Abstract
The synthesis of Fe3O4@Au nanoparticles has received much attention due to promising applications in the biomedical field. In this work, we produced Fe3O4@Au nanoparticles by using a two-step solvothermal route that employed Fe3O4 nanoparticles as seeds for the Au deposition. Although this protocol leads to highly monodisperse and reproducible Fe3O4@Au nanoparticles it was necessary to perform a systematic study to have a better understanding, improve the yield and allow us to obtain a tunable result. We demonstrated that the Au:Fe3O4 ratio is a key parameter that, contrary to what could be expected, does not influence the Au shell thickness. However, this parameter should be optimized because it strongly influences the yield. When the Au:Fe3O4 ratio was low there were plenty of uncoated Fe3O4 nanoparticles, whereas when the Au:Fe3O4 ratio was high there could be some pure Au nanoparticles together with the desired Fe3O4@Au nanoparticles. Furthermore we demonstrated that the Au shell thickness can be tuned by varying the reaction temperature. This paper describes the influence of both parameters and proposes a mechanism of the synthetic process by studying parametrically the morphological and structural evolution of the nanoparticles by TEM, DLS, SQUID and UV–vis spectroscopy.
Export citation and abstract BibTeX RIS
1. Introduction
Iron oxide nanoparticles have attracted much interest due to their potential applications in biomedicine, and have been used in imaging cancer cells [1], specific targeting [2, 3], hyperthermia treatment of solid tumors [4], and contrast enhancement agents in magnetic resonance imaging (MRI) [5]. Nevertheless, one of the major limitations of the use of these nanoparticles in biomedical applications is related to their poor colloidal stability in physiological conditions [6]. For this reason, scientists have developed different techniques for coating the surface of the magnetic nanoparticles with organic layers like surfactants or polymers [7], inorganic shells made of silica [8], or by combining both organic and inorganic coatings [9]. These methods enhance the stability of the nanoparticles and provide functional groups that can serve as a platform for immobilizing biomolecules of interest.
An interesting approach to provide colloidal stability and functionality is by covering the magnetic nanoparticles with a noble metal shell of Au, which is chemically stable under physiological conditions and can be easily functionalized throughout the well-known Au-SH chemistry [10]. The incorporation of Au can be used to enhance the hyperthermia capacity of the nanoparticles [11], and to provide additional physical properties like x-ray attenuation coefficient that can be used in computed tomography as contrast agents in Dual-Mode MR/CT Imaging systems [12]. Furthermore, plasmonic properties can be exploited for localizing hyperthermic effects, which would be tremendously important for its application toward tumor cell thermolysis and tissue ablation [13]. Thus, many research efforts have been made to develop hierarchical nanostructures that combine Au and Fe3O4 in a core@shell structure [14, 15]. Sun and coworkers [16] used hydrophobic Fe3O4 nanoparticles as seeds. The Fe3O4 nanoparticles were first transferred to an aqueous solution with CTAB and subsequently were covered with an Au shell by gently reducing hydrogen tetrachloroaurate hydrate (HAuCl4 · 3H2O). Another strategy was developed by Zhong and coworkers [14, 17] and involved the formation of the Fe3O4 core and the subsequent reduction of Au on the surface of the iron oxide nanoparticles to create the Au shell, giving as result highly-monodisperse Fe2O3@Au and Fe3O4@Au nanoparticles. The strategy starts with the synthesis of Fe3O4 seeds. After that, gold is deposited onto the surface of Fe3O4 nanoparticles by reduction of Au(CH3COO)3 using 1,2-hexadecanediol in the presence of capping agents at elevated temperature (180–190 °C). A key element of this process involves careful manipulation of the reaction temperature to control the thermally activated partial desorption of the capping layer from the core, the deposition of Au on the exposed Fe3O4 surface, and the subsequent re-encapsulation of the Au shell surface by the capping agent.
