Abstract
In this work femto-second pulsed laser ablation in liquid (PLAL) procedure for the generation of titanium oxide nanoparticles (NP) is reported with the purpose of understanding morphology and structure of the newly generated NPs. Ablation duration was varied for optimization of NP generation processes between 10 and 90 min. Surface morphology of NPs as well as their size and shape (distribution) were analysed by various complementary electron microscopy techniques, i.e. SEM, TSEM and TEM. The crystalline structure of titanium oxide particles was investigated by XRD (two instruments operated in different geometries) and HR-TEM. Concentration of generated titanium oxide NPs in liquid was analysed by ICP-MS. A mix of crystalline (mainly anatase), partly crystalline and amorphous spherical titanium oxide NPs can be reported having a mean size between 10 and 20 nm, which is rather independent of the laser ablation (LA) duration. A second component consisting of irregularly shaped, but crystalline titanium oxide nanostructures is co-generated in the LA water, with more pronounced occurrence at longer LA times. The provenance of this component is assigned to those spherical particles generated in suspension and passing through the converging laser beam, being hence subject to secondary irradiation effects, e. g. fragmentation.
Export citation and abstract BibTeX RIS
1. Introduction
Titania (TiO2) nanoparticles are a harmless, cheap, facile, bio-compatible, chemically and mechanically stable and highly efficient photo-catalyst [1, 2]. Physico-chemical properties of the titanium oxides allow their usage as photoanodes in dye-sensitized solar cells (DSSC) and as photo-catalysts for the selective synthesis of chemical compounds, for the removal of environmental pollution and sterilization (viruses, bacteria and cancer cells), as well as the preparation of self-cleaning surfaces, as gas sensors, biocompatible films covering medical implants, or simple anti-reflective layers [1, 3, 4]. Various crystalline phases (rutile, brookite, or anatase) [1, 5] with controlled particle size, shape, phase fraction and some other properties can be formed depending on the titanium precursors, as well as the synthesis technique, i.e. sol-gel method [6, 7], hydrothermal [8] and/or solvothermal synthesis [1], polyol method [5] or sol-hydrothermal method [9]. Commonly used titanium sources in the synthesis of titania are TiCl3, TiCl4, Ti(SO4)2, titanium alkoxide, and titanium complexes [10]. The main issue with all these preparation methods is that the use of toxic solvents such as HCl (aq.), H2SO4 (aq.) and organic solvents are required [1, 5, 7–10]. Consequently, for making titanium oxide preparation environment-friendly, water should be employed as a main liquid [10]. Preparation of titanium oxide nanoparticles in liquid is a promising technique, as it can be applied with low risk, and ensures compatibility with other fabrication techniques [4], i.e. spraying [11], dip or spin coating [12–14], membranes of polymeric nanocomposites preparation [15–17].
One of the most promising techniques for the synthesis of titania nanoparticles in liquid or in polymeric matrices is pulsed laser ablation in liquids (PLAL) [18–22]. The PLAL method is compatible with the environment because only a metallic titanium plate and water are used as a titanium source and in the same time as a 'green' solvent [18–21]. The formation of the mentioned oxides with certain structural and physical properties is mainly affected by the laser type (milli-/nano-/pico-/femto-second) and preparation conditions (frequency, pulse energy, duration of ablation, etc) [18, 22–25]. Some authors assume that the usage of femtosecond lasers enables effectively the control of the nanoparticle size—in contrast to nano-second laser ablation; further, it minimizes laser–plume interaction as well as reduces the heat affected zones [19, 26].
The production rate we achieved in the present study coincides with data presented in the literature. Hamad et al [27] analysed TiO2 NPs generated by three different types of lasers: nano-second, pico-second and femto-second. Resulting average production rates are similar for all of LA procedures used and are in the range of 0.05–0.07 mg min−1, despite relatively high differences in ablation conditions. Zimbone et al [4] used a very low frequency nano-second laser ablation with high laser pulse energy and obtained rates from 0.03 to 0.07 mg min−1. Other metals are reported to be ablated at similar rates. Simakin et al [28] ablated Ag and Au metals with rates of about 0.01 mg min−1 for Ag NPs and 0.02 mg min−1 for Au NPs. Similar values were obtained by Schwenke et al [29], i.e. 0.04 mg min−1 for Mg, 0.05 mg min−1 for Zn after 30 min of irradiation with picosecond laser.
