Abstract
The advancement of metal oxide nanomaterials via green synthesis protocol proved leading tools for nanotechnology applications especially as drug deliver carriers, biosensors and clinical pharmaceutical. The aim of the current study was to synthesize cerium dioxide nanoparticles (CeO2 NPs) using orange peel extract (OPE) and evaluate its enactment for the antioxidant, anticancer and photocatalytic activities. Structural and morphological investigations of green synthesized CeO2 NPs was examined by using x-ray diffraction (XRD), transmission electron Microscopy (TEM), Fourier transform infrared (FTIR) spectra, Raman spectroscopy and ultraviolet-visible spectroscopy (UV–vis). Further, cytotoxicity and ROS activity of green synthesized CeO2 NPs exposed against cervical cancerous cells (HeLa). Green synthesized CeO2 NPs revealed the considerable antioxidant activity and hold effective methylene blue (MB) dye degradation in the presence of sunlight.
Export citation and abstract BibTeX RIS
1. Introduction
Nanotechnology has been developed as a novel and targeted approach that ensured a significant development in cancer therapy [1]. Nanobiotechnology is the most prevailing and evolving interdisciplinary division of nanotechnology and cell biology [2]. Nowadays, developing awareness of green chemistry has been observed in biomedical fields which includes cell imaging, biosensors and drug delivery [3]. Synthesis of nanomaterials via green methodology is relatively stable, exceptional biocompatibility, low cost and environment friendly [3–5]. Currently, CeO2 nanoparticles have demonstrated encouraging approaches as restorative agents in biology and medicinal sciences [3, 6]. The physicochemical properties of CeO2 NPs, for example, size and surface charge always play a key role in the eventual interactions of these nanoparticles with cancerous cells. In addition, CeO2 NPs are capable to explore reactive oxygen species and inhibit SOD (superoxide dismutase) at cellular level as well [6].
Momentous chemical and physical properties of orange peel extract fulfill the requirements of highly effective concentrated polyphenolic and flavonoids compounds that make excellent antioxidant candidates for countering cancerous cells [7]. Another uniqueness of OPE is the metal associated chelating effect or reducing effect. Usually, OPE is interacted with metal oxide nanoparticles and formed sigma bonds to maintain the oxidation state of metal oxide ion which support to produce the additional antioxidant properties [7–10 ].
Numerous efforts have been adopted towards excellent use of eco-friendly methods for the preparation of metal oxide NPs and mostly are comprised of plant or fruit and its peel extract [8–11]. Orange peel extract is rich in terpenoids, flavonoid and ascorbic acid. These compounds are supposed to be responsible for the redox reactions [12–14]. Therefore, CeO2 nanoparticles mediated via orange peel extract were prepared as chemical coating and reducing agent. The current study examined the cytotoxicity by observing cellular viability loss along with ROS activity against HeLa cervical cancerous cells, antioxidant potential and revealed substantial photocatalytic activity of green synthesized CeO2 NPs.
2. Material and methods
The collected orange peel pieces were cleaned several times by deionized water and dried at 80 °C in a convection oven for 3 h. The dried orange peel was grinned and dispersed into alcohol and finally orange-peel extract was obtained after vacuum filtration. Orange peel extract (3 ml) and cerium nitrate (10 ml) were mixed in deionized water. The obtained solution was stirred on magnetic stirrer at 80 °C for one and half hours. The colour was transformed to dark brown showing the formation of CeO2 NPs.
The 2, 2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay [11] was evaluated by the following formula:

HeLa cells (1 × 105 cells/well) were collected and perceived in 96-well plates as described in previous study [15]. Hereafter, green synthesized CeO2 NPs as a dose in the range from (10–125) μg ml−1 was delivered in each well of 96-well plates. In contrast, the remaining column was used for the control [16, 17].