An alternative strategy developed also by Zhong and coworkers [18] consists of thermally activated processing of Fe3O4 nanoparticles and Au nanoparticles as precursors in a mixed solution. This strategy is based on the thermally induced homo-interparticle coalescence of metal nanoparticles [19]. The basis stems from the decrease of melting point, especially the surface melting, for many nanosized metal particles. Compared with the dramatically decreased melting temperature of gold nanoparticles, iron oxide nanoparticles did not exhibit coalescence in the range of 140–190 °C.
In this work we have used the synthesis of Fe3O4@Au nanoparticles using the methodology described by Zhong due to its high reproducibility and nanoparticle monodispersity [14].The main drawback of this method is the production of a large amount of pure Au nanoparticles and the purification of the desired Fe3O4@Au nanoparticles is very tedious. In this work, we present a systematic study for the optimization of this synthesis, demonstrating the influence of two key parameters, which are the Au:Fe3O4 molar ratio and the reaction temperature. The results obtained varying the Au:Fe3O4 ratio permitted to optimize this parameter and limit the formation of undesired pure Au nanoparticles. The TEM micrographs revealed that the Au shell thickness was independent on the Au:Fe3O4 ratio and we discuss this unexpected result as a consequence from the reaction mechanism. On contrary, the Au shell thickness was dependent on the reaction temperature. This study would permit a better understanding of this methodology for the synthesis of core@shell nanoparticles in which the shell is formed by Au. The production in high yield of hybrid Fe3O4@Au nanoparticles could be potentially used for a wide range of applications, with a special highlight in biomedicine.
2. Experimental part
2.1. Materials
All chemicals were used as received unless otherwise specified. Iron(III) chloride hexahydrate, oleic acid, 1-octadecene and diphenyl ether were purchased from Sigma-Aldrich. Gold(III) acetate (99%) was purchased from Alfa-Aesar. Deionized water was produced using Millipore Elix 20.
2.2. Methods
UV–vis absorption spectra were recorded using a spectrometer UV–vis Cary 300 Bio (Varian, USA). The measurements of the hydrophobic iron oxide and the iron oxide core—gold shell nanoparticles were acquired in hexane. Dynamic Light Scattering (DLS) measurements were recorded using Zetasizer Nano ZS instrument (Malvern Instruments, UK) and the accumulation time was determined automatically for each sample. Selecting the multimodal analysis method of the DLS software (5.0) provided by Malvern, the Z-average (intensity mean) was calculated from the correlation function, and the hydrodynamic diameter (Dh) was derived using the Einstein-Stokes equation.
TEM specimen of the inorganic nanoparticles were prepared by placing a drop of a diluted solution on a TEM copper grid coated with a Formvar film and let to dry in air. The size and morphology of the samples were observed with a JEM-1010 electron microscope working at 80 kV equipped with a digital camera GATAN megaview II (JEOL, Japan). HR-TEM, selected area electron diffraction (SAED) and energy-dispersive x-ray spectroscopy (EDS) for sample composition were performed with a JEOL JEM-2100 electron microscope working at 200 kV equipped with a digital camera CCD ORIUS SC1000 Model 832 (JEOL, Japan), and a Oxford Inca detector (Oxford Instruments, UK) for Energy Dispersive Spectroscopy (EDS) analysis. The EDS experiments were performed with a standard less method. The magnetic properties were measured with a Quantum Design SQUID magnetometer MPMS-XL (Quantum Design Inc, USA) at 298 K as a function of the external magnetic field in the range of −10 000 to 10 000 Oe. The magnetometer was calibrated prior to the measurements using metallic Pd as a standard.