The main objective of this work is to understand the morphology (size and shape) and structure of the new nanoparticulate material, spherical titanium oxide NPs, obtained by femto-second PLAL procedure as well as the effect of laser ablation duration on the generated NPs. TiO2 spherical NPs are very interesting because of application like photonic crystals [30] and seem to have different electronic and catalytic properties from faceted particles [31]. Methods for the synthesis of spherical TiO2 NPs are very scarce; other non-equilibrium methods like flame-pyrolysis do not result in spherical nanoparticles. Mou Pal et al [30] describe a hydrolysis method where the spherical TiO2 particles obtained are amorphous or, after annealing, polycrystalline.
2. Materials and methods
2.1. Materials
In this work, a solid titanium plate (purity 99.99%, Alfa Aesar GmbH & Co KG, Germany) with dimensions of 25 mm × 25 mm × 0.5 mm was used. Distilled water (DI water) was employed as the ambient liquid in experiments. Acetone (99.8%, Merck KGaA, Germany) and ethanol (99.9%, Merck KGaA, Germany) were used for cleaning of the titanium plate after polishing and laser ablation.
2.2. Preparation
In order to remove macro-level surface defects, contaminants and thin film oxide layer from titanium plates, their surfaces were polished progressively with 320, 400, 800, 1500, 2000 and 2500 grit silicate-carbon papers. Before starting the experiments, the targets were cleaned out with precision tissue wiper which was moistened in ethanol and washed ultrasonically in acetone (5 to 10 min), followed by ultrasonic cleaning in ethanol (5 to 10 min). Then, the titanium plate was placed into an open heat-resistant glass container for direct access of laser beam and filled with 7 ml distilled water enabling accumulation of the generated titanium oxide particles. The thickness of the water layer above the titanium plate was about 3.5 mm.
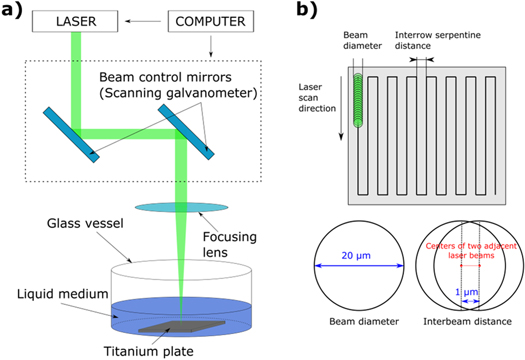
Experiments for the synthesis of titanium oxide colloidal suspensions by laser ablation in distilled water were performed using a Yb:KGW femtosecond laser of type PHAROS (Light conversion Ltd, Vilnius, Lithuania), operating at second harmonic (513 nm) with a 260 fs pulse duration and 100 mm lens focal length, see figure 1(a). The fluence at the target surface was 4.78 J cm−2. The laser system was controlled by a computer with dedicated software. The laser beam was continuously moved automatically via scanning galvanometer with a constant speed (330 mm s−1) across titanium metal plate in rows of a rectangular serpentine pattern, see figure 1(b), within time frames from 10 to 90 min. Selected laser parameters result in high temporal beam spot overlapping (duration between pulses 1 μs) as well as spatial overlapping (distance between the centres of two adjacent light spots 1 μm), see figure 1(b). Time interval between subsequent laser pulses is much smaller than the lifetime of cavitation bubble (of 100 to 700 μs) [32–34] that is generated by expansion of high temperature plasma created in a target zone of each of the pulse. Therefore, the conditions for NPs generation are a superposition of impact of multiple beams. The resulting suspensions containing the generated NPs were poured into clean containers to be used for further analyses.
Figure 1. (a) Experimental set-up of pulsed laser ablation in liquid media. (b) visualisation of the beam spot and inter-row distance, equal to 20 μm each.
Download figure:
Standard image High-resolution image2.3. Measurement methods
The size, morphology and structure of titanium oxide NPs produced by PLAL were investigated by means of scanning electron microscopy (SEM), including the transmission mode (TSEM), and scanning transmission electron microscopy (STEM) used in conjunction with high resolution transmission electron microscopy (HR-TEM). Energy dispersive x-ray spectroscopy (EDX) was used selectively to verify the elemental composition of the NPs. The analysis of phases present in the generated titanium oxide NPs was carried out by x-ray diffraction (XRD), applied in two versions on two different instruments, i.e. in the Bragg-Brentano geometry and at grazing incidence (GIXRD). Concentration measurements of the generated particulate material in water suspension were carried out with inductively coupled plasma mass spectrometry (ICP-MS).
