Abstract
In this study, the fabrication of Al-Al2O3-TiB2 hybrid composite using TiO2, B2O3, and Al5083 powders through aluminothermy method has been studied. TiO2, B2O3, and Al5083 powders were attrition milled (20:1 bullet to powder ratio, under argon atmosphere, the speed of 650 rpm for 2, 4, 6 and 20 h). After the completion of the milling process, the milled powder was added to the aluminum molten at 900 °C and finally, the Al5083-Al2O3-TiB2 composite was produced in situ. After the hot extrusion process, specimens were characterized by SEM and XRD analyses and the strength of the composite was investigated. The results of the microstructure analysis and the strength of the specimens indicate the efficiency of low milling times and the absence of TiB2 in milling. The observations from microstructure show presence of TiB2 ceramic particles less than 300 nm in size. The strength tests done on 2, 4, 6 and 20-h specimens represent values of 294, 336, 295 and 276 MPa, respectively.
Export citation and abstract BibTeX RIS
Introduction
Metal matrix composites have attracted the attention of many researchers and craftsmen due to their high strength to weight ratio.
One of the common uses of these composites is in the automobile, aerospace, chemical, and transfer industries, and they can also be used as cutting tools, attrition resistant substrates, lightweight armors and Neutron absorbents [1], [2].
Studies have shown that there are various methods for synthesizing materials in order to fabricate composites, including liquid phase processes [3], solid phase processes [4], deposition processes [5], and in situ processes [6].
In the in situ synthesis method, the reinforcement is produced during the composite process by occurring chemical reactions in the base. Different types of in situ synthesis methods are; Flux Assisted Synthesis (FAS) which is also known as the salt reaction or casting reaction [7], response surface methodology (RSM) [8], and in situ synthesis in high-energy mills such as planetary mills [9] and Cryomilling [10]. The in situ processes have some advantages over other processes including (1) the produced reinforcement is thermodynamically stable and it has a higher stability when it is under high temperatures; (2) the interfaces formed between the reinforcement and the base are distinguishable (3) The in situ method tends to produce finer size reinforcement that improves the mechanical performance of composites [11].
TiB2 is one of the materials that can be synthesized in situ due to its high elasticity modulus, fracture toughness, thermal stability, and high stiffness. This material is obtained from the reaction between the two salts of K2TiF6 and KBF4 [12] or the reaction between titanium oxide and boron oxide along with aluminum [13]. Meanwhile, the use of titanium oxide and boron oxide is interested because of cost-effectiveness reasons [14].
Chen et al [13] used TiO2, H3BO3, and Al-4.5Cu alloys as raw materials in order to synthesize TiB2 in resistance furnaces at 900 °C. In this study, an undesirable phase of Al3Ti was observed in addition to the TiB2 particles, too. Angers et al [14] reported that adding 0 to 25% volume percent of 2–6 μm Sic particles to 2024 alloy increased the tensile strength from 402 MPa to 595 MPa while decreased the flexibility from 10% to 2.4%. Growing the size of silicon carbide particles caused a further reduction up to 1.9%.
In the present work, considering the discussed issues, boron oxide and titanium oxide were used as boride and titanium precursors for the synthesis of titanium diboride. In order to increase the strength of the composite, 5083 aluminum alloy as one of the strongest aluminum grades was selected as the base alloy due to its mechanical properties and good adhesion with the lowest cost. To reduce both particle size and activation energy, the reaction between the precursors was done through an attritor ball mill. Vortex casting was used to fabricate composites.
Experimental
In this study, 5083 aluminum (table 1) was selected as a base composite and boric acid and titanium dioxide (figure 1) was used for the synthesis of titanium diboride. The raw materials used in the current study are presented in table 2.
