Abstract
Stability and hydrophilicity of TiO2 nano-coating was improved in this work. TiO2 nanoparticles were prepared via conventional, microwave radiation, surfactant, refrigerating, and silica-assisted sol-gel processes. The coated rock plates were obtained by impregnating the plates with nanofluids. The modified TiO2-based coatings shows to be more effective for wettability alteration purposes compared to the conventional TiO2 coating. The un-coated surface was superhydrophobic where the n-heptane and water droplets contact angles on the rock surface were 0° and 168° respectively. The n-heptane contact angle on the rock treated via conventional, microwave radiation, surfactant, refrigerating, and silica-assisted sol-gel synthesized nanoparticles changed to 128°, 159°, 156.8°, 151° and 163° respectively. While water contact angle was 53.5°, 0°, 24.1°, 28.6°, and 0° applying the above processes. This confirms that the superhydrophilic coatings were formed on a superhydrophobic surface. The fabricated nano-coatings exhibited high thermal, mechanical and salinity stability. The rock surface before and after treatment as well as the synthesized nanoparticles were characterized by SEM, XRD, and FTIR analyses. Moreover, the surface charge of the nanoparticles in the solution was evaluated using Zeta potential analysis. Applications of these nanocoatings vary from self-cleaning surfaces, protection of building facades and public monuments from weathering however, results of this study indicate possibility of using the materials for wettability alteration of oil-wet carbonate rock in enhanced oil recovery (EOR) process.
Export citation and abstract BibTeX RIS
1. Introduction
Hydrophilic surfaces have great importance in fundamental research and practical applications. These surfaces have many advantages in cleaning technical surfaces due to their interesting properties such as having less dirt, being easy to clean, inhibiting growth of vegetation and bacteria as well as oil-water separation [1, 2]. Furthermore, the surfaces can improve fluids flow in porous substrates such as mineral rocks however, creation of the superhydrophilic coating on porous substrates is difficult due to complexity of the process [3–5]. Moreover, practical application of the hydrophilic coatings are greatly limited by their poor mechanical and thermal stability [6, 7] and their low durability in contact with salt solution [8–10]. Therefore, there is a great challenge in developing the stable hydrophilic coatings.
Modifying the wettability of porous substrates such as rocks is an important subject due to spontaneous imbibition of different liquids into porous media [11, 12]. This has important industrial applications in oil production [12, 13] that is focused in this study.
One of the main challenges in enhanced oil recovery is oil production from the oil-wet carbonate rocks [14]. More than half of the oil reserves in the world are held in the oil-wet carbonate reservoirs [15, 16]. In these type of reservoirs, conventional water flooding technique for enhance oil recovery is not productive [17]. This is due to the oil as wetting fluid that adheres to the rock surface. Thus, water does not imbibe into the rock matrix and only oil in the fracture is produced while most of the oil traps in the pores [18]. One of the most effective mechanisms to increase oil production from oil-wet carbonate reservoirs is wettability alteration toward water-wet state [19, 20]. It causes water to imbibe into the walls of porous media and displace the oil from the pores. In a water-wet reservoir, the mobility of oil in rock matrix increases with water injection resulting oil production to increase [20, 21].
Several methods have been suggested for wettability alteration in carbonate reservoirs including injection of surfactants [15, 22–24], and alkaline/surfactants [25, 26]. However, these methods are very limited by the reservoir conditions. Some researchers have reported that wettability alteration of rock surface from hydrophobic toward hydrophilic state, can be obtained by nanoparticle adsorption on the rock surfaces [11, 27, 28].
Nanoparticles have a high surface energy and due to their nano-sized dimention, they can migrate through the micron and submicron-sized rock pores [12, 29, 30]. Since, hydrophilic surfaces can be obtained by modifying the surface energy and surface roughness of the rock, wettability of the rock will change by adsorption of nanoparticles on the rock surface [16, 31, 32].