SEM analysis has been performed with a Zeiss Supra 40 (Carl Zeiss, Germany), which can be operated also in transmission mode (TSEM). The transmission mode is enabled at this instrument by means of a dedicated sample holder described in detail elsewhere [35]. The analytical advantages of the complementary imaging modes SEM and TSEM, including EDX for elemental analysis, can be applied successfully for comprehensive high-resolution analysis of NPs, as demonstrated recently in [36–39]. Thus, it is useful to analyse the morphology of NPs in terms of particle shape and surface morphology (roughness) by high-resolution SEM imaging with an In-Lens detector and asses accurately the size (distribution) of the nanoparticles by TSEM [35–39].
The TEM investigations were performed using a STEM of type JEM 2200FS (JEOL, Japan), operated at 200 kV acceleration voltage. It is equipped with an in-column energy filter, a scanning unit with bright field and High Angle Annular Dark Field (HAADF) detectors, and an EDX system. Specimens were prepared by depositing a drop either on a carbon coated copper TEM grid or Lacey carbon coated copper TEM grid, and absorbing excess liquid with laboratory tissue after a few minutes.
XRD measurements in Bragg-Brentano geometry were performed with a Bruker-AXS D5000 diffractometer at room temperature using Cu Kα1,2 radiation, a 1.0 mm aperture, a 0.1 mm receiving slit, a sample spinner (0 or 15 rpm) and a scintillation counter. A corundum plate (NIST SRM 1976) was used to check and monitor the performance of the instrument. The sealed x-ray tube was operated at 30 kV and 40 mA.
A specimen of titanium oxide NPs generated by 90 min laser ablation was prepared by dispersing 0.4 ml of the liquid suspension (total available amount 0.8 ml) on the polished and clean surface of a flat, low background specimen holder (off-cut Si wafer, Ø 50 mm) and consecutive drying in a dry-box at 46 °C for 30 min. This procedure was repeated once and led finally to a specimen with a net dry weight of 0.7 mg titanium oxide spread uniformly within about 0.6 μm thick layer over an area of about 18 mm × 18 mm. For comparison only, a reference specimen was prepared by putting 35 mg of a well-defined nanocrystalline anatase powder on top of the low background sample holder.
Diffraction data of the sample-free, low background sample holder and of the sample '90 min' were collected from 5° to 80° in steps of 0.04° for 10 s per step in each run. The total data accumulation time was increased to 320 s per step by repeating the measurement of the complete 2θ range 32 times and, after checking the individual diffraction curves for consistency, adding them up. In addition, a diffraction pattern of the above described reference specimen was collected using the same instrumental conditions, but within a much shorter total data accumulation time of just 5 s per step. In this diffraction pattern, the 101 anatase reflection peak at about 25.3° is characterized by a very satisfactory value for both the signal-to–background ratio and the signal-to-noise ratio of about 36.
XRD diffractograms were measured also with a second diffractometer system of type SEIFERT XRD 3000 TT, operating at 40 kV, 40 mA, using secondary HOPG monochromator filtered Cu Kα radiation in a parallel beam configuration, at a grazing incidence angle of 1.3° and with 2θ steps of 0.08° and 0.2°, respectively. The samples '20 min' and '90 min' were prepared by depositing each of the suspension on the cleaned surface of a Si wafer with (100) orientation as specimen holder and subsequent drying in air. This procedure was repeated three times. After that, a specimen with a net dry weight of less than 1 mg titanium oxide distributed over an area of approximately 10 mm × 20 mm was obtained.
For the analysis of the x-ray powder diffraction patterns, the DiffracPLUS software package [40] as well as the Inorganic Crystal Structure Database (ICSD) issued in 2016 by Fachinformationszentrum (FIZ) and National Institute of Standards (NIST) [41] and the Powder Diffraction File (PDF) [42] were used.
3. Results and discussion
First, the morphology of the NPs generated by PLAL for the set of LA parameters selected in this work (see section 2.2.) has been analysed by electron microscopy. SEM was used with focus on the NP shape and surface morphology and TSEM for determining the particle size distribution. A sequence of eight ablation times, e.g. of 10, 15, 20, 25, 30, 45, 60, and 90 min has been selected in order to analyse the behaviour of NP morphology with the LA time. Representative electron micrographs of samples '10 min', '25 min' and '90 min', are shown in figure 2. SEM and TSEM micrographs of the rest of the samples, i.e. of '15 min', '20 min', '30 min', '45 min' and '60 min', are illustrated in figure S1 is available online at stacks.iop.org/MRX/5/045015/mmedia (Supplementary Material). The electron micrographs as in figures 2 and S1 suggest that the generated particulate material consists of (i) NPs of a spherical or nearly spherical shape, but also (ii) of a non-spherical, irregular nano-particulate fraction, which is more pronounced with increasing LA time. The two differently shaped components of particulate material are present as co-existing phases on the sample substrates used for analysis in all electron micrographs.
