Abstract
Polyvinyl alcohol (PVA) is a synthetic, semi-crystalline, biodegradable and water soluble polymer having moderate mechanical properties. In the present investigation, restriction in water absorption of PVA is achieved by cross-linking with hydrochloric acid (HCl) which is confirmed by Fourier transform infrared spectroscopy (FTIR) and water uptake test. Results suggest that due to the formation of cross-linked bonds thermal and mechanical properties of PVA based cross-linked composite are improved as compared to pristine PVA. Further improvement in physical, mechanical and thermal properties is achieved by reinforcing basalt fibers. Tensile test results show that ultimate tensile strength (UTS) of basalt fiber reinforced composites increased by 79.4% as compared to the cross-linked PVA. Dynamic mechanical analysis of fabricated composites has been carried out to determine the storage modulus, glass transition temperature and activation energy. Effect of stress and temperature on creep and recovery behavior of cross-linked PVA and basalt fiber reinforced composite are studied and burger model is used to study the creep data.
Export citation and abstract BibTeX RIS
Introduction
In past years, demands of biodegradable composite have been increasing due to environmental impact. The backbone of polymer matrix composites is matrix (polymer) which needs to be biodegradable for environment friendly. Biodegradable polymers are classified as natural such as polysaccharides, proteins, polyesters etc and synthetic such as poly amides, poly anhydrides, poly amide-enamines, polyvinyl alcohol etc. The major disadvantage of natural bio-polymer is low mechanical properties that is why research has been shifted toward synthetic bio-polymer. Polyvinyl alcohol (PVA) is the synthetic [1–4], thermoplastic [5, 6] and biodegradable [7–9] polymer which is soluble in water. PVA can be used as matrix because of its good mechanical properties, low cost, processability etc. Because of high interfacial adhesion of PVA with reinforcing material it can be used for fabrication of composite which were mechanically stronger and tougher and have a potential for use in several applications. It is known that properties such as physical, thermal, mechanical and biodegradability depend upon the degree of hydrolysis. Due to the presence of hydroxyl group, PVA is hydrophilic [10–12] in nature which promotes biodegradability through solubility in water. PVA has excellent film making ability [13–16], gas barrier properties [14, 17, 18] and high interfacial bonding which results in application of PVA in many different field such as fabrication of composite [19–21], packaging material [9], coating material [22], drug delivery system [23, 24] etc. However, hydrophilic nature of PVA restricts its application and to overcome this limitation blending [2, 7, 9, 25] and cross-linking [1, 26] of PVA were carried out by many researchers in past years. For instant in 2009, Hyder and Chen [25] improved the mechanical, swelling and thermal properties by fabricating PVA–Chitosan blend films cross-linked with trimesoyl chloride (TMC)/hexane. In 2010, Limpan et al [3] blended PVA with fish myofibrillar protein which resulted in reduction in the solubility of films. In 2016 sonker et al [26] fabricated composite in which cross-linking of PVA was performed by glutaric acid to reduce the water absorption and enhance the mechanical properties.
Use of eco-friendly fiber has gain interest in recent years and basalt fiber emerges out as new promising reinforcement material [27–30]. Jalal et al [31] fabricated concrete by reinforcing the hybrid PVA-basalt fiber at different volume fraction. Result shows that crack toughness and flexure toughness of concrete is increased as volume fraction of PVA-basalt fiber increases. Moreover, PVA-basalt fiber increases ductility of the fabricated concrete. In 2014 Farsani et al [32] studied the tensile properties of polypropylene-clay nano-composites reinforced with chopped basalt fiber. Yield strength and Young's modulus of chopped basalt fiber reinforced composites increased by 17%, 30% and 68% at room temperature, low temperature and high temperature respectively, due to restriction in mobility of polymer chains by fiber under loading. Shafiq et al [33] used basalt and PVA fibers to improved flexural strength of concrete. Basalt fiber result in improvement in deflection-softening behaviour whereas PVA fiber improve post cracking flexural response. In 2018 Gulsan et al [34] studied the effect of acidic environment on mechanical properties of cementitious composites reinforced with carbon and basalt polymer fabrics. Result shows that compressive strength basalt fiber reinforced concrete improved by 200% whereas their on 6% reduction in the compressive strength when exposed to acidic environment. Basalt fiber can be used as an alternate of glass fiber due to its excellent mechanical and thermal properties, biodegradability and non abrasive qualities. Basalt fiber is fabricated from molten igneous rock as it is cools in atmosphere air followed by extrusion. The major classification [35] of basalt fiber is on the SiO2 content. For instance, acidic basalt (Tholeiites) when SiO2 content higher than 46% and its magma is developed at shallow depth at ocean floor and island arc or continental arc. Mildly acidic basalt has SiO2 content 43 to 46% and its magma developed at intermediate depth. Alkaline basalt, SiO2 content up to 42% (nepheline basalt) and its magma developed at greater depth. It has been observed that chemically, it consists of oxides of magnesium, calcium, sodium, potassium, silicon and iron, along with traces of alumina [36, 37].