2.3. Procedures
2.3.1. Synthesis of hydrophobic iron oxide nanoparticles
Monodisperse iron oxide nanoparticles were synthesized by thermal decomposition of iron (III) oleate complex [20]. Iron (III) oleate complex was prepared as follows: 480 mg of NaOH (12 mmol) were dissolved in 50 mL of water and 80 mL of EtOH. Next, 4 mL of oleic acid (12 mmol) were added under vigorous stirring to the mixture, and the pH was adjusted to 7. Afterwards, 1.08 g of iron(III) chloride hexahydrate (4 mmol) were dissolved in 10 mL of water and added under stirring. Then, 140 mL of hexane were poured and the mixture was stirred and refluxed at 70 °C for 4 h. After this time, the upper layer of hexane showed a red brownish color due to the presence of the iron (III) oleate complex. The organic phase was washed three times against 30 mL of water in a separation funnel. Finally, the iron (III) oleate complex was collected after the evaporation of the organic phase in a rotary evaporator under reduced pressure. To produce monodisperse hydrophobic Fe3O4 nanoparticles, 900 mg of the as-prepared iron oleate complex (1 mmol) and 1156.56 mg of oleic acid (4 mmol) were dissolved in 8 mL of 1-octadecene in a three necked round bottom flask equipped with a thermometer, nitrogen gas flow and a reflux condenser. The flask was placed in a heating mantle and heated at 120 °C for 1 h without the reflux condenser to remove all trace solvents. After this time, the reflux condenser was placed and set at 40 °C and the reaction temperature was raised to 320 °C and kept at this temperature for 45 min. Afterwards, the reaction was cooled down to room temperature and the nanoparticles were precipitated from the reaction mixture by centrifugation with acetone and re-dispersed in hexane. The cleaning procedure was repeated at least three times and nanoparticles were re-dispersed and stored in hexane.
2.3.2. Synthesis of hydrophobic core@shell Fe3O4@Au nanoparticles
Monodisperse and individual hydrophobic core@shell Fe3O4@Au nanoparticles were synthesized by a modification of the method reported by Zhong and coworkers [14]. In a typical synthesis, 500 μL of hexane solution of Fe3O4 nanoparticles (0.05 mmol of Fe content), 282 mg of oleic acid (1 mmol), 1600 mg of oleylamine (6 mmol) and different amount of gold (III) acetate ranging from 0.38 to 3.8 mmol were dissolved in 10 mL of diphenyl ether and poured into a three necked round bottom flask, equipped with a thermometer, nitrogen gas flow and a reflux condenser. The flask was placed in an oil bath and the temperature was set to 160 °C and let to react for 90 min. The reaction was then cooled down to room temperature and the Fe3O4@Au nanoparticles were extracted by centrifugation with ethanol and re-dispersed in hexane. The cleaning procedure was repeated at least three times and the Fe3O4@Au nanoparticles were re-dispersed and stored in hexane.
3. Results
3.1. Characterization of hydrophobic iron oxide nanoparticles
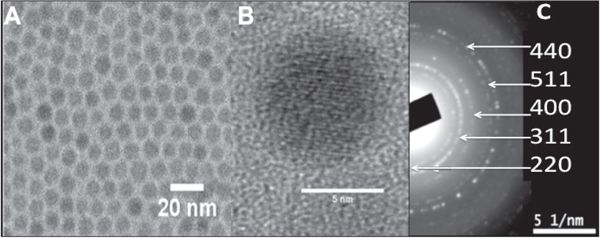
Iron oxide nanoparticles were prepared by the thermal decomposition of iron (III) oleate complex in the presence of oleic acid and 1-octadecene at high temperature, as detailed in the experimental section [20]. The solution turned from brownish to dark brown color, which corresponds with the formation of the iron oxide nanoparticles. The presence of monodisperse and individual iron oxide nanoparticles with a narrow size distribution was confirmed by TEM micrographs, as shown in figure 1(a). Figure 1(b) shows the HR-TEM image of Fe3O4 nanoparticles, and figure 1(c) depicts the SAED pattern of the nanoparticles.
Figure 1. (a) Monodisperse iron oxide nanoparticles obtained by thermal decomposition, (b) HR-TEM of a single iron oxide nanoparticle, (c) SAED pattern of the nanoparticles.
Download figure:
Standard image High-resolution imageThe size distribution obtained from the TEM picture gave as result a mean diameter of 7.5 ± 0.6 nm. Furthermore, the hexane dispersion of the hydrophobic iron oxide nanoparticles was stable for over a year without any signs of precipitation. The HR-TEM micrograph shows the lattice fringe of the 〈220〉 lattice plane (3,01 Å) and the SAED analysis showed the typical reflections attributed to the FCC spinel Fe3O4 [21].