Despite the vital importance of carbonate reservoirs, previous studies have focused on sandstone formations to obtain more hydrophilicity in hydrophilic surfaces [33–38], or wettability alteration in gas condensate reservoirs [39–41] while limited reports are available for oil-wet carbonate reservoirs [21, 42]. Furthermore, the thermal, mechanical and salinity stability of the coatings has not been studied in detail. Table 1 shows a summary of studies published for wettability alteration of mineral rock by nanoparticles.
Table 1. Summary of some conducted studies for wettability alteration of mineral rock surface by nanoparticles.
| References | Nanoparticle type | Contact angle change | Rock |
|---|---|---|---|
| [12] | SiO2 | Water contact angle in oil medium from 54° to 38° | Sandstone |
| Al2O3 + PVP | Water contact angle in oil medium from 54° to 28° | ||
| TiO2 + PVP | Water contact angle in oil medium from 54° to 22° | ||
| [33] | Al2O3 + Surfactant (PRNS) | Water contact angle in air medium from 104°−142° to 0° | |
| [37] | TiO2 | Water contact angle in oil medium from 125° to 90° | |
| [38] | SiO2 | Oil contact angle in water medium from 54° to 22° | |
| [40] | Fluorinated silica | Water contact angle in air medium from 0° to 147 | |
| Oil contact angle in air medium from 0° to 61° | |||
| [43] | SiO2 | Oil contact angle in water medium from 35° to 130° | |
| TiO2 | Water contact angles in air medium from 0° to 161 | ||
| Oil contact angle in air medium from 0° to 144° | |||
| [44] | SiO2 | Water contact angles in air medium from 0° to 164° | Carbonate |
| Oil contact angle in air medium from 0° to 151° | |||
| CNT | Water contact angles in air medium from 0° to 163° | ||
| Oil contact angle in air medium from 0° to 147° | |||
| [42] | ZrO2 | Water contact angle in air medium from 118° to 35° | |
| Oil contact angle in water medium from 180° to 0° | |||
| [21] | SiO2 | Water contact angle in oil medium from 122° to 18° |
The aim of this work is to increase the performance of TiO2 nano-coating for wettability alteration of rock surface from superhydrophobic to superhydrophilic state. For this purpose TiO2 nanoparticles hydrophilicity was increased by various processes, including microwave radiation, cooling, and increasing functional groups on the surface of nanoparticles using silica and surfactant during the synthesis stage. Effect of nanoparticles on wettability of carbonate rock was investigated via contact angle measurement. Thus, nanofluids with various concentrations were prepared and the rocks were impregnated with nanofluids. The synthesized nanoparticles were characterized by SEM, XRD and FTIR analyses. The surfaces of the rocks before and after coating were characterized utilising XRD analysis and SEM images. The surface charge of the synthesized nanoparticles in different pH values was determined using Zeta potential analysis. Thermal, mechanical, and salinity stability of the coatings that are important in industrial applications were also examined.
2. Materials and methods
2.1. Materials
Absolute ethanol (EtOH, 99.5%), nitric acid (HNO3, 65% in water), ammonium hydroxide (NH4OH), tween80 (C64H124O26), titanium isopropoxide (TTIP, Ti(OCH(CH3)2)4, >98%), tetraethyl orthosilicate (TEOS, Si(OC2H5)4, >98%), and n-heptane (C7H16, 99%), as oil representative, were purchased from Merck (Germany).
2.2. Preparation of nanoparticles
TiO2 is the first choice for large-scale and relatively inexpensive applications due to its strong oxidizing power, chemical inertness, and nontoxicity. Despite the popularity of TiO2 nano-coatings in wettability alteration of different surfaces, their stability and performance in tough environmental conditions are always challenging. In this study, TiO2 nanoparticles with various hydrophilic properties were synthesized via conventional, microwave radiation, surfactant, refrigerating, and silica-assisted sol-gel methods as described in the following:
2.2.1. Sol-gel method
After adding 10 ml titanium isopropoxide (TTIP) to 30 ml ethanol and stirring for 30 min, 3 ml HNO3 was added to 150 ml deionized water and the aqueous solution was injected drop-wise into the mixture of TTIP and ethanol for hydrolysis reaction. The mixture was stirred for 2 h.Next, the obtained viscous and opaque solution was heated at 100 °C until the solvents were evaporated. After annealing at 600 °C for 4 h, TiO2 nanoparticles were obtained.