Table 1. The elements in used Al5083 alloy.
| Element | Mn | Fe | Cu | Si | Zn | Cr | Ti | Mg | Al |
|---|---|---|---|---|---|---|---|---|---|
| Wt% | 0.04–1.0 | 0.04 | 0.01 | 0.04 | 0.52 | 0.50–0.52 | 0.15 | 4.00–4.09 | remain |
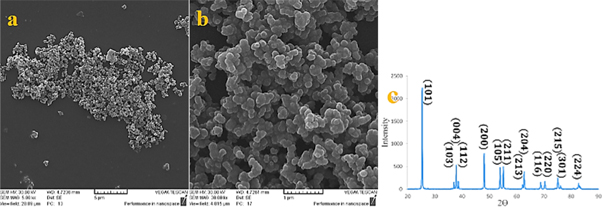
Figure 1. (a) and (b) Image of scanning electron microscope (c) x-ray diffraction pattern of TiO2 powder.
Download figure:
Standard image High-resolution imageTable 2. Raw materials and properties of the raw materials used in this research.
| Raw materials | Purity | Particle size | Purpose of use |
|---|---|---|---|
| Titanium dioxide | 99 | <300 nm | As a titanium precursor |
| 5083 Aluminum powder | 99 | 100 μm | Aluminothermy of raw materials in the mill |
| 5083 Aluminum ingot | 99 | — | Base of the composite |
| boric acid powder | 99 | <0.5 mm | As a boride precursor |
| Argon gas | 99.99 | — | Milling environment |
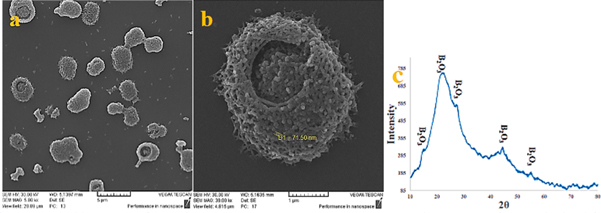
Boric acid was used to prepare boron oxide. Boric acid was placed in a resistance furnace at 650 °C and dehydrated. Boron dioxide balk was crushed. Then, boron dioxide powder attrition milled by speed 360 RPM for 30 min Finally, the pseudo-crystal phase was obtained from B2O3 (figure 2). In order to obtain a powder with a uniform particle size, the crystal phase was transferred to a bullet-shaped milling, and then sifted through a 100 sieve mesh.
Figure 2. (a) and (b) Image of scanning electron microscope (c) x-ray diffraction pattern of B2O3 obtained from boric acid dehydration.
Download figure:
Standard image High-resolution imageThe amount of powders was determined according to the stoichiometric ratio of reaction (1). In addition, based on conducted studies indicating high vapor pressure of B2O3, as well as to prevent the formation of an undesirable Al3Ti phase (in term of reduction in mechanical properties) [14], the content of this material was 10% higher than the stoichiometric ratio.
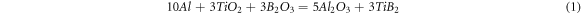
Milling was done within an attritor ball mill having 20:1 bullet to powder ratio under argon gas with 99.9% purity and 8 psi pressure. 6 millimeters Stainless Steel Bullets were selected. The powders were milled at the rate of 650 rpm for 2, 4, 6 and 20 h. After milling, the mill chamber was transferred to the glove box and powders were discharged from the mill under argon atmosphere.
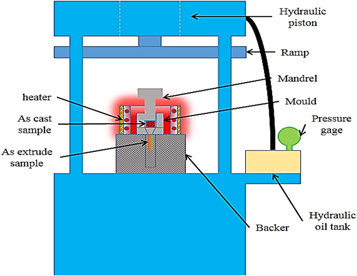
Aluminum 5083 ingot was melted in a resistance furnace at 900 °C. After melting, the aluminum powders were added to the molten material as foils at 2, 4, 6 and 20 h. The molten material was stirred by the graphitic stirrer (figure 3) at the speed of 350 rpm for 5 min and then it was rest for 5 min to complete the crystalline structures and do chemical reactions, and then the molten product was again stirred for 3 min to obtain a uniform distribution of reinforcement particles.