3.2. Characterization of hydrophobic core@shell Fe3O4@Au nanoparticles
The hydrophobic core@shell Fe3O4@Au nanoparticles were synthesized by using the iron oxide nanoparticles as seeding points by a briefly modified procedure which was previously published [14]. In our experimental conditions we did not use 1,2-hexadecanediol because we found that it was unnecessary for the synthesis. We observed that Au and Fe3O4@Au nanoparticles could be obtained by using oleylamine both as reducing and capping agent and Gold (III) acetate as Au precursor. The mixture of oleic acid and oleylamine and the high temperature in diphenyl ether favored the Au deposition on the surface of Fe3O4 nanoparticles [22]. The Fe3O4@Au nanoparticles were characterized by TEM, EDS, UV–vis, DLS and magnetization measurements.
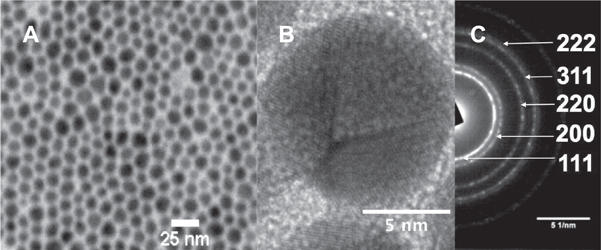
Figure 2(a) shows a representative TEM micrograph of the as obtained Fe3O4@Au nanoparticles, figure 2(b) shows the HR-TEM image of Fe3O4@Au nanoparticles, and figure 2(c) depicts the SAED pattern of the nanoparticles.
Figure 2. (a) Monodisperse Fe3O4@Au nanoparticles (Au:Fe3O4 ratio = 3.7), (b) HR-TEM of a single Fe3O4@Au nanoparticle, (c) SAED pattern of the nanoparticles.
Download figure:
Standard image High-resolution imageThe size distribution obtained from the TEM picture gave as result a mean diameter of 11 ± 2.2 nm. In the HR-TEM micrograph it is observed the crystalline structure of a single Fe3O4@Au nanoparticle in which the Au 〈111〉 lattice plane is predominant (2.4 Å) and the SAED analysis showed the typical reflections attributed to the Au crystalline structure [23]. Moreover, in figure 2(b) the presence of twinned planes in the Au shell, which were frequently observed, is noticeable. The reflections of the Fe3O4 core could not be observed by this technique due to the well-known heavy atom effect [24]. Figure 3 reveals the results of different experiments carried out on the previously synthesized nanoparticles.
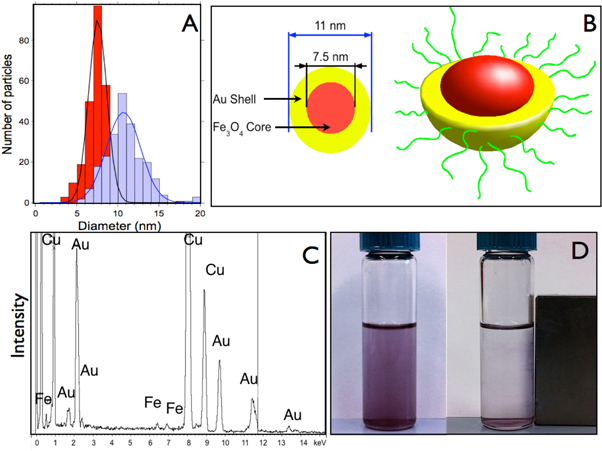
Figure 3. (a) Size distribution of Fe3O4 nanoparticles (in red) and Fe3O4@Au (blue) nanoparticles measured by TEM. (b) Idealized scheme of the Fe3O4@Au nanoparticles. (c) Energy Dispersive Spectroscopy spectrum of the Fe3O4@Au nanoparticles. (d) Fe3O4@Au nanoparticle dispersion in hexane/ethanol in the absence and presence of a magnetic field (B = 0.4 T). The Fe3O4@Au nanoparticles were synthesized with a Au: Fe3O4 ratio equal to 3.7.