Figure 2. Representative SEM (a), (c), (e) and TSEM (b), (d), (f) micrographs of TiO2 NPs produced via pulsed laser ablation in liquid (PLAL) of titanium plate in DI water with increasing laser ablation duration: (a), (b)—10 min, (c), (d)—25 min, (e), (f)—90 min. Rest of sequence of samples, i.e. generated after 15, 20, 30, 45 and 60 min LA time, are presented in figure S1.
Download figure:
Standard image High-resolution imageMoreover, at a more careful inspection of the surface morphology of the spherical NPs, it can be observed that some of them are provided with surface areas of increased roughness, in contrast to the rest of particles which have a well-defined spherical shape and a smooth surface. The rough structure visible on many NPs is similar in morphology with the irregularly shaped nano-particulate component present in all images mostly on the sample substrate around the NPs. This observation leads us to the conclusion that the generation of spherical titanium oxide NPs is accompanied by the formation of ultrafine nano-particulate matter constituted of irregularly shaped structures of titanium oxide. This ultrafine component could have been produced when spherical titanium oxide particles generated in suspension pass through the converging laser beam, so that secondary irradiation effects, e. g. fragmentation, take place. After drop deposition on the sample support (carbon foil of TEM grid) for electron microscopy analysis, the ultra-fine nano-particulate component, present initially in the ambient water hosting the LA generated spherical particles, covers—during drop drying process—areas of both the sample support and spherical NPs surface. The result is the presence of a nanometre thin, but rough sheet/shell of ultrafine LA debris.
Further to the morphological investigations by SEM, analysis regarding particle size has been performed by TSEM on the eight samples corresponding to ablation times from 10 to 90 min. For that, the diameter of several hundred up to 2000 of the spherical particles has been traced manually in the TSEM images. The ultrafine particulate component of irregular shape with individual structures of maximum of a few nm has not been included. Although rather well distinguished in both SEM and TSEM micrographs, the accurate dimensional evaluation of these agglomerated nano-structures is hindered due to proximity to the limit of detection of the high-resolution capability of the SEM used [35]. Thus, only spherical NPs with a size above 10 nm have been counted. Figure 3 shows the particle size distributions of all eight NP samples corresponding to different ablation periods as extracted from TSEM data. Most of the NPs have sizes in the range of 10 to 20 nm. A quantitative differentiation between the size distributions of the NPs constituting the eight samples becomes possible only after statistical evaluation of the particles size distributions as in table 1. While all the mode, median and mean values tend to decrease slightly with increasing LA time, the evaluation of size polydispersity of the NPs, as the square of the standard deviation divided to mean value (see last row in table 1), indicates firstly a narrowing of the size distribution with LA times up to 35 min, followed by a broadening of the size distribution for longer LA times.
Figure 3. Size distribution of titanium oxide NPs produced by femtosecond laser treatment of a titanium plate after different ablation duration of: 10 min, 15 min, 20 min, 25 min, 35 min, 45 min, 60 min and 90 min.
Download figure:
Standard image High-resolution imageTable 1. Characteristics of particle size distributions of the eight titanium oxide NP samples as generated with increasing LA time (from 10 min to 90 min).
| LA duration | 10 min | 15 min | 20 min | 25 min | 35 min | 45 min | 60 min | 90 min |
|---|---|---|---|---|---|---|---|---|
| Number of analysed particles | 237 | 991 | 1947 | 834 | 958 | 652 | 223 | 2121 |
| Minimum size/nm | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Maximum size/nm | 178 | 146 | 110 | 122 | 91 | 127 | 197 | 241 |
| Mode/nm | 17 | 11 | 21 | 12 | 11 | 15 | 14 | 11 |
| Median/nm | 18 | 18 | 22 | 15 | 17 | 16 | 14 | 16 |
| Mean size/nm | 25 | 22 | 26 | 19 | 21 | 21 | 18 | 20 |
| Standard deviation (SD)/nm | 19 | 16 | 15 | 11 | 12 | 15 | 16 | 15 |
| Polydispersity (calculated as (SD/mean size)2) | 0.6 | 0.5 | 0.3 | 0.3 | 0.3 | 0.5 | 0.8 | 0.6 |
The inner structure and potential crystallinity of the generated titanium oxide NPs have been investigated in detail by XRD as an integral analysis, and by HR-TEM whereby individual representative NPs have been considered for analysis. Due to the high amount of analyses necessary to characterize systematically all the eight samples generated with various LA durations, with both methods and based on the findings above, only two samples have been selected for XRD and HR-TEM analyses, namely one representing short time LA ablation, i.e. '20 min', and the other being the sample generated after the longest LA duration, i.e. '90 min'.