Cytotoxicity of HeLa cancerous cells were evaluated by MTT (3-(4,5-dimethyl thiazol-2yl)- 2,5-diphenyl tetrazolium bromide) assay. Various concentration of green synthesized CeO2 NPs (10, 25, 50, 75, 100 and 125 μg ml−1) were used to treat HeLa cells after incubation of 24 h. 30 μl of MTT was added in each well and further incubated for 12 h at 37 °C with 5% CO2. [18]. The observed absorbance spectrum of obtained specimens were recorded at 595 nm wavelength using microplate reader.
Morphological variations induced by green synthesized CeO2 NPs was obtained using inverted phase contrast microscopy at 20× magnification against HeLa cells. Intracellular reactive oxygen species was sensed using a non-fluorescent CMH2DCFDA (Invitrogen Partnership, USA) compound. The compound is distributed via the cell membrane and applies demobilization by esterase's, which generates the nonfluorescent CMH2DCF, which reacts with oxygen species in the cells. After that, HeLa cells were treated with the several doses of green synthesize CeO2 at 37 °C for 12 h [19, 20]. Finally, strength of fluorescence was observed using a Gemini EM spectrofluorometer.
Photocatalytic activity of green synthesized CeO2 NPs was examined on methylene blue (MB) dye under solar irradiation [19].
Surface morphology and crystal structure of green synthesized CeO2 NPs was studied by TEM and XRD while UV spectroscopy attained by the spectrophotometer (Shimadzu, UV-2450). The particle size of green synthesized CeO2 NPs was analyzed by a particle size analyzer (Nanotrac, USA). The existence of biomolecules of green synthesized CeO2 via OPE NPs was confirmed by FTIR study.
3. Results and discussion
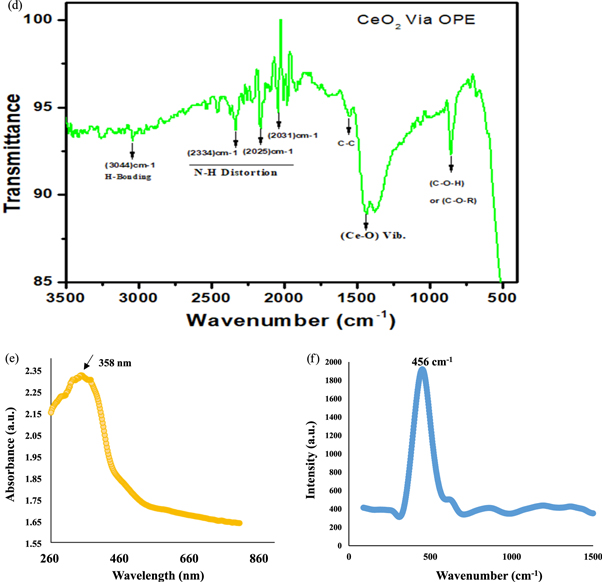
The strong diffraction peaks at 2θ values of 28.43°, 33.62°, 48.38°, 54.03°, 63.2°, 73.40°, 76.69°, and 85.09° are showing the associated results with the structures (111), (200), (202) and (311), (222), (400), (313), and (402) crystal planes as shown in figure 1(a). These XRD results confirmed the cubic structure for green synthesized CeO2 NPs that was matched with associated card number in COD library [20]. Crystallite size of CeO2 NPs was calculated about 20 nm by using Scherer's equation. TEM analysis certainly confirmed that the prepared CeO2 NPs via green protocol using OPE have cubic nanostructure with variations in crystallite size of 20 to 25 nm as revealed in figure 1(b). The average diameter of all particles of CeO2 NPs mediated via OPE was calculated using dynamic light scattering (DLS) techniques. Figure 1(c) reveals that majority of CeO2 NPs exhibits diameter of 23 nm approximately. In figure 1(d), observed spectra of FTIR ensured Ce-O vibrations that was attributed through band location at 1460 cm−1. The above results revealed the presence of orange peel associated band at 1045 cm−1 which indicates the alcohol or ester linkage (C–O–H or C–O–R) [20, 21]. There is no extra sharp peak of water molecule in the range of (3000 to 3600 cm−1), which confirm the purity of the prepared CeO2 NPs via orange peel extract. At 2361–1840 cm−1 range, N–H bond creates a small distortion which is basically due to orange peel extract. The involvement of hydrogen bonding reflected at 2918 cm−1 and 2850 cm−1 as constituents of orange peel extract [20, 22]. The maximum absorption is observed at 358 nm, which is associated with the progression of green synthesized CeO2 NPs as seen in figure 1(e). Oxidized polyphenols were confirmed with the existence of expansion band that leading towards the prevention of agglomeration which is accountable of successive CeO2 NPs mediated via OPE as revealed in figure 1(e). The Raman spectrum of CeO2 via OPE shows a sharp band at 456 cm−1 as prescribed in figure 1(f), which attributed to symmetrical stretching mode of the Ce-O vibrational unit that confirmed the strong interaction between CeO2 and orange-peel extract [21–23].