Figure 3. An 8-blade 90° angle turbine stirrer (left) and the type of generated flow in the fluid (right).
Download figure:
Standard image High-resolution imageThe resulting melt was cast within a steel mold (figures 4 and 5).
Figure 4. A simulated schematic figure of the process and used vortex casting system.
Download figure:
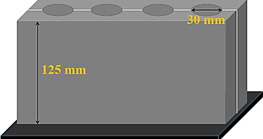
Standard image High-resolution imageFigure 5. Schematic figure of the steel mold used to cast the specimens.
Download figure:
Standard image High-resolution imageSpecimens were prepared for extrusion operation. They were extruded at 300 °C with a ratio of 3:1 (figure 6).
Figure 6. A simulated schematic figure of the process done for extruding casting specimens.
Download figure:
Standard image High-resolution imageAfter completing the casting and extruding process, metallographic and then etch process of the specimens were performed in 50 ml of Paulson reagent (3 ml HCl + 2 ml HF + 20 ml HNO3 + 175 ml water) + 50 ml of HNO3 + 40 ml of solution of 3gr of Chromic Acid per 10 ml of water Became.
The specimens were prepared according to ASTM E8 standard for the tensile test. The tensile test was done at an ambient temperature at a speed of 1 mm min−1 in the same direction of the extrusion process.
In order to study the phase status of the powders obtained from milling and leaching (with HF) processes, x-ray diffraction (XRD) analysis was performed using Cu kα radiation on the casting specimens. The DSC analysis was used to characterize the thermal specimens. The microstructure characterization of the specimens was done using a field emission scanning electron microscope (FE- SEM) (MIRAJ TESCAN model).
Results and discussion
XRD
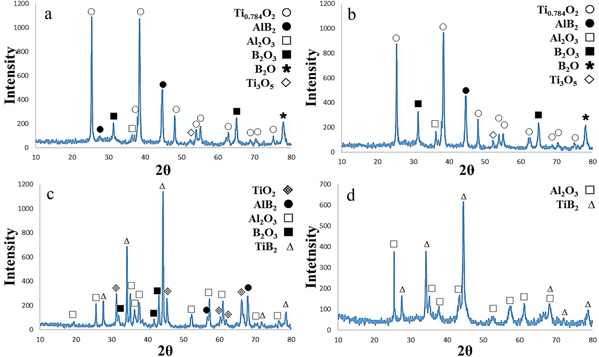
Figure 7(a) illustrates the XRD pattern of a two-hour milling. As shown in the figure, AlB2 phase with two interphase combinations is visible by performing 2 h of milling.
Figure 7. An image of x-ray diffraction of TiO2-B2O3-Al powders in milling time of (a) 2 h (b) 4 h (c) 6 h d) 20 h.
Download figure:
Standard image High-resolution imageFigure 7(b) shows the XRD pattern of specimen milled for 4 h. As shown in the figure, the diffraction pattern is similar to the two-hour milling and there is no change in phases except that the peak intensity of alumina has increased slightly.
Contrary to Loo et al's report in which TiB2 cannot be formed during the milling process of Al-Ti-B even after 30 h [15], in the present work, after a 5.5-hour milling, the peaks of TiB2 were appeared indicating the initiation of the reaction (figure 7(c)). This is attributed to small-sized particles of the raw materials, especially the boron oxide, the high speed of the milling rotation and the high ratio of the bullets to powder.
As shown in figure 7(d), the peaks of the middle phases have been decreased and the peaks of Al2O3 and TiB2 have remained. Should be noted that, the interfacial area of Al in contact with TiO2 and B2O3 increases as the milling time increases, thereby the efficiency of the reactions increases as well. From the thermodynamic and kinetic viewpoint, by milling process barriers of activation of the reactions decrease while the energy of the reactants increases [16].