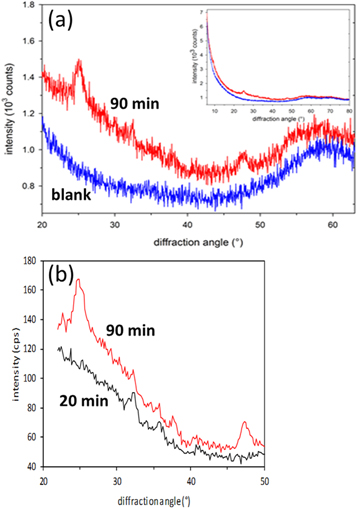
XRD measurements of the two selected samples, '20 min' and '90 min', have been carried out in two different instrumental geometries, namely Bragg-Brentano and grazing incidence. The results have been summarized in figure 4. For measurements, sample dispersion was dropped and dried on a single crystal Si wafer. Diffractograms of both samples reveal only a small number of reflections, which are broadened and of poor signal-to-background ratio as compared to the diffraction pattern of the (reference) nanocrystalline titanium oxide powder. This indicates that less than a half of the dry mass in the suspension belongs to the nanocrystalline fracture, while the major fraction is amorphous. A major contribution to the line broadening is caused by the very small size of the nano-crystals, which has been confirmed by SEM and TEM. However, other factors may contribute as well to this broadening: micro-strain caused by deviations from stoichiometry or partial overlap of nearly coinciding neighbouring lines, belonging to different nanocrystalline components. Low intensities of reflections could be ascribed to the low amount of sample material available for analysis (less than 1 mg on the sample holder) as well as to the low percentage of nano-crystalline component(s) in the mainly x-ray amorphous sample. The named factors, low net intensities and broadening of reflections, result in the low number of observed diffraction lines. This, together with the presumably poor crystallite orientation statistics, hinders an unambiguous crystalline phase identification.
Figure 4. (a) section of the diffraction patterns of the '90 min' sample and blank sample holder collected in Bragg-Brentano geometry. The inset demonstrates that both curves converge at small and large diffraction angles, (b) diffraction patterns acquired in grazing incidence geometry of the '90 min' and '20 min' samples.
Download figure:
Standard image High-resolution imageThe '90 min' sample shows in both XRD analysis geometries (Bragg-Brentano and GIXRD) two prominent reflections at about 25° and 48° and one weak reflection at about 38°. All these three reflections, which are considerably broadened, could be assigned to anatase [40–42], originating from reflections by the {101}, {200} and {004} crystallographic planes, respectively. As the peak positions of one of the polymorphs of titania, brookite [40–42], are close to those of the anatase mentioned above, they could not be resolved due to the considerable broadening of the observed reflections. Further difficulty is given by the lower reflection intensity of each lattice plane due to the orthorhombic crystal structure of brookite, i.e. 17 low-Miller-index Bragg reflections within analysed angle range, compared to 5 for tetragonal anatase and rutile, and 3 to 4 for hexagonal titania phases. Hence, based on the available diffractograms, the presence of a minor fraction of brookite cannot be ruled out for the '90 min' sample. The same holds true for the Magnéli phase Ti3O5 [40–42]. There are two additional reflections present in the diffractogram at about 32° and 36° and a signified one at about 41°, whereby the former could not be assigned to a TiO2 polymorph. However, this reflection at 32° further suggests the presence of Ti3O5. The other two reflections could correspond to rutile [40–42]. On the other hand, the absence of the rutile 110 reflection is rather opposed to this assignment. It should be noted that the profile of the reflex at 25° suggests more than one phase, which could be at about 24.9°, e.g. β-TiO2 [40–42].
In comparison to the '90 min' sample, the diffractogram of the '20 min' specimen, which has been obtained by measurement at grazing incidence, see figure 4(b), contains reflections, too, even though their number is smaller compared to the '90 min' sample. The reflections at 25°, 38° and 48° are missing, but reflections at 32°, 36° and 41° are present, similar to the ones of the '90 min' sample. The lack of reflections might be due to the lower concentration of sample dispersion as well as to a lower crystalline fraction, i.e. a smaller number of crystalline or partly crystalline particles.