From past few years, composites fabricated from biodegradable polymers and natural fibers have attracted more interest among researchers and scientists, as there is increasing demand for developing environmental friendly materials. As it is known, Polyvinyl alcohol is a nontoxic and thermoplastic polymer which is completely biodegradable. The major disadvantage of PVA based composites/films is higher water uptake or solubility in water. To overcome this negative aspect in present investigation biodegradable polymer PVA is been cross-linked. The cross-linked composite was further reinforced with fibers to overcome the today's world challenges. In present investigation, the limitation of solubility and improvement in mechanical, physical and thermal properties of PVA based composites has been done through cross-linking of PVA with HCl. Which can extend the applications of PVA based composites to polybag, packaging industry, coating, etc. To improve the mechanical properties, PVA has been cross-linked by researchers such as Sonkar et al [26] who reported an improvement in tensile strength of PVA matrix (about 50.7%) by cross-linking with glutatic acid. Cross-linking of PVA with borax had been done by Tanpichai and Oksman [38] which result in enhancement in tensile strength by 60.2%. In 2013 Heydari et al [39] also improved the mechanical properties of PVA by using formic acid as cross-linking agent. To confirm the formation of cross-linked bond FTIR and water uptake tests were performed. Further enhancement in mechanical and thermal properties was carried out by reinforcing with nano-basalt fiber. Thermo-gravimetric (TGA) and dynamic mechanical analysis (DMA) were performed to determine thermal stability, glass transient temperature and activation energy of fabricated composite. Creep and recovery behavior of composite were studied to determine the visco-elastic behavior of composite and effect of cross-linking on creep strain is also studied. Burger model is been employed to developed mathematically model. Effect of temperature was studied by maintaining constant stress (4 MPa) at three different temperature level (20 °C, 40 °C & 60 °C) whereas effect of stress was studied at three different stress value (2, 4 and 8 MPa) for the constant temperature of 20 °C.
Experiment
Material
Polyvinyl alcohol cold M.W. 850000–124000 with degree of hydrolysis 99% having viscosity (4% solution at 20 °C) 23–38 cP with purity greater than 99% was supplied by HPLC, India; Basalt fibers having diameter of 12–8 nm was purchased from NIKUNJ, India and Hydrochloric acid (HCl) M.W. 36.46 with acidimetric 35%–36% was purchased from MOLYCHEM, India.
Methods
PVA based cross-linked composite reinforced with basalt fiber was fabricated through solution casting method. To prepare PVA based cross-linked matrix, 5 g of PVA was added to 100 ml distilled water at 80 °C to obtained 5 wt% PVA aqueous solution, which was then stirred on a magnetic stirrer for 1 h at 80 °C with 350 RPM. After that 2 ml HCl having different normality 3.03, 3.49 and 3.96 was added to the solution which was again stirred at the similar condition. The above mention different normality solution of HCl was obtained by adding 13 ml, 15 ml and 17 ml concentrated HCl to 50 ml distilled water at room temperature. The solution was casted into the petri dish and dried in an oven at 70 °C for different time 24, 48 and 72 h to study the effect of reaction time on the amount of cross-linking. To prepare basalt fiber reinforced composite 5, 10, 15 and 20 wt% (with respect to PVA) basalt fibers were added to above fabricated cross-linked PVA solution. The casting and drying method for composites were same as mentioned above. Table 1 represents nomenclature of the composite.
Table 1. Nomenclature of composite.
