the process due to special features such as abundance, cleanness, renewability, low cost, and safety. The interest in exploring and developing the photocatalysts with visible-light efficiency is considered strong support for utilizing solar energy in water purification [219, 220].
The design process of suitable photocatalysts has been involved in many key requirements for the effective usage of neutral and sustainable solar energy for wastewater degradation and environment protection. Primarily, the material should have a smaller band gap [221], highly active system [222], and good stability [223]. Additionally, the degree of porosity and surface geometry also exhibit an essential effect on the photocatalytic efficiency as well as the fact that the adsorption of pollutants is a critical step [224]. From past decades, many photocatalysts such as TiO2, ZnO, metal complexes or transition metal oxides within cavities have been the focus of researchers in water treatment.
8.1. Functionalization and design of photocatalytic systems within non/transition-metal oxides
Titanium dioxide, zinc oxide, tungsten oxide and magnesium oxide have been widely used as photocatalysts due to their unique advantages, e.g., high stability, low cost, high photocatalytic activity, and nontoxicity. However, the large band gap, absorption in/or near UV light range, and the need for neutral energy such as sunlight, were drawbacks in using these pure/individual photocatalysts [225, 226]. Several pathways have been used to modify these pure semiconductors, such as narrowing the band-gap of semiconductor coupling [227], implantation of ions [228], composition of π-conjugated structure [229], and doping of transition ions [230, 231] within a substrate (table 4). A comparison was made between P25-TiO2 and ZnO as a photocatalyst for dye degradation, where ZnO exhibits a higher activity due to lack of defects and better micro-scaling than P25-TiO2. The modification of ZnO with TiO2 is a superior dye photodegradation catalyst and promising renewable energy source.
Table 4. Comparison of degradation efficiency % of various modifying photocatalysts.
| Photocatalyst | Modifying group | Pollutant | Absorption region | Time (min) | Degradation efficiency % | References |
|---|---|---|---|---|---|---|
| TiO2 | non-doped | UV light | 150 | 13.00% | [231] | |
| P25 TiO2 | commercial | Orange G (OG) | 42.55% | |||
| TiO2 | N-doped | UV/Vis light | 96.29% | |||
| TiO2 | C-doped | visible light | — | — | [232] | |
| Bi2O3 | non-doped | Methyl Orang (MO) | 42.00% | [233] | ||
| C-doped | 95.00% | |||||
| ZnO | multi-walled carbon nanotube | 0.00% | [234] | |||
| dumbbell-shaped ZnO | Crystal Violet | UV light | 75 | 43.20% | [235] | |
| Methyl Violet | — | 59.40% | ||||
| Methylene Blue | — | 70.60% | ||||
| TiO2 | N-doped | — | 360 | 15% | [236] | |
| TiO20 | Bi12 | acid orange 7 (AO7) | visible light | 90% | ||
| ZnO | non-doped | Methylene Blue (MB) | 80 | 0.0517 min−1 | [237] | |
| CdSe | 0.0779 min−1 |
Generally, any improvement in the surface features of a photocatalyst will be responsible for increased photocatalytic degradation efficiency. One of these features is the adsorption ability of implanted materials and/or a photocatalyst considered to be an essential factor that is useful to transfer the charges between the semiconductor and the target pollutant. Likewise, the high surface area of the modified photocatalyst is also an essential parameter in the design process. A good example is the bi-synergetic effect of noble metals (Pt, Ni, Ag, Fe) and nitrogen with TiO2 that enhanced the photodegradation of phenolic compounds, which was 5 times higher under UV–vis light than P25-TiO2. The decomposition of a pollutant was very difficult without a catalyst but increased to 35.6% after 120 min by adding the prepared TiO2. The decomposition of phenol by Degussa P25 exhibited a higher efficiency than the prepared TiO2 and was 63.9% after 120 min. On the other hand, improvement by the synergy of noble metals and nitrogen with TiO2 led to 100% of pollutant removal at absorption at a visible region of approximately 520 nm as strong evidence for a promising strategy for highly efficient photocatalysts for the treatment of water pollutants [238].