ICP-MS measurements indicate a linear dependence of total amount of titanium on the LA time from 10 min to 90 min, see figure 5. Experimental diffractograms (figure 4) revealed no indications of the presence of crystalline metallic Ti, therefore, we assume that at least no considerable amount of metallic Ti exists in our samples and mass spectrometry measurements provide the amount of TixOy. Therefore, in figure 5 the dependence of concentration of TixOy in arbitrary units on LA time is presented. The production rate of titanium oxide NPs calculated from the linear approximation (R2 = 0.9461) of concentration curve from figure 5 equals about 0.07 mg min−1.
Figure 5. TixOy concentration change after different durations (t = 10; 15; 20; 25; 35; 45; 60; 90 min) of LA as measured with ICP-MS. Parameters of LA: 260 fs, 513 nm, 4.9 W, 330 kHz, 15 μJ, 330 mm s−1.
Download figure:
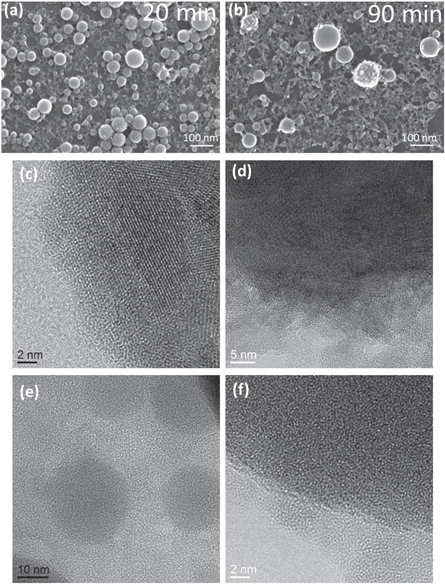
Standard image High-resolution imageHigh-resolution electron microscopy measurements have been carried out on the two selected samples, '20 min' and '90 min', in order to further investigate the morphology and structural nature of individual titanium oxide NPs, see figure 6. For an overview observation, high-resolution SEM images are also shown in figures 6(a) and (b). From the acquired TEM images, several observations regarding crystallinity of NPs can be made.
Figure 6. SEM and HR-TEM images of TiO2 NPs after different durations of laser ablation, left column, images (a), (c) and (e): LA time = 20 min and right column, images (a), (c) and (e): LA time = 90 min. Parameters of LA: 260 fs, 513 nm, 4.9 W, 330 kHz, 15 μJ, 330 mm s−1.
Download figure:
Standard image High-resolution imageParticles constituting the '20 min' sample exhibit lattice planes, as can be seen in figure 6(c), but also in figures S2(a) and (b). A first category of NPs, of which a representative outer region is shown in figure 6(c), shows clearly lattice planes and is thus completely crystalline. On the other hand, amorphous surface regions of particles are also visible (see figure S2(a)). A third type of particles, as those captured in figure 6(e), is completely amorphous. It can be concluded that the '20 min' sample is a mixture of roughly three types of particles: (i) fully crystalline, (ii) partly crystalline, and (iii) amorphous. Furthermore, several polycrystalline NPs have been identified. The surfaces of crystalline particles are not very compact, but rather frayed, as can be observed in figure S2(b). Compared to e.g. facets of pure anatase titania NPs with bipyramidal shape [43, 44], no well-defined crystallographic facets have been formed. Nevertheless, as it is marked in figure S2(c), there are rough steps discernible which are formed by the lattice planes and thus build up the surface morphology.
By applying Fast Fourier Transformation (FFT) to selected HR-TEM images of NPs of sample '20 min', d-spacings have been measured and the results are displayed in table 2. A number of d-spacings matches with those of titanium(IV) oxide. Due to the broadness of the diffraction spots and the incomplete FFT patterns, a clear discrimination between the TiO2 polymorphs rutile, anatase and brookite is rather difficult. A hint to the existence of the latter is given by the detection of a reflection at 0.29 nm, fitting to 121 reflection of the brookite structure. This indication is rather vague because of the multitude of Ti-O phases containing reflections at 0.29 nm. It was possible to index a FFT of one of the larger particles of the sample produced after 20 min of laser ablation. This FFT fits well to anatase in [−1 −3 1]-orientation, see figure 7. Remarkably, intense diffraction spot of rutile structure with d = 0.3247 nm which corresponds to (110) lattice planes did not appear in the FFTs. Other authors have also reported predominance of anatase and brookite phases in case of laser ablated NPs, despite bulk rutile being the most stable form of titanium oxide under ambient conditions [45, 46]. Furthermore, Magnéli phase Ti3O5 or triclinic members of the homologous series TinO2n−1 cannot be excluded, due to the correspondence of detected d-values with literature values and especially as the inter-planar distance of 0.27 nm indicates strong reflection of those phases. Moreover, only in some of the FFTs a reflection with a d of 0.37 nm is present. This diffraction spot matches with 012 reflection of Ti2O3 as well as with 201 of β-TiO2. It should be mentioned that the TEM method is not integrative, but allows inferences from a number of random crystallites, only.