| Abbreviations | HCl Normality | Time | Basalt fiber content |
|---|---|---|---|
| PVA-3.03HCl-24 | 3.03 | 24 | — |
| PVA-3.03HCl-48 | 3.03 | 48 | — |
| PVA-3.03HCl-72 | 3.03 | 72 | — |
| PVA-3.49HCl-24 | 3.49 | 24 | — |
| PVA-3.49HCl-48 | 3.49 | 48 | — |
| PVA-3.49HCl-72 | 3.49 | 72 | — |
| PVA-3.96HCl-24 | 3.96 | 24 | — |
| PVA-3.96HCl-48 | 3.96 | 48 | — |
| PVA-3.96HCl-72 | 3.96 | 72 | — |
| PVA-3.49HCl-B5 | 3.49 | 48 | 5 |
| PVA-3.49HCl-B10 | 3.49 | 48 | 10 |
| PVA-3.49HCl-B15 | 3.49 | 48 | 15 |
| PVA-3.49HCl-B20 | 3.49 | 48 | 20 |
Characterization and testing of composite
To confirm the formation of cross-linking bonds Fourier transform infrared spectroscopy (FTIR) and water absorption test were performed. FTIR testing was performed by using ECO-ATR ALPHA, Bruker spectrometer with taking 24 scans at the resolution of 2 cm−1. A spectra was recorded in transmittance mode varying from 600 cm−1 to 4000 cm−1. The morphology of the PVA based cross-linked composite reinforced with basalt fiber was characterized by scanning electron microscopy (SEM), using a JEOL JSM-6490LA microscope working in high vacuum mode, with an acceleration voltage of 5 kV. Water absorption test was performed to determine the water uptake of different composites by immersing the sample of size 1 cm × 2 cm into 100 ml distilled water for 24 h at room temperature. For water absorption test three sample were taken of each composition. Excess water from the sample surface was soaked through tissue paper before measuring the weight of the sample at the different time interval. Water absorption (WA) is calculated by the formula given below:
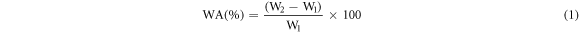
Where, W1 and W2 are initial and after water uptake weight of the specimen, respectively.
Mechanical testing of the specimen was carried out as per ASTM D882-12 standard and five specimen of similar composition were used. Tensile specimen gauge length was taken 40 mm and testing was performed at speed of 2 mm min−1 for all samples using universal testing machine AMT-SC-01521. Before performing the test, samples were dried into the oven at 70 °C for 1 h.
Thermo-gravimetric analysis (TGA) and derivative thermo-gravimetric (DTG) were performed to study the thermal stability of sample using STA7300, HITACHI. For that first, samples of weight about 3–6 mg were placed on the platinum crucible. The analysis was conducted for 25 °C to 500 °C at the heating ramp rate of 10 °C min−1 under the nitrogen atmosphere at the flow rate of 200 ml min−1. To determine the visco-elasticity response of composites, dynamic mechanical analysis (DMA) was performed on DMA7100, HITACHI under sinusoidal tensile mode. Sample size of 20 mm × 10 mm and temperature range from 25 °C to 180 °C with heating rate of 2 °C min−1 were taken. Creep and recovery behavior of fabricated composites were performed under force-control module in tensile mode. Effect of temperature was studied by maintaining constant stress (4 MPa) for 15 min and recovery behavior was studied for 15 min after removal of stress. The effect of stress on creep behavior was studied at three different stress value (2, 4 and 8 MPa) for the constant temperature of 20 °C.
Proposed chemical reaction and bond formations
The major disadvantage of PVA is its solubility in water which restricts its applications. It is known that PVA is obtained by hydrolysis of polyvinyl acetate in which replacement of acetate group (C2H3O2) by hydroxyl group (O-H) occurs. This leads to formation of hydrogen bond between hydroxyl group of PVA and water molecules which result in the solubility of PVA. To overcome solubility and improve mechanical strength, cross-linking by Zimmer's Hydrogenesis (dehydration reaction) and esterification were proposed. Similar esterification reaction between PVA and citric acid also proposed by Brick et al [40]. It was mention that, the major factors which affected the cross-linking were concentration, time and temperature. In present investigation temperature of the reaction maintained constant at 70 °C and the effect of concentration and reaction time on cross-linking has been studied. In dehydration reaction, hydroxyl group (O-H) is replaced by chlorine (Cl) as shown in figure 1 which inhibits the solubility of PVA. Moreover in esterification reaction, two{-C-OH} bonds of same PVA chain (intermolecular) or different PVA chains (intramolecular) react with two HCl to form {-C-O-C-} bonds as shown in figure 2. This results in the interlocking of PVA chains which imparts high mechanical strength. When basalt fibers were introduced in PVA cross-linked matrix, hydrogen bonds are formed between Si of basalt fiber and hydroxyl group of PVA. This results in further decrease in the water absorption of the composites.
Figure 1. Cross-linking of PVA with HCl and formation of hydrogen bounding with basalt fiber.
Download figure:
Standard image High-resolution imageFigure 2. FTIR spectra for PVA based cross-linked composite.