Widening of the XRD peaks can be attributed to the size of the crystals and the increase in the strain amount of the network generated due to the milling process. Therefore, the XRD analysis led to this conclusion that interactions in the Al-Ti-B system occur within the mill.
Thermodynamics review
As it can be seen (figure 8), there are several endothermic peaks in the DTA curve. It seems that the first endothermic peak near to 110 °C is related to the reduction region of TG curve, which can be due to the loss of absorbed water in the mixture resulting in rapid reduction in the TG curve. The second endothermic peak is near to 150 °C, which can be attributed to the thermal decomposition of unstable phases. The last endothermic peak near to about 650 °C, which is as a result of the melting of the Al metal powder, remained in the milled powder mixture.
Figure 8. TG-DTA results for milled powder after 4 h under argon atmosphere and 10 °C / min heating rate.
Download figure:
Standard image High-resolution imageAn exothermic peak is observed about 800 °C. This peak indicates that a highly exothermic reaction occurs during two following reactions on the system:
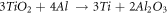
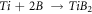
Based on the TG-DTA combined analysis, Al2O3-TiB2 composite is produced at a temperature range of 730–960 °C. As stated in the reports, the temperature for synthesis of the TiB2 particles without milling is 1100 °C [17].
TiB2 cannot be produced through a direct reaction between TiO2 and B2O3 because Gibbs free energy of TiB2 is much higher than those of TiO2 and B2O3, but because of low Gibbs free energy of Al2O3, TiO2 and B2O3 first react with aluminum and then form TiB2.
The thermodynamic calculations show that the change of Gibbs free energy due to the reduction of boron oxide and titanium dioxide with aluminum (reactions 2 and 3) at ambient temperature is desirable [2].
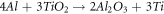
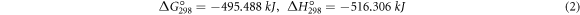
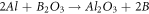
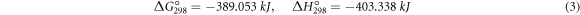
Then, the free atoms of Ti and B react with each other and create the form of Ti-B and interphase compounds of Al-B and Al-Ti.
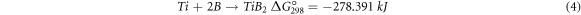
The activation time of the reaction depends on involving exothermic process, the mill condition, and the mechanical properties of the raw material; in other words, if the reaction is highly exothermic, the mill bullets can initiate a mechanical reaction.
When the ratio of titanium dioxide to boron oxide is high, the below reaction occurs and the Al3Ti needle shape forms in a composite [13].
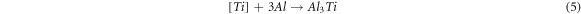
As the value of boron oxide increases, these particles react with aluminum and create the AlB2 phase:
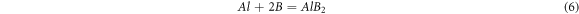
By increasing the amount of boron oxide and the occurrence of reaction 6, titanium diboride may possibly be generated due to reactions between Al-Ti and Al-B (as in reaction 7) [17].
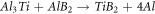
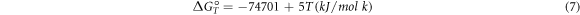
Microstructure
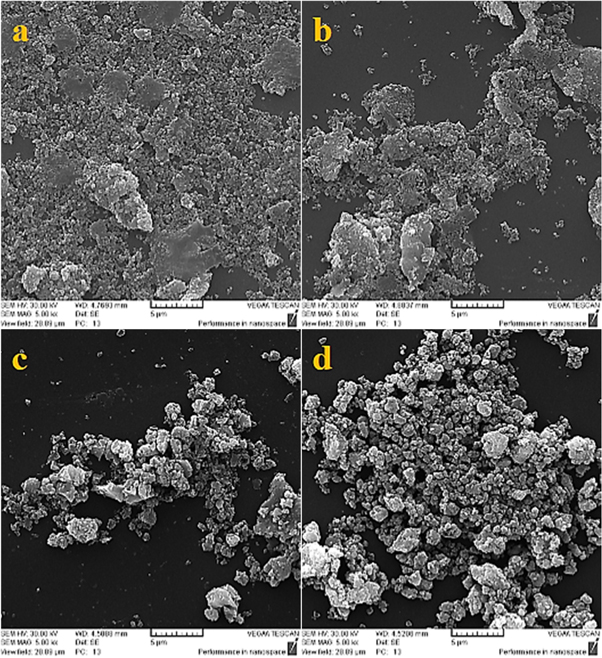
A microstructural analysis was carried to better understand the mechanical alloying process of the powders. Figures 9(a) and (b) show the SEM images of powders before the blast in milling Process. As seen in figure 9(a), after 2 h of milling time, flattening is a predominant phenomenon at this stage. The trend of flattening in the particle size during the mill process occurs more easily in soft materials than hard materials. On the other hand, because of the high flexibility of aluminum, at first, it is widened as observed in the figure. After 4 h, the phenomenon of fracture occurs and the flat particles break and only the particle size and their distribution become more uniform (figure 9(b)).