2.2.2. Microwave radiation-assisted process
The obtained opaque solution was heat-treated for 30 min using a microwave oven (900 W, 2.45 GH, Sharp, Thailand) to produce a homogenous gel. The obtained gel was subsequently heated at 100 °C for 24 h and the highly homogeneous 'TiO2-MW' nanoparticles were obtained without heat treatment for crystallization.
2.2.3. Surfactant-assisted process
10 ml Tween80 was added to the mentioned opaque solution and stirred for about 2 h. It was then heated at 100 °C for 24 h until the solvent was evaporated. Annealing of the gel at 600 °C for 4 h yields the 'TiO2-Tween80' nanoparticles.
2.2.4. Silica-assisted process
4 ml tetraethyl orthosilicate (TEOS) was added to a solution of 1 ml ammonia and 18 ml EtOH. Next, the solution was added drop-wise to the hydrolyzed TTIP solution. It was then heated at 100 °C until the solvent was evaporated and after annealing at 600 °C for 4 h 'TiO2/SiO2' hybrid nanoparticles were obtained. In this method, the second precursor (TEOS) was used to increase the Si−O functional groups on the surface of the TiO2 nanoparticles.
2.2.5. Refrigerating-assisted process
The above mentioned opaque suspension was kept in refrigerator at 4 °C for 24 h. It was subsequently heated at 100 °C for 24 h until the solvent was evaporated and the 'TiO2-Ref' nanoparticles were obtained without heat treatment for crystallization.
2.3. Nanofluid preparation
Nanofluids having concentrations of 0.033, 0.07 and 0.2 wt% were prepared. At first, a base fluid was prepared adding diluted HNO3 and NH4OH solution to the deionized water to achieve different pH values for the best condition stabilizing the nanoparticles in nanofluids. Next, the nanoparticles were added to the solution and the suspension was stirred for 30 min. It was then put in the ultrasonic bath for 30 min to ensure the stability of nanofluids. The nanofluids were stable for 60 days during the experiments.
2.4. Cleaning and preparing of the rock samples
Superhydrophobic carbonate rocks were used in all experiments. At first a slab saw was used to cut the large pieces of rocks into perfectly smooth small plates of 2 × 2 × 0.2 cm. Next, an end face grinder was used to flatten the thin pieces of the stone polishing their surface. The plates were then washed with distilled water and toluene. Moreover, that the rock plates were submerged in the prepared nanofluids at 80 °C for 48 h. Finally the plates were prepared for characterization, wettability measurement and stability tests.
2.5. Surface wettability measurement
Contact angle value is employed by researchers to quantify the wettability of a solid surface by a liquid phase. In this study, contact angle was measured to investigate the effect of nanoparticles on wettability of the rocks [45]. The contact angle measurement setup consists a glass cell container. The rock plate was placed in the cell and surrounded by water/n-heptane, while a droplet of n-heptane/water with a micro-syringe was dropped on the plate. A camera magnifies the droplet and captures the image of both of the droplet and rock on a connected computer. The Image J software was employed to measure the exact contact angle values.
For each experiment, 2 rock samples have been impregnated with the nanofluids and used for wettability alteration experiments. The average of 4 measurements on the samples at different places was reported as mean contact angle. It is noteworthy that the contact angles in each place were the mean value of contact angle measurements on both the left and right sides of a droplet, as shown in figure 1.
Figure 1. Contact angle value.
Download figure:
Standard image High-resolution image2.6. Characterization
Fourier transform spectrometry, by Perkin-Elmer spectrum RX1, was used to identify the existent bonds in the synthesized nanoparticles. Morphology of the synthesized nanoparticles and the surfaces of rocks before and after coating were studied via SEM using a digital scanning electron microscope VEGA//TESCAN model. Mineralogy of synthesized nanoparticles and the rock surface before and after the nano-treatment were studied by XRD using JOEL JDX-8030 x-ray diffractometer with Cu Kα radiation, while the zeta potentials were measured by a Malvern Panalytical Zetasizer.