Download figure:
Standard image High-resolution imageCharacterization
Proposed chemical reaction and restriction in solubility of PVA was supported by FTIR spectroscopy. As shown in figure 2(d) the major peaks which characterized the PVA were observed to be 3400–3600 cm−1 (broad), 2970 cm−1, 1260 cm−1, 1026 cm−1 and 1093 cm−1 attributed to H- bond and O-H stretching of hydroxyl group, stretching of aliphatic (C-H) bonding of alkyl group, stretching of skeletal C-C bond and stretching of primary and secondary alcohol C-O bound [41, 42] respectively. Figures 2(a)–(c) represents the cross-linked composites having 3.49 N HCl concentration dried at 70 °C for the different time period (24 h, 48 h and 72 h). With increase in time there was removal of O-H group which can be represented by decrease in amplitude of the peak of hydroxyl group as shown in figure 2. It can be seen that the new peak emerges out at 775 cm−1 in all cross-linked composite which could be attributed to the formation of C-Cl bond i.e. replacement of O-H with Cl as shown in figure 1. Similar observation had been shown by soner et al in 2018 [43] in which intensity of O-H bond peak decreases whereas as new new intensity band of C=O was appeared when PVA cross-linked with dicarboxylic acid. Chanthad and Wootthikanokhan [44] also observed new peak when PVA modified with sulphothatic acid. Moreover two peaks at 1026 cm−1 and 1093 cm−1 observed in the case of PVA whereas single peak can be noticed at 1059 cm−1 in all cross-linked composites which could be attributed to the stretching of C–O–C bond [41].
It should also be noticed that the Zimmer's Hydrogenesis (dehydration reaction) results in depolymerization of PVA into vinyl alcohol or acyclic alkenes which are characterized by peaks at 1695 cm-1 and 1438 cm−1 represent alkenyl C=C stretch and Vinyl C-H in-plane bend [41] respectively.
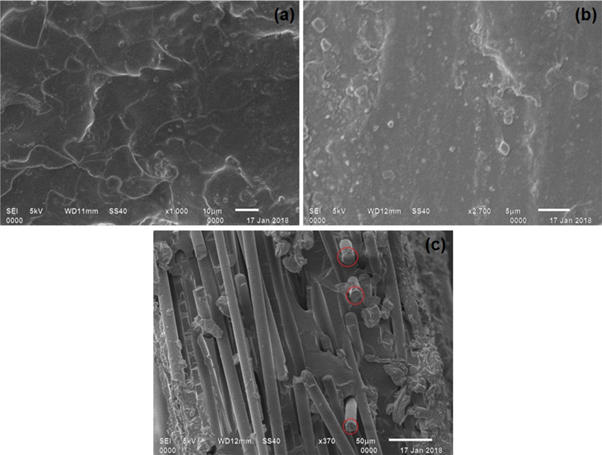
The SEM images of fracture surface after tensile testing of neat PVA, cross-linked PVA (PVA-3.49HCl-48) and cross-linked PVA reinforced with 15 wt% basalt fiber (PVA-3.49HCl-B15) are shown in figures 3(a)–(c) respectively. It can be observed that the cross-linking results in more smoother surface as compared to PVA as shown in figure 3(b). Moreover, there is no internal cracking and phase separation for the cross-linked case, which results in the higher tensile strength of the cross-linked film as compared to neat PVA. Figure 3(c) shows that basalt fibers are randomly distributed in PVA matrix with some fiber oriented in the direction of loading as marked with the circle. It should also be noted that there is no aggregation of basalt fiber which results in the proper adhesion between matrix and fiber. Similar observation was also found by Sonker et al [26]. This leads in the successful transfer of load from matrix to the fiber which results in better mechanical properties.
Figure 3. SEM morphology of fracture surface of tensile test (a) PVA (b) PVA-3.49HCl-48 and (c) PVA-3.49HCl-B15.
Download figure:
Standard image High-resolution imageMechanical properties
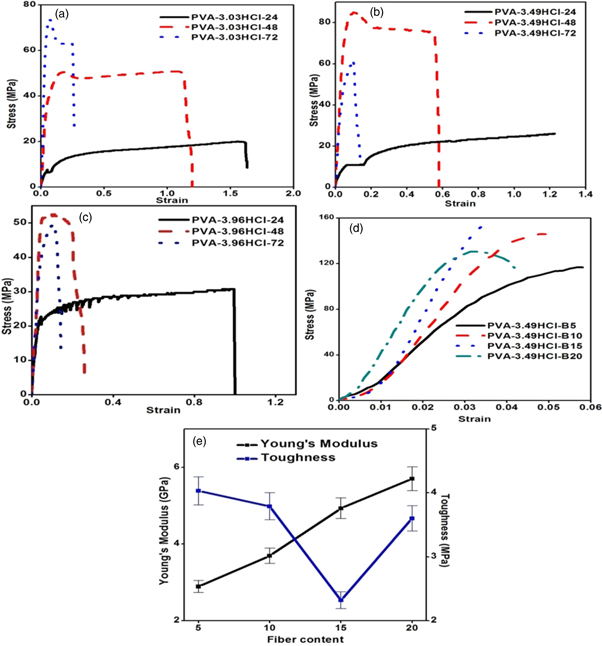
Figure 4 represents the behavior of PVA based cross-linked composite reinforced with basalt fiber under tensile loading. In the present investigation characterization of composites has been carried out on the cross-linked PVA-HCl composite on the basis of tensile properties. In present investigation, the effect of time and normality of HCl on ultimate tensile strength (UTS) and elongation have been studied. It can be observed in figure 4 that the tensile strength of cross-linked PVA with 3.03 N HCl composite increased with increase in time. Whereas, for cross-linked composites with 3.49 N and 3.98 N HCl UTS first increases with time and then reduces. Moreover, UTS of cross-linked composites is almost similar after 24 h as HCl interacts very less with PVA in the liquid state. This is attributed to present of water molecules which result in the low amount of cross-linking. It is observed that with the increase in the normality of HCl, cross-linking increases which result in restriction of movement of PVA chain this lead in the increase of UTS and decreases elongation. With further increase in the normality of HCl, the depolarization of PVA chain occurs causes the decrease in UTS. The same phenomenon is occurring in case of time. It is also observed that when normality remains constant UTS first increases with increase in time and later decreases. From the above results, it has been found that PVA-3.49HCl shows highest UTS of 85 MPa after 48 h.