Download figure:
Standard image High-resolution imageWhen the Au:Fe3O4 ratio was enough (this is discussed further in the text) we could observe the formation of Fe3O4@Au nanoparticles. One of the most important results was the increment of the nanoparticle diameter, which increased an average of 3.5 nm (figures 3(a) and (b)). In addition, the absence of uncoated Fe3O4 nanoparticles in the TEM micrographs would indicate that the structure of the Fe3O4@Au nanoparticles is a core@shell structure. The composition of the Fe3O4@Au nanoparticles was studied by EDS microanalysis that provided qualitative determinations of their elemental composition (figure 3(c)). The EDS spectrum revealed the presence of iron, with a Kα peak at 6.400 keV and a Kβ peak at 7.059 keV and gold with a Mα peak at 2.1 keV, Lα at 9.7 keV and Lβ at 11.4 keV. The analysis of the peak intensities provided the atomic percentage between gold and iron, which were 90 and 10% respectively. These results are in agreement with those calculated theoretically for spherical core@shell particles with a core of 7 nm and a shell of 3 nm [22].
The inner magnetic properties of the Fe3O4 cores remain in the Fe3O4@Au nanoparticles and can be directly observed using a magnet. Figure 3(d) shows that in the presence of a magnetic field the nanoparticles migrate towards the magnet. The magnetic properties of the Fe3O4@Au nanoparticles were studied using a superconducting quantum interference device (SQUID). The comparison of the magnetization curves between Fe3O4 and Fe3O4@Au displayed a significant difference shown in figure 4.
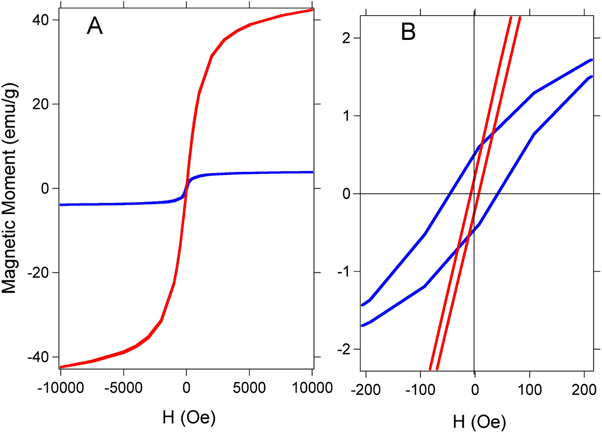
Figure 4. (a) Magnetization curve as a function of the applied field for Fe3O4 nanoparticles (red curve) and Fe3O4@Au nanoparticles (blue curve) at room temperature. (b) Magnification of the curves. The Fe3O4@Au nanoparticles were synthesized with a Au: Fe3O4 ratio equal to 3.7.
Download figure:
Standard image High-resolution imageTo prove that the magnetic properties of the core@shell nanoparticles were due effectively to the presence of a Fe3O4 core within the Fe3O4@Au, we treat a dispersion of Fe3O4@Au in chloroform with 0.1 mL of HCl. The addition of HCl etched the uncovered Fe3O4, rendering the Fe3O4@Au nanoparticles, which resist the acidic attack due to the protect effect of the Au shell. After that, the dispersion was centrifuged and the Fe3O4@Au nanoparticles dispersed in hexane.
The saturation magnetization and coercivity were determined from SQUID measurements. The magnetic data displayed two major differences between Fe3O4 and Fe3O4@Au nanoparticles. First, the maximum magnetization values are 45 and 4 emu g−1 for Fe3O4 and Fe3O4@Au, respectively. Second, the coercivities of Fe3O4 and Fe3O4@Au nanoparticles were 19 and 80 Oe, respectively. The reduction of the maximum magnetization in the Fe3O4@Au indicates that there is less magnetic material per gram [25]. Furthermore, the coercivity showed a clear increase for the Fe3O4@Au nanoparticles, which can be attributed to the larger size of Fe3O4@Au that leads to a less-effective coupling of the magnetic dipole moments. In contrast, the magnetic dipole moments in the case of uncoated Fe3O4 nanoparticles are coupled more effectively, and hence the particles tend to have a lower coercivity [26].