Download figure:
Standard image High-resolution imageFigure 1. Green synthesized CeO2 nanoparticles using orange peel extract (a) Represents the XRD spectra which confirms the cubic crystal structure of prepared specimen (b) Transmission electron microscopy analysis (c) DLS analysis of CeO2 nanoparticles (d) UV–vis spectra (e) FTIR spectra revealed the presence of CeO2 NPs stretching (f) Raman Scattering spectra shows the CeO2 NPs energy shift.
Download figure:
Standard image High-resolution imageRadical scavenging activity confirmed the antioxidant activity of green synthesized CeO2 NPs that was originated with the gradual increase in concentration ranging from 40 μg ml−1 to 100 μg ml−1 as seen in figure 2(a). The observed investigations perceived the superior antioxidant activity of CeO2 NPs via orange peel extract as compared to ascorbic acid [24, 25]. Figure 2(b) shows the fluorescence stability of CeO2 NPs in aqueous solution at 4 °C and 37 °C, respectively. After 3 weeks of storage at 4 °C and 37 °C, the fluorescence intensity of CeO2 NPs remained 92% and 88% of the initial intensities that indicate a consistent stability.
Download figure:
Standard image High-resolution imageFigure 2. (a) DPPH radical scavenging activity (b) Fluorescence stability and stability in water of CeO2 NPs (c) Loss in cell viability of Green synthesized CeO2 nanoparticles against HeLa cells, t-test (*p < 0.05) (d) Representation of Linear calibration plot of CeO2 concentration versus cell viability (e) Cellular destruction of HeLa cells by CeO2 (scale bar 50 μm; magnification ×40).
Download figure:
Standard image High-resolution imageThe appraisal of cytotoxic results using MTT assay of the green synthesized CeO2 NPs was evaluated on the HeLa cell line. In figure 2(c), it is seen that the cell viability was lessened from 92%–33% when Hela cells were displayed to green synthesized CeO2 NPs at the concentrations of 10, 25, 50, 75, 100 and 125 μg ml−1. In figure 2(d), the value for the R2 = 0.99 shows that the analytical outcomes confirmed the correctness of the preliminary outcomes. In another study, biosynthesis of CeO2 NPs by carrageenan proved the metabolic activity, which was reduced in a concentration-dependent way against the WEHI 164 malignant cells [26]. CeO2 NPs hindered the lung melanoma cells that is in accordance with our results [27]. Cellular destruction of HeLa cells revealed the morphological variations using inverted phase contrast microscopy induced by green synthesized CeO2 at different doses as illustrated in the figure 2(e).