Table 2. Measured d-spacings from FFTs of different particles in HR-TEM images of the '20 min' sample (an example is shown in figure 7) in comparison to those of titanium oxide phases.
| Measured d (nm) | Anatase, dhkl (nm) | Brookite, dhkl (nm) | Rutile, dhkl (nm) | β-TiO2, dhkl (nm) | Ti3O5, dhkl (nm) |
|---|---|---|---|---|---|
| 0.35 | 0.3520, (101) | 0.3512, (120) | |||
| 0.34 | 0.3465, (111) | 0.3376, (002) | |||
| 0.27 | 0.2729, (200) | 0.2697, (310) | 0.2661, (310) | ||
| 0.2687, (−311) | |||||
| 0.24 | 0.2378, (004) | 0.2409, (201) | 0.2381, (401) | 0.2465, (−312) | |
| 0.2383, (202) | |||||
| 0.22 | 0.2244, (022) | 0.2188, (111) | 0.2249, (112) | 0.2240, (121) | |
| 0.2237, (−411) | |||||
| 0.17 | 0.1699, (105) | 0.1691, (320) | 0.1687, (211) | 0.1729, (113) | 0.1759, (510) |
| 0.1667, (211) | 0.1662, (241) | 0.1715, (601) | 0.1737, (222) | ||
| 0.1693, (−603) | 0.1722, (420) | ||||
| 0.1671, (−114) | |||||
| 0.14 | 0.1364, (116) | 0.1434, (213) | 0.1424, (221) | 0.1427, (−713) | 0.1451, (−332) |
| 0.1417, (161) | 0.1419, (−223) | 0.1440, (204) | |||
| 0.1392, (023) | 0.1410, (−604) | ||||
| 0.1379, (−621) | 0.1392, (−424) | ||||
| 0.1389, (512) | |||||
| 0.12 | 0.1189, (008) | 0.1238, (024) | 0.1201, (212) | 0.1211, (−424) | |
| 0.1173, (303) | 0.1211, (124) | ||||
| 0.1207, (124) | |||||
| JCPDS card no. | [21–1272] | [29–1360] | [21–1276] | [46–1238] | [40–806] |
Figure 7. (a) HR-TEM image of a large particle belonging to sample after 20 min of laser ablation, with visible lattice fringes and Moiré patterns. Small amorphous particles are attached; (b) FFT of the marked region (in red) on the crystalline particle with (c) simulation, fitting to anatase in [−1 − 3 1] orientation.
Download figure:
Standard image High-resolution imageIncreasing the LA duration from 20 min to 90 min results in generation of TixOy particles, which at first sight look similar to the particles obtained after 20 min of LA, as can be observed in the SEM images in figures 6(a) and (b), but also quantitatively by the corresponding particle size distributions displayed in figure 3 and described in table 1. Regarding the surface roughness of the NPs generated after 90 min LA time, it behaves similarly as in the case of '20 min' sample, i.e. some NPs are smooth, rest of NPs is provided with surface asperities very similar in morphology to the nano-structured material present on the TEM grid support surrounding the NPs. Moreover, the roughly irregular shape of these nano-structures is even more sharp-edged. TEM images of such particles, see e.g. figure S2(e), reveal their partial crystallinity by visible lattice planes. With respect to crystallinity, particles of a rather large size in the '90 min' sample exhibit lattice planes visible in HR-TEM micrograph in figure 6(d) and are of polycrystalline structure. This is indicated by the presence of different lattice planes within one particle and the clear distinction of smaller particles which are visible within a larger one, but also by the occurrence of Moiré patterns suggesting that the larger NPs consist partly of former single smaller ones. The prolonged energy input of the laser might cause a selective-laser-sintering of particles forming larger ones, see figure 8. In this example, it can be observed that size of the small particles occupying the particle surface is smaller than the size of crystalline domains within the particle, which are visible by lattice planes. Sintering may involve recrystallization and coalescence. Furthermore, Ostwald ripening, at the expense of smaller particles, could have taken place in suspension. The surface of (crystalline) larger particles is very frayed, this being more pronounced than for the NPs of the '20 min' sample, and there are no well-defined crystallographic facets. FFTs calculated from HR-TEM images revealed broad diffraction spots with various d-spacings, suggesting different Ti-O phases to be present after 90 min of LA, which is identical to the sample after 20 min duration, as described above. However, 110 diffraction spot of rutile [40–42] with an interplanar distance of 0.32 nm has been identified after prolonged time of 90 min laser treatment. This 110 spot has not been observed in the FFTs of the sample with much shorter LA duration. In addition to the titanium oxide phases present in the '90 min' sample, detected d-spacing of 0.45 nm could possibly be assigned to one or more sub-oxides, e.g. (110) of Ti3O5 [40–42]. Particles generated after 90 min LA time which appear as nearly perfect spheres, showing a very smooth surface, are amorphous, see e.g. TEM image in figure 6(f). In the fraction comprised by the small particles different states of crystallinity were detected. They are amorphous, crystalline or exist in an intermediate state; as an example, they can be seen in the gap between the two particles in figure S2(d). EDX spectra taken on a rough particle as that imaged in figure 8(a) show that only presence of Ti and O, see figure S3.