Moreover, we look now to the porosity, which is also considered an important factor having a great effect on a photocatalyst activity, which contributes to increasing surface active-sites as well as helping pollutant ion diffusion within the photocatalyst.
8.2. Functionalization and design of photocatalytic systems within mesoporous materials
Design of the photocatalytic system by a combination of photocatalysts and mesoporous materials has been extensively used for several photocatalytic applications such as water treatment. The above combination provided a special solution for problems which resulting in photocatalysts in a form of nanoparticles powder such as agglomeration in aqueous solution [239].
In this regard, the nature of supports, which play a role as anchoring bulk materials, greatly affects the adsorption efficiency and the crystallinity of the photocatalyst [240]. To the best of our knowledge, the porous silica and mesoporous silica-supports play two key roles in designing the photocatalytic systems via their own photocatalytic activity and as superior hosts [241, 242]. However, using porous/microporous silica, e.g., clays [243, 244] or zeolites [245, 246], as a support with TiO2 photocatalysts has intrinsic drawbacks, resulting in limitations of large/organic pollutant adsorption due to narrow channels and weak accessibility. On the other hand, the mesoporous supports have unique advantages that can improve the efficiency of output of the photocatalytic systems. For instance, control of pore size in the range 2 to 50 nm, high surface area up to 1200 m2 g−1 with large numbers of silanol species available for suitable functionalization, the open dimensions with an amorphous framework displaying extra facilities of organic pollutants transfer, and a clear transparency to UV and visible irradiation range [247]. Therefore, there are multiple synthesis pathways to the incorporation of photocatalytic materials such as TiO2 in a mesoporous-silica-based support, e.g., sol-gel/hydrothermal processes [248, 249], wet impregnation [250], co-condensation [251], co-hydrolysis and inner-pore hydrolysis [252]. In some cases, it may be desired to combine these materials with minerals [253] and non-carbon components [254] or organic groups, ignoring the drawback of hosts such as high affinity towards water or weak selectivity to organic components. Herein, photocatalysis may be considered as a process that occurs simultaneously with adsorption in order for contamination removal by photocatalysts. Thus, the active sites of adsorption must be close to electron/hole centres, where the produced radicals such as radicals of hydroxyl can contact them rapidly and easily. Accordingly, the degradation of organic pollutants or heavy metals on the photocatalytic mesoporous system depends on its physical support properties, especially surface chemistry properties such as surface charge or hydrophobicity. Moreover, nanoparticle metals such as silver, palladium, copper, chromium, gold, and titanium, which may be loaded on various silica supports, direct the heterogenous catalytic process, whereas the dielectric environment, shape and the size control the efficiency of bond formation or cleavage of the photocatalyst and enhance the catalytic performance [255, 256].
8.2.1. Photocatalytic degradation of organic pollutants on mesoporous silica
Organic pollutants such as dyes and industrial wastes are classified as the most problematic pollutants for human health and the environment. The treatment of wastewater containing these pollutants is very demanding due to their intractability and resistance to photolytic degradation. Using a photocatalytic system is considered one of the superior solutions for the removal of such pollutants. The removal strategy is based on the destructive nature of the target and leads in most cases to total mineralization of water and carbon dioxide. Additionally, this method avoids the mass transfer of pollutants from water to another medium [257].
The titanium dioxide and zinc oxide/mesoporous silica system possesses compelling advantages, including high stability, unique surface chemistry engineering, low-cost, and especially the destruction of pollutants via an environmentally friendly technique [258–264]. For example, the photocatalytic degradation efficiency of methylene blue dye (MB) from wastewater clearly depends on the surface thickness of the SiO2 support in TiO2/SiO2 as a core/shell system. Additionally, the system of ZnO/SiO2, mesoporous silica used as core with a shell of ZnO as a photocatalyst removed the organic dye-adsorbed by degeneration via dark adsorption/photocatalysis reaction. Further, using mesoporous silica as a shell in the ZnO/SiO2 system improved the stability and photocatalytic activity of the ZnO photocatalyst [265–267]. Moreover, the photocatalytic degradation of azo-dye acid orange 7 (AO7) under visible light irradiation increased approximately 2.3 to 12.3 times when replacing commercial TiO2 with TiO2/SiO2 [268].