Figure 9. Images of powders obtained after (a) 2 h (b) 4 h (c) 6 h (d) 20 h (a, b before and c, d after blast).
Download figure:
Standard image High-resolution imageFigures 9(c) and 9(d) show the SEM images of powders after the blast in milling Process. From this stage, the type of alloying mechanism is changed from composite to ceramic. Figure 9(c) shows the SEM image of the powder after 6 h of milling time (blast moment). Increasing the temperature because of the collision among the bullets and the bullets to powder, provide the required energy for the formation of TiB2 particles and crystalline phases [17]. Since the blast occurs at the break stage of the powder, and on the other hand, since the break not been carried completely, the limited size of the reinforcements are widened. So the fine and coarse grain can be seen beside each other. Because the penetration rate of fine grain is greater than the coarse grain material, it can be said that most fine grain become the final material (Al2O3 & TiB2) after the blast. Therefore, it can be concluded that the reaction will occur in a limit of milling time and the fine grain will react earlier and the coarse grain will react later.
Figure 9(d) shows the morphology of the powder after 20 h of milling time. As can be seen, the powders is reached a steady state that the balance is between the agglomeration and the break.
The images obtained from the scanning electron microscope of casting specimens show non-uniform distribution and lack of a good connection between reinforcement and matrix (figure 10). The TiB2 synthesized particles are observed in cubic and hexagonal forms in the size of 200 nm to 600 nm (figure 11(b)). There was also no trace of the Al3Ti undesirable phase as mentioned in the references [18]. This is due to an increase of the boron value to react. The second phases (MgAl2O4 and MgSiO3) because of the freezing process of Al 5083 alloy, which is inevitably present [19], [20], also are observed in figures 10 and 12. These phases can be broken during deformation and formation processes, such as extrusion (figure 12(a)) and have uniform distribution at the surface [21].
Figure 10. Images obtained from field emission scanning electron microscope for as cast specimens containing powders milled for (a) 2 h (b) 4 h (c) 6 h (d) 20 h.
Download figure:
Standard image High-resolution imageFigure 11. Images of field emission scanning electron microscope for TiB2 particles.
Download figure:
Standard image High-resolution imageFigure 12. Images obtained from field emission scanning electron microscope for extruded specimens containing powders milled for (a) 2 h (b) 4 h (c) 6 h (d) 20 h.