Figure 8. (a) Crystalline particle of the sample after 90 min laser ablation at the edge of TEM grid carbon film, (b) HR-TEM image of the same particle with location indicated by red arrow and revealing that size of the small particles occupying the particle surface is smaller than the size of crystalline domains within the particle, which are visible by lattice planes.
Download figure:
Standard image High-resolution imageSumming up the HR-TEM results on structure of synthesized NPs, short (20 min) as well as long (90 min) duration of femto-second LA in water generates Ti-O NPs, which are not phase-pure. This finding agrees well with the XRD findings described in corresponding part above. Moreover, the results are consistent with similar reports in literature, e.g. [32], in terms of PLAL technique producing nano-scaled polymorphs of titanium(IV) oxide.
4. Conclusions and outlook
A series of optimized nano-particulate titanium oxide samples obtained with increasing femto-second pulse duration laser ablation (LA) times between 10 min to 90 min has been systematically investigated with the aim of understanding their surface morphology, size (distribution) and shape, and inner structure. Various types of electron microscopy (SEM, TSEM and TEM) applied in a complementary approach have been completed by x-ray diffraction analysis (with two instrumental geometries) in order to resolve the inner structure of the generated nanoparticulate material. The result of the variable LA duration is the generation of titanium oxide nanoparticles of spherical shape with a diameter of most particles in the range of 10 to 20 nm. The statistical evaluation of the particle size measurement demonstrates a rather constant mean particle size with increased LA duration, but a decrease of size polydispersity up to 30 min LA time followed by a broadening of the moderate polydispersity with LA durations longer than 45 min. Further, the NP surface morphology is either very smooth or providing fine, but measurable [47] asperities which are similar with the 'rest' of co-generated nano-structured material present outside the particles on the sample substrate. The morphology of these debris seems to become more pronounced with longer LA times. Regarding the crystalline structure of the particulate material generated by LA, no clear distinction could be concluded between shorter and longer LA times; the analysis of individual nanoparticles by high-resolution TEM, but also the integral investigation by x-ray diffraction have demonstrated the presence of non-phase-pure particles. A mix of fully (poly-)crystalline, partially crystalline as well as amorphous particles has been identified as not being strongly dependent on the LA time. Further, the nano-structured material component complementing the spherical particles has been proven to be of crystalline nature.
Additionally, ICP-MS analysis carried out on the series of eight samples with increasing LA duration has enabled to determine the generation rate of titanium oxide as being about 0.07 mg min−1.
Spherical TiO2 NPs seem to have different electronic and catalytic properties from faceted particles [31]. To our knowledge, there are no systematic studies on catalytic features of round TiO2 particles. However, to investigate the catalytic behaviour, we need to have at least ten times higher concentration than we have succeeded in the present work. One solution can be offered by repeating the current LA procedure for a few days. Another solution could be a method based on use of multiple laser beams. Once succeeding to scale up the NP generation procedure, the electronic properties of spherical TiO2 NPs could be investigated. Further, the use of spherical TiO2 NPs as new, unique nano certified reference materials (at least for size shape distribution) is also attractive.
Acknowledgments
This work was supported by the SETNanoMetro Seventh Framework Programme project (project number 604577; call identifier FP7-NMP-2013_LARGE-7).