3. Results and discussions
3.1. Characterization
SEM images of the synthesized nanoparticles are shown in figure 2. It can be seen that the synthesized nanoparticles have a nearly spherical shape. According to the SEM images and by Image j software, the sizes of TiO2, TiO2-Tween80, TiO2-MW, TiO2-Ref and TiO2/SiO2 nanoparticles are 60 ± 5, 87 ± 8, 50 ± 7, 10 ± 2 and 20 ± 3 nm respectively.
Figure 2. SEM Images of (a) TiO2 (b) TiO2-Tween80 (c) TiO2-MW (d) TiO2-Ref and (e) TiO2/SiO2 nanoparticles.
Download figure:
Standard image High-resolution imageFigure 3 represents the XRD patterns of the synthesized nanoparticles. For TiO2 and TiO2-Tween80 nanoparticles, diffractions were observed at 27° (110), 36° (101), 41° (111),44° (210), 54° (211), 56° (220), 62° (002), 64° (310) and 69° (301) that indicate the major crystalline phase of the nanoparticles was rutile [46–48] as shown in figures 3(a) and (b). Figures 3(c)–(e) show the XRD pattern of TiO2-MW, TiO2-Ref and TiO2/SiO2 hybrid nanoparticles. The diffraction peaks are observed at 25° (101) and 48° (200) that indicate the formation of TiO2 nanoparticles in anatase phase [49–51]. Moreover the formation of rutile phase was due to the heat treatment at 600 °C in the TiO2 and TiO2-Tween80 XRD patterns. The nanoparticles in anatase phase were obtained as shown in TiO2-MW and TiO2-Ref XRD patterns. The nanoparticles were synthesized without heat treatment for crystallization. Furthermore, suppression of anatase to rutile phase transformation, despite the heat treatment at 600 °C in TiO2/SiO2 XRD pattern, was due to the formation of Ti−O−Si bonding that prevented the nucleation of rutile phase by impeding TiO2 nanoparticles [52–54].
Figure 3. XRD pattern of (a) TiO2 (b) TiO2-Tween80 (c) TiO2-MW (d) TiO2-Ref and (e) TiO2/SiO2 nanoparticles.
Download figure:
Standard image High-resolution imageThe Scherrer equation is used to determine the average crystallite size of the nanoparticles in x-ray diffraction.
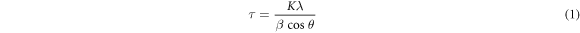
Where τ is the mean crystallite size of particles, that may be equal to or smaller than the particle size, K is the crystallite shape factor (about 0.9), λ is the wavelength of x-ray (1.548 A for Cu KR), β is the line broadening at half of the maximum intensity (FWHM) and θ is the Bragg angle [47].
Mean crystallite size of the synthesized nanoparticles obtained from Scherrer equation are presented in table 2.
Table 2. Size of the synthesized nanoparticles.
| TiO2 | TiO2-Tween80 | TiO2-MW | TiO2-Ref | TiO2/SiO2 | |
|---|---|---|---|---|---|
| The mean crystallite size (nm) (by Scherrer equation) | 56 | 85 | 41 | 8 | 11 |
| Size of nanoparticles (nm) (by SEM image) | 60 ± 5 | 87 ± 8 | 50 ± 7 | 10 ± 2 | 20 ± 3 |
Comparing all the synthesized nanoparticles, the refrigerating-assisted process produced TiO2 nanoparticles with the smallest size. A higher annealing temperature leads to larger crystallite size. In a nanoparticles sample, the size of crystallites ranges from small to large dimentions. The smallest crystallites, having the largest surface area to volume ratio, will dissolve by increasing temperature. The dissolved crystals then deposit on the larger crystals remained. The larger crystals become larger and the small crystals tend to smaller size or disappear [55–57]. On the other hand, the crystallite size decreases with increasing cooling rate, since the cooling limits growth of the crystallite. Moreover, the refrigerator-assisted method leads to produce nanoparticles with smaller crystallite size. The adsorption of 'TiO2-Ref' nanoparticles on the rock surface increases due to the large surface area and creates more hydrophilic coating in comparison with conventional TiO2 nanoparticles.