The technological applications of core@shell nanoparticles will often require a precise control of the shell thickness. For this, it is necessary to carefully set the reaction parameters that might influence the production of the desired nanoparticles. In this work we studied the influence of a key parameter of this synthesis that is the Au:Fe3O4 ratio. A set of syntheses varying the Au:Fe3O4 molar ratio from 0.3 to 8 were performed. The results of these experiments were analyzed by TEM (see figure 5).
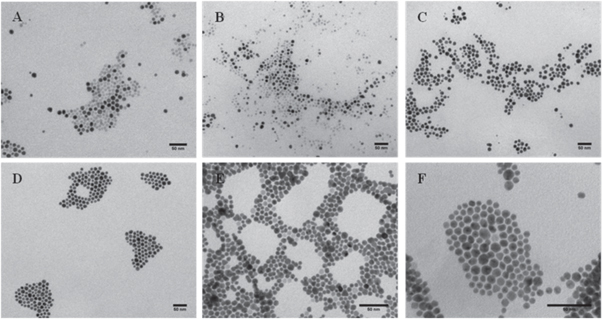
Figure 5. Representative TEM micrographs of the as obtained nanoparticles synthesized with different Au:Fe3O4 ratios (a) 0.3, (b) 1, (c) 2.5, (d) 3.7, (e) 5.5, (f) 8. All scale bars are 50 nm.
Download figure:
Standard image High-resolution imageIn figures 5(a) and (b) it is noticeable the presence of two different types of nanoparticles, which differ mainly in their contrast in TEM operated in bright field imaging mode. Iron oxide has less contrast in TEM than Au and therefore the bright nanoparticles were considered as Fe3O4. However the darker nanoparticles could not be only attributed to Fe3O4@Au nanoparticles because there could be pure Au nanoparticles which would not be differentiated by their contrast. For this reason, the population of darker nanoparticles was considered as 'Au-containing nanoparticles'. As can be seen in figure 5, as the Au:Fe3O4 ratio increased, the number of uncoated Fe3O4 nanoparticles was reduced. This reduction was concomitant with an increment in the number of Au-containing nanoparticles. However, these Au-containing nanoparticles appeared even at low ratios of Au:Fe3O4 and showed a similar size and shape. The proportion of Au-containing nanoparticles was defined as χ (number of Au containing nanoparticles/total number of nanoparticles) and was represented as a function of the Au:Fe3O4 ratio, as shown in figure 6(a).
Figure 6. (a) Proportion of Au containing nanoparticles versus the Au:Fe3O4 ratio. (b) UV–vis spectra of the nanoparticles obtained with different Au:Fe3O4 ratio: 0 (black), 0.3 (red), 3.7 (blue) and 7 (green).
Download figure:
Standard image High-resolution imageFigure 6(a) depicts that χ increased with the Au:Fe3O4 ratio until it reached a plateau close to 1, which is the maximum possible value, for ratios of 3.7 and above. The figure 6(b) shows the UV–vis spectra of representative nanoparticles obtained at different Au:Fe3O4 ratios. The absorption band with a maximum at 521 nm corresponded to the surface plasmon resonance (SPR) of Au nanospheres in hexane [27] except for the sample of uncoated Fe3O4 nanoparticles that did not show that band. The SPR band showed a similar profile and maximum wavelength independently from the Au:Fe3O4 ratio, which only influenced the maximum intensity. Both the TEM micrographs and the UV–vis measurements demonstrated that the Au shell thickness was not influenced by the Au:Fe3O4 ratio. However, we found that this parameter had a strong influence on the yield of Fe3O4@Au nanoparticles. This result is of key relevance for the optimization of the synthesis of Fe3O4@Au nanoparticles, because if the synthesis is performed using a high Au:Fe3O4 ratio, the Au shell thickness would remain constant and therefore the excess of Au reagent would provoke a homogeneous nucleation, leading to pure Au nanoparticles without a Fe3O4 core. We performed a control experiment using the same experimental conditions without Fe3O4 seeds and we could observe the formation of polydisperse Au nanoparticles (figure S1, supporting information). This result demonstrates the relevance of using the optimized Au:Fe3O4 ratio, because otherwise it would be tedious to purify the desired Fe3O4@Au nanoparticles from the pure and less homogeneous Au nanoparticles. Our experiments revealed that the optimum Au:Fe3O4 ratio was 3.7 for our synthetic conditions. However, the uncoated Fe3O4 nanoparticles that we used had a mean diameter of 7.5 nm, and therefore the optimized Au:Fe3O4 ratio would be different for nanoparticles of different size.