Figure 4. Tensile test result for PVA cross-linked composite (a) 3.03 N HCl, (b) 3.49 N HCl, (c) 4.98 N HCl, (d) 3.49 N HCl and different percentage of basalt fiber (e) Young's modulus and toughness with basalt fiber content.
Download figure:
Standard image High-resolution imageFurther enhancement of UTS has been achieved by fabricating composites reinforced with nano basalt fiber. It has been found that the UTS of composite increases to 152.5 MPa for 15 wt% content of basalt fiber which is 79.4% higher than PVA-3.49HCl composite and later reduces to 130.45 MPa at 20 wt% content of filer. It is known that basalt fiber consists of metal oxides of Al, Fe, Ca, Mg etc. Further, when it is added to HCl cross-linked risen/matrix it reacts with HCl, result in the replacement of H- with metals ions of fiber [44–46]. Due to which amount of cross-linking decreases which results in the reduction of binding strength of matrix and hence at higher content UTS of composite decreases. The Modulus of toughness of basalt fiber cross-linked composite had been calculated through the area under stress-strain curve and value are represented in figure 4(e). It can be observed that the toughness decreases with increase in fiber content this can be rationalized in terms of drastic reduction in ductility of the composite.
Analysis of variance is used to investigate the effect of normality of HCl and time on mechanical properties (UTS and elongation) of the cross-linked composite. The result after ANOVA test is presented in 2. It is also noticed that time contributes substantially 70.44% on UTS followed by normality with a mere of 8.23% as in table 2.
Table 2. Linear model: UTS versus normality and time.
| DF | Square of sum | Mean Square | F | P | Contribution % | |
|---|---|---|---|---|---|---|
| Normality | 2 | 301.40027 | 150.70013 | 0.77109 | 0.5209 | 8.23 |
| Time | 2 | 2581.20847 | 1290.60423 | 6.60368 | 0.05404 | 70.44 |
| Error | 4 | 781.74827 | 195.43707 | 21.33 | ||
| Total | 8 | 3664.357 | 100 |
Water absorption test
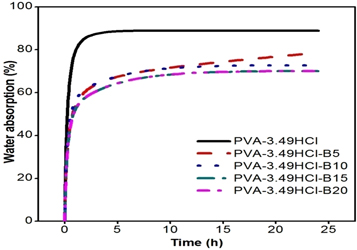
Figure 5 represents the variation of water absorption of PVA based cross-linked films and cross-linked composites reinforced with basalt fiber. It is known that pristine PVA is completely soluble in water because of formation of hydrogen bond between the hydroxyl group and water molecules. As discussed before that the cross-linking results in the replacement of C-OH bond into C-Cl and intermolecular/intramolecular {C-O-C} bonds as shown in figure 1. This leads to decrease in the availability of hydroxyl group to form hydrogen bond which causes the restriction in the solubility. It can be noticed the maximum water absorption takes place within the 2 h of time and then remains nearly constant. Moreover, it is also observed that the PVA based cross-linked film absorbed around 128.6% of water whereas, water absorption decreases with increase in the content of basalt fiber. It is suggested that the water absorption of composite depends upon the uncross-linked hydroxyl group. When basalt fibers are introduced in PVA based cross-linked matrix as reinforce material the hydrogen bonds are formed between basalt fiber and hydroxyl group, which cause the further reduction in the availability of hydroxyl group to interact with water molecules. Moreover, with increase in the content of basalt fiber hydrogen bonds are formed between basalt fiber and hydroxyl group which results in the further reduction of water intake of composite as shown in figure 5.
Figure 5. Water absorption test.