FTIR analysis, in the range of 450–4000 cm−1, was used to identify the structure and respective bonds in the synthesized nanoparticles as presented in figure 4. In figures 4(a)–(d) the strong peak between 450 and 800 cm−1 is due to the Ti−O bond that indicates the formation of TiO2 [58, 59]. Furthermore, the peaks observed at 480, 970 and 1000–1200 cm−1 in figure 4(e) are assigned to Ti–O, Ti–O–Si, and Si–O bonds respectively [60, 61]. The absorption peaks around 1600 and 3400 in all FTIR spectra are attributed to H–O–H bending and hydroxyl groups (Ti–OH and Si–OH) respectively[61] while peaks between 2840 and 3000 cm–1 are due to C−H bond of alkane groups [62].
Figure 4. FTIR spectrum of (a) TiO2 (b) TiO2-Tween80 (c) TiO2-MW (d) TiO2-Ref and (e) TiO2/SiO2 nanoparticles.
Download figure:
Standard image High-resolution imageWhen the nanoparticles are exposed to humidity, OH groups are generated on the surface of the partcles [63]. The TiO2 nanoparticles after humidification were characterized via FTIR in order to determine the amount of OH groups. Figure 5 shows the FTIR spectra of the nanoparticles after exposing to the 95% relative humidity. It was observed that the FTIR spectra shows stronger OH peaks after humidification.
Figure 5. FTIR spectrum of (a) TiO2 (b) TiO2-Tween80 (c) TiO2-MW (d) TiO2-Ref and (e) TiO2/SiO2 nanoparticles after exposing to 95% relative humidity.
Download figure:
Standard image High-resolution imageThe surface morphology of the rocks before and after nano-coating was observed by SEM images. The image of the untreated rock surface, as shown in figure 6(a), portrays a rough surface with pores and cracks. Figure 6(b) shows the untreated rock surface after impregnation with oil. It can be seen that the oil was adsorbed onto the rock surface.
Figure 6. SEM images of untreated rock (a) before, and (b) after impregnation with oil.
Download figure:
Standard image High-resolution imageFigure 7 shows the SEM images of the coated rocks surface. From this figure, it can be seen that the nanoparticles were adsorbed onto the rock surface.
Figure 7. SEM images of treated rock with 0.2 wt% (a) TiO2 (b) TiO2-Tween80 (c) TiO2-MW (d) TiO2-Ref and (e) TiO2/SiO2 nanofluids.
Download figure:
Standard image High-resolution imageXRD pattern was used to determine the chemical composition of the rock surface before and after treatment with nanofluids. In XRD pattern of untreated carbonate rock, as shown in figure 8, strong peak at 29° (104), 36° (110), 39° (113), 43° (202), 47° (018) and 48° (116) are assigned to calcite (CaCO3) [64].
Figure 8. XRD pattern of carbonate rock.
Download figure:
Standard image High-resolution imageFigure 9 shows the XRD patterns of rock surface after coating. From comparing the XRD patterns of nanoparticles and rock surface, it can be seen that the peaks of TiO2 in rutile phase and CaCO3 were still observed in coated plates with TiO2 and TiO2-Tween80 (figures 9(a) and (b)). The peaks of TiO2 in anatase phase and CaCO3 were still observed in coated plates with TiO2-MW, TiO2-Ref and TiO2/SiO2 nanoparticles as shown in figures 9(c)–(e).
Figure 9. XRD patterns of treated rock surface with (a) TiO2 (b) TiO2-Tween80 (c) TiO2-MW (d) TiO2-Ref and (e) TiO2/SiO2 nanofluids.
Download figure:
Standard image High-resolution image3.2. Contact angle tests
Wettability of the coated plates with the synthesized nanoparticles at three different concentrations, 0.033, 0.07 and 0.2 wt%, was investigated. For this purpose, the contact angle of n-heptane and water droplets on the coated plates was measured in water and n-heptane media respectively. The n-heptane droplet was instantly spread on the untreated plate and its contact angle with the rock was 0°. Moreover, the water contact angle was 168° as presented in figure 10(a), therefore the untreated rock was superhydrophobic.