Generally, the synthesis of new catalysts via loading the photocatalysts on ordered or disordered mesoporous silica is considered one of the most critical and efficient photocatalysis techniques for the adsorption and degradation of various organic pollutants under visible light irradiation. Suitable processes to achieve these optimal outcomes will develop by applying adsorbents with a high surface area as supporting materials (table 5).
Table 5. The photodegradation of various photocatalysts depot on mesoporous silica.
| Doped Catalyst | Target pollutant | Degradation % | References |
|---|---|---|---|
| ZnO-KIL2 | 76.40% | [269] | |
| ZnO-SBA15 | Reactive Blue 19 (RB19) | 85.90% | [269] |
| TiO2-KIL2 | 97.90% | [269] | |
| Ti-MCM-41 | 95% | [270] | |
| TiO2/MCM-41 | 60% | [270] | |
| TiO2/MCM-48 | 75% | [270] | |
| TiO2/MCM-4 | methylene blue (MB) | 99% | [271] |
| Ti-MCM-41 | 98% | ||
| TiO2-SBA-15 | 60% | [272] | |
| Bi/Ti-MCM-41 | 100% | [273] | |
| AuCu/SBA-15 | 100% | [274] | |
| AuCu/TiSi | methylene blue (MB) | 100% | [274] |
| TiO2/SiO2 | reactive 15 (R15) | 100% | [275] |
| TiO2/SiO2 | cationic blue X-GRL (CBX) | 100% | |
| Cr-TiO2/TUD-1 | Methylene Blue (MB) | 75% | [276] |
| Co–Fe/Al2O3–MCM-41 | Methylene blue (MB), Congo red (CR) | 100% | [277] |
| ZnO/SBA-15 | methylene blue (MB) | — | [278] |
| ZnO/MCM-41 | methylene blue (MB) | — | |
| Mo/SBA-15 | aromatic pollutants | 95% | [279] |
| phenolic pollutants | 69% | [280] | |
| Dyes | 14% | [281] |
9. Conclusion
This paper reviewed the catalytic processes of using porous materials, a defined category from materials science, in the context of removing pollutants during wastewater treatment. As is known, the porous materials involve three types, micro-, meso-, and macroporous material, with different pore sizes that offer different properties and provide different effects on contaminant removal. Likewise, the simple comparison between them showed the excellent performance of mesoporous materials. The review presented a well-known example of this family, mesoporous silica, and introduced different examples of the pore's structures, 1-, 2- or 3-dimensional properties, and their effects on catalyst performance in water treatment. Historically, several materials have been used to remove the contaminants, including charcoal, metal oxides, and zeolites. However, the unique advantages of mesoporous silica as an adsorbent matter or as a superior host material in a photocatalysis system has attracted researchers to improve the properties of mesoporous silica, especially the 3- dimensional properties. Moreover, this paper presented some of the functionalized strategies of this category. Finally, this review showed two examples of using functionalized mesoporous silica in water purification as adsorbent and/or photocatalyst with high catalytic performance. The future aspects of using mesoporous silica may take various lines to develop the water treatment scope. The research of novelty in synthetic methods of materials in the industrial scale will continue by a combining the benefits of several methods to find an eco-friendly, simple, low-cost and general technique. The attention of textural properties of materials surface such as the pore volume and shape will be spreading. Additionally, the challenging in the field of developing of the in-situ characterization tools via the combination and miniaturization of techniques for mesoporous materials identifying will continue. Recently studies also focused on using small molecules alternate of polymers or big molecules for the synthesis of mesoporous silica in nanoscale with multi-function of its active sites, which act as perfect carriers for several applications.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through research group program under grant number R.G.P.1/45/40.