Download figure:
Standard image High-resolution imageThermo-gravimetric analysis
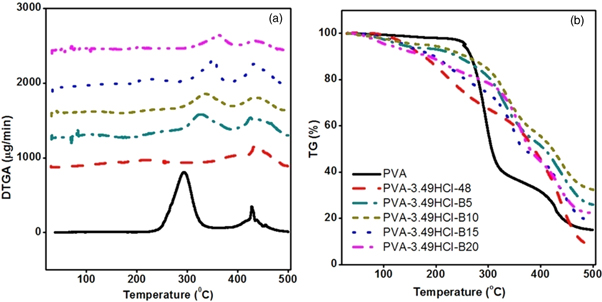
Figure 6 shows the effect of cross-linking and basalt fiber as reinforcement material on thermal stability of PVA based cross-linked composites. It can be observed that pristine PVA experience weight loss in three temperature regions [39], i.e. 80 °C–150 °C, 230 °C–350 °C and 400 °C–450 °C. It can be attributed to moisture or dehydration of neat PVA in first range, the thermal degradation of PVA into acetone, aldehydes, ethanol etc as the major product and carbon dioxide and carbon monoxide as the major gasses [47] in second temperature range and the further degradation of second range products into carbons and hydrocarbons in third temperature range.
Figure 6. (a) DTGA and (b) TG thermo-grams for PVA based composite.
Download figure:
Standard image High-resolution imageThermo-gram (figure 6(b)) of cross-linked composite shows that in second temperature range only 10% of weight loss occurs as compared to Pristine PVA where weight loss is 60%. Moreover cross-linking results in modification of PVA structure which causes the thermal degradation of cross-linked composite occurs only in one step as compared to PVA where it occurs in two steps as shown in figure 6(b). It could also be noticed that the thermal stability of PVA based cross-linked composites reinforced with basalt fiber is higher as compared to PVA. Thermo-grams of composites reinforced with basalt fiber show the shifting in the peak for the second temperature range from 230 °C–350 °C to 300 °C–450 °C as compared to Pristine PVA, this can be rationalized in terms of the formation of hydrogen bonding between basalt fiber and hydroxyl group Si...OH.
Dynamic mechanical analysis
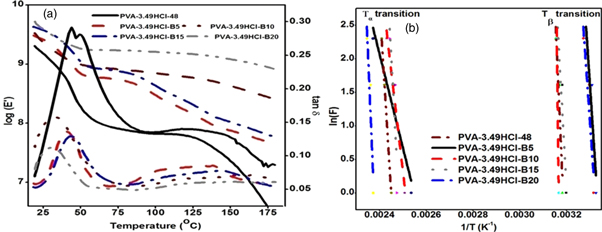
Typical DMA thermo-grams of PVA cross-linked films and cross-linked composites loading with different basalt fiber content are represented in figure 7(a). It is observed that tan δ curve represent two peaks, first from 20 °C to 55 °C temperature range and second 120 °C to 150 °C. Similarly two peaks in tan δ were observed when PVA is cross-linked with citric acid in study of Brick et al [40]. The first peak is referred to beta relaxation, also known as beta transition (Tβ) which represents the motion of side chains developed in composite due to cross-linking of main PVA chain during the esterification reaction as shown in figure 1. It is suggested that with increase in temperature material get warm up results in increase of free volume which facilitates the movement of side chains. The movement of the side chain is also favorable by the presence of moisture molecules which act as voids and on heating results in free volume. Moreover, HCl which act as the plasticizer also imparts free space by getting in between the PVA chain. The Tβ transition might also be associated with glass transition temperature of small polyvinyl chloride chains which are formed by replacement of C–OH bond into C-Cl. The second peak which is of low intensity and wider than the first one is associated with the alpha transition which represents the movement of main PVA chain. With further increase in temperature free volume increased more which results in large scale movement of main PVA chain. The temperature at which it occurs is known as glass transition temperature (Tg). It is noticed that the intensity of this peak is very low due to the presence of cross-linking bond and basalt fiber which restricts the movement of PVA chain.
Figure 7. (a) DMA analysis of PVA based composite (b) Arrhenius plot to determine the activation energy.
Download figure:
Standard image High-resolution imageTanδ curve for basalt fiber reinforced cross-linked composites has lower value as compared to cross-inked composite which represents that the present of basalt fiber increases elasticity of composite. Storage modulus (E') of basalt fiber reinforced cross-linked composites is found to be higher than that of cross-linked composite which infers that mechanical strength of composite increases with basalt fiber loading. In Tg-transition region for cross-linked composite, value of storage modulus decreases drastically as compared to basalt fiber reinforced composite which concludes that presence of basalt fiber increases the mechanical strength of composite for higher temperature.