Figure 10. (a) n-heptane and water CAs on the untreated rock plate, (b) n-heptane CAs on the rock plate after treatment and (c) water CAs on the rock plate after treatment.
Download figure:
Standard image High-resolution imageFigures 10(b) and (c) shows the contact angle values of the n-heptane and water droplets on the coated rocks. A comparison between contact angle values show that increasing the nanoparticles concentrations result in the n-heptane contact angle to increase while the water contact angle decreases. The most changes in contact angle values are in the concentration of 0.2 wt%. For the rocks treated with 0.2 wt% TiO2, TiO2-MW, TiO2-Tween80, TiO2/SiO2 and TiO2-Ref nanoparticles, the n-heptane droplet contact angle changed to 128°, 159°, 156.8°, 163° and 151° while the water droplet contact angle changed to 53.5°, 0°, 24°, 0° and 28.6° respectively. According to the results, we assume that increase of hydrophilicity property of the surfaces can be continued with increasing the nanoparticles concentration however, more research is still needed.
The results confirmed that the produced coatings were successfully modified the wettability of carbonate rock from superhydrophobic to hydrophilic/superhydrophilic state.
Several authors [65–68] have studied variation of of zeta potential on calcite (CaCO3) surfaces. Based on their observations calcite is positively charged almost in all ranges of pH.
The acidic and basic characters of the mineral surfaces are indicated by the value of zero potential charge (pHzpc). At pHzpc, the surface is electrically neutral at a particular pH [69]. The pHzpc for calcite is between pH 8 and 10 whereas above this value the calcite surface is negatively charged and below that is positively charged [67]. Therefore in most ranges of pH, carbonate surface have a positive charge and is more likely to be basic, hence it has affinity for acid species [65, 66].
In this study we determined the surface charge of the synthesized nanoparticles in solution by Zeta potential analysis. For this purpose, nanofluids with concentration of 0.2 wt% in four different pH values (pH = 3, 4, 5, and 8) were prepared and the zeta potential of every nanofluid was measured. Figure 11 shows the zeta potential of the prepared nanofluids as a function of pH. The general dividing line between stable and unstable suspensions is generally taken at either +30 or −30 mV. Particles with zeta potentials of more positive than +30 mV or more negative than −30 mV are normally considered to be stable. According to figure 11, TiO2-Ref, TiO2-Tween80, TiO2-MCW and TiO2/SiO2 nanoparticles have zeta potentials more negative than −30 mV. Therefore, they are considered to be stable with negative surface charge since for a specific pH more negative zeta potential indicates more negative surface charge. In pH values lower than 8, TiO2, TiO2-REF, TiO2-TWEEN80, TiO2-MW and TiO2/SiO2, nanoparticles have more negative zeta potential respectively and as a result they create more hydrophilic coatings. Comparing figures 10 and 11 it is clear that the nanoparticles with more negative zeta potential values create more hydrophilic coatings.
Figure 11. Zeta potential of 0.2 wt% of different type of the synthesised nanoparticles in suspension as a function of pH.
Download figure:
Standard image High-resolution imageAccording to the above explanations, since carbonate surface has positive surface charge and the nanoparticles have negative surface charge, the nanoparticles will be adsorbed on the rock surface and hydroxyl group (Ti−OH) will be formed in contact with water. Hydroxyl groups are polar and hydrophilic. Therefore, with adsorption of nanoparticles on the rock surface, the wettability of rock will change toward the hydrophilic state.
From figures 10(b) and (c), it is observed that the TiO2/SiO2 nanoparticles produce the most hydrophilic surfaces. According to figure 11, TiO2/SiO2 nanoparticles have the most negative surface charge in a specific pH value. It is postulated that the increase in acidity of the Si−O−Ti bonds at the interfaces may induce a greater amount of OH groups and it is the main reason behind improved hydrophilicity of the TiO2/SiO2 coatings. Tanabe et al [70] postulated that the enhanced surface acidity leads to a greater absorption of hydroxyl radicals. In this manner the TiO2/SiO2 surface has a greater concentration of hydroxyl radicals than a pure TiO2 coated surface. Consequently, the improved hydrophilicity of the TiO2/SiO2 coating is postulated to be a result of the increased surface hydroxyl groups. It has been proposed by Tanabe et al [70] that acid sites on binary metal oxides are formed by an excess of negative charges in the mixed oxides. The enhanced hydrophilicity of the TiO2/SiO2 mixed oxides has been explained by this increase of the surface acidity by several authors [71–73].