Download figure:
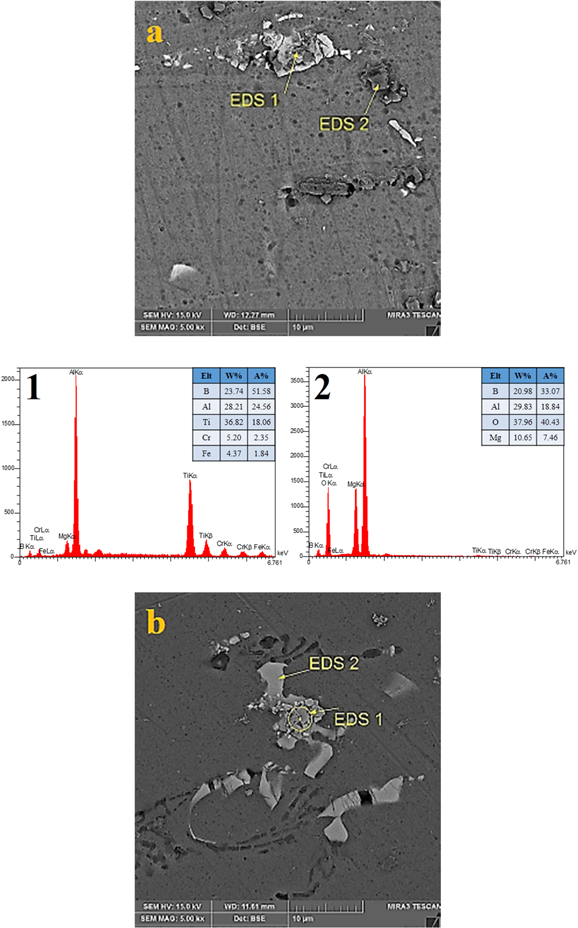
Standard image High-resolution imageAs stated before, in situ method leads to hard surface connection, but in the specimen containing powder milled for 20 h, this connection and wettability were decreased because the powders were formed in milling rather than in the melt, and the powders were agglomerated in sizes more than 20 microns (figure 10(d)), which was not observed in figure 10(a) and figure 10(b). in contrast, for specimens in which the reinforcements were formed within the melt, the particles are separated while in the specimens containing powders milled for 2 h, particles are not well distributed (figure 10(a)). As shown in the images of the extruded specimens, the agglomerates were broken and the particles were distributed along the extruded direction (figure 12). Unlike claims of references, the second phases not fractured (figure 12(b)) and remained at the dendritic shape. In figure 13, EDS analysis on 2 and 4-hour specimens shows that the reinforcement particles are formed on the second phases.
Download figure:
Standard image High-resolution imageFigure 13. EDS analysis of extruded specimens containing powders milled for (a) 2 h (b) 4 h.
Download figure:
Standard image High-resolution imageStrength
Table 3 shows the ultimate tensile strength variations of the Al5083 alloy and in situ composites. The ultimate tensile strengths (UTS) of a, b, c and d specimens were 294, 336, 295 and 276 MPa respectively, which was improved in comparison with the base alloy in the specimens containing 4-hour milled powder. Ramesh et al [18] achieved a maximum strength of 140 Mpa for Al6063-TiB2 metal-based composite. In specimen d, the least properties were observed, so the results are consistent with the images obtained by the electron microscope. As seen in the figure, the specimen d has the highest porosity and agglomerate particles. Also, the tensile properties of the specimens whose reinforcement particles have been synthesized within the melt are better than those in which their reinforcement particles are not synthesized in milling. This improvement in the ultimate tensile strength is mainly attributed to the presence of TiB2 and Al2O3 particles in the base alloy. Other reasons for the improvement of strength can be the presence of some dislocations around TiB2 particles due to the difference in the thermal expansion coefficients between the base and the TiB2 particles, as well as the interactions between the TiB2 particles and dislocations and the phases of the 5083 alloy casting during composite load bearing by the composite. So TiB2 particles and phases act as obstacles to dislocation during the tensile test [22]. Also, bearing high stress by reinforcement phase during the tensile test (regarding the stiffness of TiB2 particles) leads to an increase of UTS [23]. But these strengths are beyond expectations. The main reason for it is the elimination of alloy Mg from the Al5083 alloy. This can be explained by quantometry analysis of cast alloys. The result of the specimens' analysis indicating the decrease of the Mg element is represented in table 4 (this result is the mean of 8 times analyses). Therefore, it can be said that the exothermic reaction as a result of a reaction between the powders in the melt increases the localized temperature inside the molten to an extent which evaporates the magnesium in the alloy.