At β-relaxation and α-transition polymer absorbs energy in degradation of polymer chain molecules under the action of load, which is referred as activation energy and determined by Arrhenius equation [48] given below:
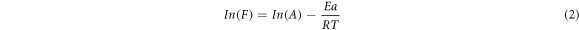
Where F represents the frequency in Hz, Ea is the activation energy (kJ/mol), A is pre-exponential factor, R is the gas constant (kJ/mol-K) and T is the temperature (K). The value of activation energy is presented in table 3 at Tβ and Tα is obtained from the slope of curve between In(F) and 1/T graph as shown in figure 7(b). From the result it has been found that the total activation energy (i.e. combining the Ea value for Tβ and Tα transition) of composites increases continuously with increase in fiber loading and maximum value is obtained at 15% fiber content.
Table 3. Activation energy of PVA based composite.
| Ea at Tβ (kJ mol−1) | Ea at Tg (kJ mol−1) | Total | |
|---|---|---|---|
| PVA-3.49HCl-48 | 239.2935 | 249.8527 | 489.1462 |
| PVA-3.49HCl-B5 | 516.3562 | 531.8271 | 1048.183 |
| PVA-3.49HCl-B10 | 417.0965 | 694.9674 | 1112.064 |
| PVA-3.49HCl-B15 | 664.219 | 468.0413 | 1132.26 |
| PVA-3.49HCl-B20 | 434.6279 | 116.201 | 550.8289 |
Creep and recovery behavior
Creep analysis of polyvinyl alcohol based composite has been shown in figure 8. There are mainly three stages in creep behavior that include instantaneous deformation also known as elastic strain (εe), visco-elastic strain (εv) and viscous strain (ε∞). In recovery phase, on removal of stress there is instantaneous recovery of strain which represents elastic nature of composite. In addition there is time dependent recovery which represents visco-elastic behavior which is followed by permanent deformation depicting the viscous nature of composite. The constant stress is applied to the sample directly for 15 min and recovery behavior is studied for 15 min after removal of stress. To characterize the creep data Burgers model [49–51] is been used, it consists of Maxwell and Kelvin-Voigt element in series combination.
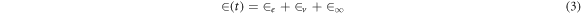
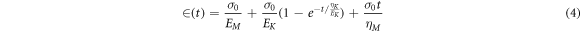
Where  is creep strain, E1 and E2 represent Maxwell and Kelvin spring elastic modulus, and η1 and η2 represents viscosities of Maxwell and Kelvin dashpots. Time required to generate 63.2% of deformation in Kelvin unit known as retardation time
is creep strain, E1 and E2 represent Maxwell and Kelvin spring elastic modulus, and η1 and η2 represents viscosities of Maxwell and Kelvin dashpots. Time required to generate 63.2% of deformation in Kelvin unit known as retardation time  The value of above parameters has been calculated and presented in table 4.
The value of above parameters has been calculated and presented in table 4.
Figure 8. Creep and recovery behavior (a)–(c) effect of stress and (d) and (e) effect of temperature.
Download figure:
Standard image High-resolution imageTable 4. Burger model parameters for PVA based films.
| Effect of Stress (Temperature at 20 °C) | |||||||
|---|---|---|---|---|---|---|---|
| Burgers model parameter | E1 (GPa) | E2 (GPa) | η1 (GPa.min) | η2 (GPa.min) | τ (min) | R2 | |
| PVA | 2 MPa | 6.2 ± 1.32 | 2.12 ± 0.11 | 31.55 ± 6.99 | 8.98 ± 0.72 | 4.22 ± 0.17 | 0.9983 |
| 4 MPa | 4.99 ± 0.87 | 5.78 ± 0.18 | 71.11 ± 7.69 | 14.43 ± 4.74 | 2.21 ± 0.69 | 0.9966 | |
| 8 MPa | 4.94 ± 0.84 | 5.8 ± 0.66 | 72.13 ± 6.68 | 13.43 ± 5.27 | 2.12 ± 0.74 | 0.997 | |
| PVA-3.49HCl-48 | 2 MPa | 2.1 ± 0.23 | 11.28 ± 3.72 | 156.67 ± 57.73 | 17.39 ± 6.72 | 1.2 ± 0.59 | 0.9932 |
| 4 MPa | 3.56 ± 0.53 | 8.16 ± 0.56 | 213.34 ± 11.55 | 14.4 ± 4.11 | 1.4 ± 0.55 | 0.991 | |
| 8 MPa | 2.91 ± 0.32 | 6.96 ± 0.6 | 164.45 ± 27.4 | 12.67 ± 3.34 | 1.5 ± 0.81 | 0.9939 | |
| PVA-3.49HCl-B15 | 2 MPa | 2.2 ± 0.42 | 7.92 ± 0.21 | 484.5 ± 50.92 | 7.72 ± 1.57 | 0.61 ± 0.17 | 0.9807 |
| 4 MPa | 4.04 ± 0.84 | 5.78 ± 0.51 | 333.4 ± 45.5 | 9.21 ± 2.09 | 1.41 ± 0.51 | 0.9783 | |
| 8 MPa | 3.04 ± 0.69 | 5.78 ± 0.24 | 244.5 ± 38.49 | 11.06 ± 2.39 | 1.6 ± 0.3 | 0.9906 | |
| Effect of temperature (Stress 4 MPa) | |||||||
| Burgers model Parameter | E1 (MPa) | E2 (MPa) | η1 (GPa.min) | η2 (MPa.min) | τ (min) | ||
| PVA | 40 °C | 90.83 ± 8.23 | 298.75 ± 16.83 | 19.08 ± 0.91 | 635.89 ± 49.96 | 1.9 ±0.15 | 0.9947 |