On the other hand, in the microwave-assisted sol-gel process, the solution is irradiated with a microwave source. Since microwave generates homogeneous volumetric heating and induces rapid and uniform heating, it increases the reaction rate and produces small and uniform particle size [63]. Here, the microwave radiation produces TiO2 nanoparticles without heat treatment for crystallization. It is suggested that the hydrophilicity is obtained by increasing the amount of surface hydroxyl groups (−OH) on the TiO2 surface. Proposed mechanism for hydrophilicity of the TiO2 nanoparticles by microwave radiation is based on photo-generated electron-hole caused by the microwave radiation. The microwave radiation creates structural changes in TiO2 (transformation of Ti4+ sites to Ti3+ sites) [74, 75]. Consequently, the bond between titanium atom and the lattice oxygen is weakened and can be broken to react with water molecules from the moist air forming newly hydroxyl groups that effectively transforms the wettability of TiO2 surface towards more hydrophilic state [76]. This is called as photo-induced hydrophilicity on TiO2 surface [77, 78]. Thus, one OH group doubly coordinated to two Ti atoms converts at the oxygen defect site to form two OH groups singly bonded to each Ti atom [63]. Figure 12(a) presents a schematic of the TiO2 surface according to the above mentioned mechanism.
Figure 12. Schematic of (a) photo-induced electron-hole caused by microwave radiation on the surface of TiO2 nanoparticles, and (b) adsorption of surfactant on the nanoparticles surface.
Download figure:
Standard image High-resolution imageIn the surfactant-assisted sol-gel method, the surfactant is added to the solution during the preparation process. Two mechanisms have been proposed for adsorption of the surfactants on nanoparticle surface: (1) covalent bonding between the surfactant and nanoparticles, and (2) electrostatic interactions between the surfactant and nanoparticles. In this study Tween80 was added after hydrolysis of the precursor and they were adsorbed on the nanoparticles surface to increase the hydrophilicity of TiO2 nanoparticles. Since hydrophilic-lipophilic balance (HLB) for this surfactant is 15, the molecular mass of the hydrophilic portion of molecule is higher than its hydrophobic portion. Thus, it can be used for the purpose of enhancing hydrophilicity. Figure 12(b) shows schematic of adsorption of the surfactant on surface of nanoparticles.
Figure 13 shows the effect of aging time on the hydrophilicity of the treated plates with 0.2 wt% TiO2-based nanofluids. The aging time is a key factor in wettability alteration of the rocks surface. The data show that for obtaining hydrophilic and superhydrophilic plates about 48 h is required and then the n-heptane contact angle maintain a relatively stable value.
Figure 13. Effect of aging time on the n-heptane contact angle of the treated rock plates with 0.2 wt% TiO2-based nanofluids
Download figure:
Standard image High-resolution imageAccording to the results, it is suggested that there are four factors to control the hydrophilicity property of nano-coated rock plates, (1) concentration of the nanoparticles in the based fluid, (2) aging time, (3) amount of hydrophilic heads (O−H) of nanoparticles, and (4) size of nanoparticles.
3.3. Thermal stability of the coatings
Thermal stability of the coatings is an important issue in practical applications where the coatings have been used in high temperature environments such as high temperature oil reservoirs. Thermal stability of the coatings was studied at 150 °C, maximum temperature of the world's known oil reserves. For this purpose, the coated plates were heated at 150 °C for 30 days and the n-heptane contact angle was measured every 5 days. Figure 14 shows the contact angle versus time. It can be seen that, there is a low temperature dependency in n-heptane contact angles with the plates.