Table 3. Tensile properties of specimens cast with milled powder at different times.
| Sample | Milling time | Reinforcement (%) | Stress yield (MPa) | U.T.S (MPa) | Stress at break (MPa) | Strain (%) | Young's modulus |
|---|---|---|---|---|---|---|---|
| 1 | 0 | 5 | — | 304 | — | 18.14 | — |
| 2 | 2 | 5 | 204.9 | 294.2 | 165.6 | 6.26 | 7.23 |
| 3 | 4 | 5 | 245.2 | 336.2 | 212.8 | 6.99 | 6.94 |
| 4 | 6 | 5 | 223.3 | 295.7 | 104.1 | 5 | 6.72 |
| 5 | 20 | 5 | 227.5 | 276.2 | 244.3 | 3.21 | 7.38 |
Table 4. The result of the quantometry analysis on cast specimens.
| Element | Mn | Fe | Cu | Si | Zn | Cr | Ti | Mg | Al |
|---|---|---|---|---|---|---|---|---|---|
| Wt% | 0.15 | 0.061 | 0.075 | 0.0005 | 0.12 | 0.030 | 0.068 | 2.03 | remain |
Fracture surfaces
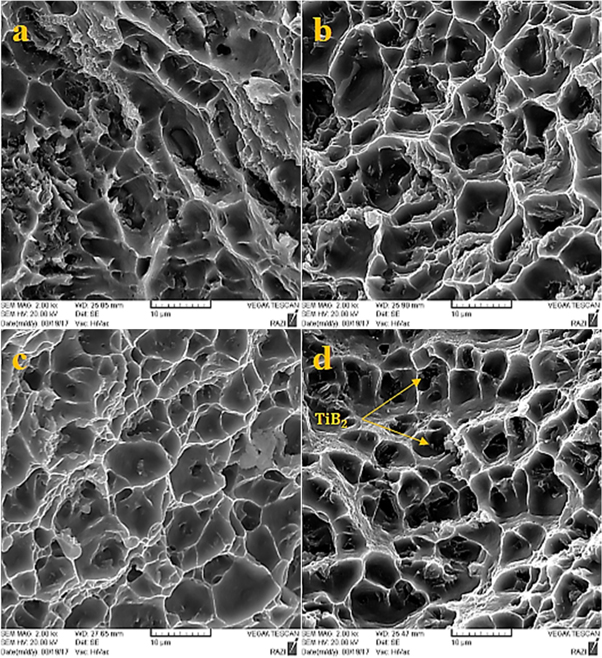
At high temperatures, the fluidity of the molten and the rising possibility of porosity increases. The fracture path continues from the porous regions [24].
Formation of dimples is evident from figure 14. Small and large dimples are observed in samples, although specimens reinforced with synthesized particles in molten have smaller dimples than those reinforced with synthesized by milling. As local damage initiates in agglomerates, the strength and ductility of particles reduced intensely (figure 14(d)). In figure 14(b), the presence of TiB2 particles at the fracture surface indicates a strong bond between the base and the reinforcement.
Figure 14. Fracture surfaces of Al5083-TiB2 composite fabricated with powders milled (a) 2 h (b) 4 h (c) 6 h (d) 20 h.
Download figure:
Standard image High-resolution imageConclusion
- 1.The milling provides the required activation energy for the reaction between the particles. By doing this process, the working temperature for the synthesis of the particles within the melt can be reduced.
- 2.Longer milling times lead to the synthesis of particles in the milling so the use of these powders in the melt results in loss of properties in the final composite.
- 3.The best strength is related to specimens in which synthesis of the reinforcement has been done in the molten product (330 MPa), but in general, there was no significant improvement in the strength of the 5083 alloy. This is due to the removal of alloy Mg in the 5083 alloy and non-uniform distribution of particles in the composite.
- 4.For a synthesis of the particle at Nanoscale, stirring speed should be increased and the ultrasonic device should be used to break agglomerate particles.