| 60 °C | 68.82 ± 5.43 | 321.8 ± 15.32 | 57.7 ± 0.97 | 730 ± 101.06 | 1.4 ± 0.34 | 0.9986 | |
| PVA-3.49HCl-48 | 40 °C | 281.77 ± 17.23 | 791.86 ± 21.98 | 211.11 ± 16.45 | 1623.68 ± 359.96 | 1.6 ± 0.4 | 0.9848 |
| 60 °C | 238.85 ± 13.21 | 549.7 ± 20.03 | 36.19 ± 6.6 | 1284.16 ± 235.48 | 1.65 ± 0.5 | 0.9921 | |
| PVA-3.49HCl-B15 | 40 °C | 954.43 ± 52.31 | 5987.1 ± 268.7 | 397.8 ± 76.98 | 9602.28 ± 349.41 | 1.42 ± 0.66 | 0.9522 |
| 60 °C | 937.11 ± 45.12 | 4362.78 ± 6.55 | 321.03 ± 1.01 | 7940.26 ± 177.18 | 1.32 ± 0.42 | 0.9875 | |
Figures 8(a)–(c) shows the effect of stress (2 MPa, 4 MPa and 8 MPa) on the creep and recovery behavior of PVA based composite at 20 °C temperature. Results show that composites have varying response for different stress level. It can be observed that creep deformation is accelerated with increase in stress level. Moreover, cross-linked and reinforced composites resist creep deformation at each level of stress which results in the higher stiffness to creep deformation as compared to neat PVA. This is attributed to the formation of dense structure in composites as shown in figure 1 which causes the restriction in the movement of PVA chain. As it is also noticed that the creep deformation is further reduced for basalt fiber reinforced composite due to the restriction of the main chain. The response of basalt reinforced composite under stress depends upon the interfacial fiber/matrix bonding. The low creep deformation can be attributed to the higher interfacial adhesion between PVA and basalt fiber which also results in the better mechanical properties than PVA. Unrecovered creep deformation of cross-linked and reinforced composite is found to be lower than PVA which shows the higher elastic nature of cross-linked and reinforced composite. Effect of temperature on creep and recovery behavior has also been studied as shown in figures 8(d)–(e). It is noticed that with increase in temperature stiffness of composite is decreased which results in higher instantaneous deformation and creep strain. With increase in temperature, creep strains of PVA increases extensively whereas, cross-linking largely reduces creep deformation followed by reinforcement with basalt fiber which shows less temperature dependence of these composites. During unloading unrecovered creep strain of PVA is higher as compared to cross-linked and reinforced composite which shows the higher viscous behavior of PVA as compared to cross-linked and reinforced composite.
Conclusions
In the present work, reaction parameters are optimized to obtain PVA based composite cross-linked with HCl. Due to the formation of cross-linked bonds mechanical and thermal properties of pristine PVA are improved and further enhancement in properties is achieved by reinforcing basalt fibers. The results suggest that the major bonds formed during the reaction are first one -C-Cl bond which inhibits the solubility of PVA and the second one intermolecular/intramolecular –C–O–C– bond which imparts strength in the composite. The presence of both kinds of bonds is confirmed by FTIR spectroscopy. It is also noticed that the pristine PVA is thermally decomposed in two steps whereas, cross-linking which causes the modification of PVA structure results in the occurrence of thermal degradation only in one step. Moreover, due to the formation of the cross-linked network in PVA chain, composite shows two transitions region in dynamic mechanical analysis i.e. beta transition from 20 °C to 55 °C and glass transition from 120 °C to 150 °C. The maximum activation energy of composite is increased up to 15 wt% basalt fiber loading which also supports the ultimate tensile strength result. From creep test results, it is found that the cross-linked and reinforced composites show higher stiffness to creep deformation as compared to pristine PVA at all level of stress. Moreover, at higher temperature levels, creep strain increases extensively for PVA whereas cross-linking largely reduces creep deformation followed by reinforcement with basalt fiber. Unrecovered creep strain of PVA was higher as compared to cross-linked and reinforced composite which shows higher viscous nature of PVA.