The prescribed photomicrograph as shown in figure 2(e) exposed the hatched HeLa cells for 24 h in the presence and absence (control) of CeO2 NPs. The untreated or control HeLa cells looks like a long armed, epithelial and bulky adherent cells along blurry cell borders. On the other hand, when cervical Hela cells treated with green synthesized CeO2 NP at 25, 50 and 100 μg ml−1, notable effects in cellular morphology has been observed which is not present in other untreated control group. In figure 2(e), another unique behavior of this investigation is the appearance of green synthesized CeO2 NPs treated cells is very less dense along contracted arms.
ROS accumulates on mitochondria, which aids cellular apoptosis by means of vascular blockade [28, 29]. The prominence of metal oxide NPs declined with size and expanded oxygen vacancies [30, 31]. Thus, countless electron–hole pairs would results which could move around to the NPs surface and lead to the ROS generation. In fact, electrons and holes existing in the aqueous environment of CeO2 NPs that could associate with the oxygen and hydroxyl particles. This created excessive amount of highly reactive free radicals, for example, the superoxide anion radical (from electrons) and the hydroxyl radical (from holes) which could oxidize and diminish macromolecules (DNA, lipids, proteins) prompting the oxidative destruction to the cell [31–33]. In this study, H2DCFDA staining was used to confirm the existence of ROS and distinguished as the cell-permeate indicator for ROS generation. It is non-fluorescent dye until the acetate groups are unresponsive by intracellular esterase to deliver the green fluorescence which gives an indication of the locality of intracellular ROS inside cells. Therefore, green synthesized CeO2 NPs at variable concentration showed the significant accumulation as revealed in figure 3(a). Moreover, we also perceived an inverse linear relationship between the ROS and cell viability as seen in figure 3(b). Green fluorescence micrographs represented the existence of intracellular ROS in the HeLa cells which created by CeO2 NPs accumulation in HeLa cells and enhanced the fluorescence Intensity as demonstrated in figure 3(c). Methylene blue (MB) dye degradation in presence of green synthesized CeO2 NPs was confirmed during 30-min exposure in sunlight with the decline of peak intensity at 657 nm as shown in figure 3(d). In the meanwhile, color of degraded MB dye was gradually transformed from deep blue color to colorless dye solution. In this photocatalytic activity, the core dynamic species are holes, hydroxyl and superoxide radical [33, 34].
Figure 3. (a) ROS (% of control) generation in HeLa cells caused by CeO2 accumulation. (b) A significant inverse correlation between ROS and cell viability (c) ROS micrographs (d) Photocatalytic activity of CeO2 NPs for methylene blue dye degradation (e) the corresponding plots of ln (Co/C) versus irradiation time.
Download figure:
Standard image High-resolution imagePhotodegradation reaction is found to follow a pseudo-first-order reaction. The photodegradation rate constant for this reaction was determined by using following equation:

where Co and C represent the initial and final concentrations respectively at time t and k is the apparent first order rate constant. Graphical representation of ln Co/C versus time appears as a straight line as shown in figure 3(e), whereas the slope this straight line upon linear regression is equal to the apparent first-order rate constant k [33]. The degradation rates were found to have values 0.03 min−1 for CeO2 NPs mediated via OPE and found to be consistent with the results obtained from the photodegradation. As a result, it is evident that CeO2 NPs synthesized via orange peel extract is exceptionally potential photocatalytic agent for MB dye degradation under sunlight.
4. Conclusion
CeO2 NPs were successfully synthesized via orange peel extract using a simple and eco-friendly green approach for the first time. MTT assay assessments and cellular morphological scrutiny indicated that green synthesized CeO2 NPs created a noticeable cytotoxicity against the HeLa cells, which revealed the reliance of ROS release and obvious HeLa cancerous cells devastation by cell necrosis/apoptosis. Moreover, photocatalytic activity of degraded MB dye indicated that these nanoparticles further appreciates to advance research in the field of textile industry and wastewater treatment as well.
Acknowledgments
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University.
Author contributions
All authors significantly contributed in the manuscript.
Conflicts of interest
The authors declare no conflict of interest.