Figure 14. Change in the n-heptane contact angle for treated rocks with 0.2 wt% (a) TiO2 (b) TiO2-Tween80 (c) TiO2-MW (d) TiO2-Ref and (e) TiO2/SiO2 nanofluids after putting in oven at 150 °C for 30 days.
Download figure:
Standard image High-resolution image3.4. Stability of the coatings in a salt solution
The fabricated hydrophilic coatings may lose their property in high salinity condition for example in saline reservoirs [17]. The coated plates obtained by impregnation of the plate with 0.2 wt% nanofluid were put in 3 wt% NaCl solution for 30 days and the n-heptane contact angle was measured every 5 days. The results shown in figure 15 confirms that there is a high salinity stability of the coatings.
Figure 15. Changes in n-heptane contact angle for treated rock plates with 0.2 wt% (a) TiO2 (b) TiO2-Tween80 (c) TiO2-MW (d) TiO2-Ref and (e) TiO2/SiO2 nanofluids after aging in 3 wt% NaCl solution for 30 days.
Download figure:
Standard image High-resolution image3.5. Mechanical stability of the synthesized coatings
Mechanical stability of the coatings is an important issue due to the existing damages in practical applications [6]. While hydrophilic coatings with superior mechanical stability have not been designed yet [79], low mechanical stability of the coatings is due to the fragility of hydrophilic coating and weak binding force between the coating and the substrate [10, 80]. In this study hydrophilic TiO2-based coating with high mechanical stability has been developed. The abrasion test was used to evaluate the mechanical stability of the prepared TiO2- coated rock. The test was carried out on the treated rocks with 0.2 wt% nanofluid. For this purpose, the coated plates having 2 × 2 cm2 surface area, were placed onto a 180 grit sandpaper with 30 cm length under pressure of 6.125 kPa (250 g). It was moved and returned back for 3 times and after each time the n-heptane contact angle was measured. Figure 16 shows the n-heptane contact angle as a function of moving times. The n-heptane contact angles on TiO2-based coating slightly reduces after 2 times of abrasion while it maintain a relatively stable value, indicating the hydrophilicity of the coatings remains well after the 2 times of abrasion. Figure 17 shows the SEM images of the coated rock surface after abrasion.
Figure 16. The n-heptane droplet contact angle on the treated rock plates with 0.2 wt% (a) TiO2 (b) TiO2-Tween80 (c) TiO2-MW (d) TiO2-Ref and (e) TiO2/SiO2 nanofluids after abrasion test.
Download figure:
Standard image High-resolution imageFigure 17. SEM images of coated rock surface with 0.2 wt% (a) TiO2 (b) TiO2-Tween80 (c) TiO2-MW (d) TiO2-Ref and (e) TiO2/SiO2 nanofluids after abrasion test.
Download figure:
Standard image High-resolution image4. Conclusions
In this study, TiO2-based nano-coating in wettability alteration of rock surface from superhydrophobic to superhydrophilic state was applied. For this purpose, TiO2 nanoparticles were prepared by conventional, surfactant-assisted, microwave radiation-assisted, refrigerating assisted and silica-assisted sol-gel processes. In the next stage, wettability alteration on superhydrophobic rocks was studied. Contact angle tests showed that nanoparticle adsorption on the rock surfaces can alter the wettability of the surface from superhydrophobic to hydrophilic condition. It was observed that, the modified TiO2-based coatings in comparison with conventional TiO2 coating, were more effective to wettability alteration process. The n-heptane and water droplet contact angle with the uncoated rock was 0° and 168° respectively. For the rock treated with conventional, microwave radiation-assisted, surfactant-assisted, refrigerating-assisted and silica-assisted sol-gel synthesized nanoparticles, the n-heptane contact angle changed to 128°,159°,156.8°, 151° and 163° while the water contact angle changed to 53.5°, 0°, 24.1°, 28.6° and 0° respectively. Characterization of the nanoparticles was confirmed by SEM, XRD and FTIR analyses. SEM images and XRD patterns confirmed adsorption of the nanoparticles on the rock surface.



