A possible explanation for these results could be given in terms of the reaction mechanism proposed by Zhong and coworkers [14, 15]. In these two works, the Au nanoshell was formed either by the deposition of the Au reagent directly onto the Fe3O4 seeds or by the heterogeneous coalescence of melted Au nanocolloids at high temperature. In our work experimental conditions it would be possible that the Au nanocolloids were formed in situ by the reaction between the Gold (III) acetate and the oleylamine. These nanocolloids could be melted at high temperature thus allowing the heterogeneous coalescence. In this way, the Au shell formation mechanism would require the following steps: i) formation of Au nanocolloids, ii) capping shell desorption, iii) the deposition of melted Au nanocolloids on the exposed Fe3O4 surface and iv) heterogeneous coalescence to form the final Au shell.
Our experimental results could be explained by this mechanism with two requirements: First, the deposition of the melted Au nanocolloids onto the Fe3O4 seeds should be the slowest step whereas the following processes should be faster and irreversible. Under these conditions, once an initial amount of Au nanocolloids are deposited on the surface of Fe3O4 nanoparticle, the system evolves rapidly towards a core@shell structure. This would explain that at low Au:Fe3O4 ratios, we observed fully coated and non-coated nanoparticles and we could not observe patchy or dumbbell like hetero-nanoparticles. By contrast, when the Au:Fe3O4 ratios were in the plateau region, the quantity of Au precursor was enough for the deposition on the majority of the Fe3O4 seeds and therefore we could observe a higher degree of coating. However, a large excess of Au:Fe3O4 ratio would also lead to homogeneous coalescence of Au nanocolloids, as we could not observe an increment of the Au shell thickness. In addition, we observed in the HR-TEM images a large quantity of nanoparticles with twinned planes, which could be explained also by this mechanism (figure 2(b)).
Although the Au shell thickness could not be controlled with the Au:Fe3O4 ratio, there are other parameters that could play an important role in the control of the Au shell thickness. For instance, some authors have extensively studied the influence of the chain length of the capping agents [28, 29], which demonstrated that the shorter the chain length, the bigger the nanoparticles. In this work we have studied the influence of the reaction temperature because preliminary works seemed to indicate that the melting temperature of the nanoparticles is intrinsically related to their size [19, 30, 31].
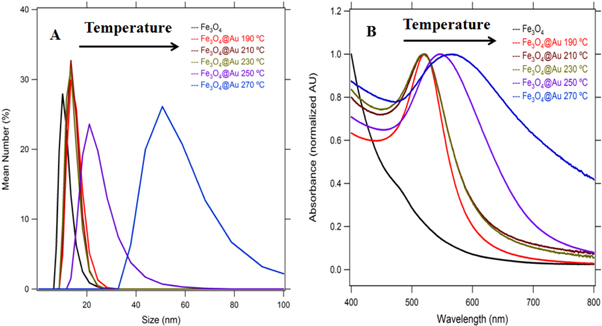
The influence of the temperature in the evolution of gold nanoparticles has been studied by Zhong and coworkers [19]. In their study, they observed a size and shape evolution of thiolate-encapsulated gold nanoparticles upon increasing the reaction temperature from 100 to 138 °C. In this work we have studied the influence of the reaction temperature, in the range between 190 to 270 °C, on the formation of Fe3O4@Au nanoparticles. Figure 7(a) depicts the size distribution analysis obtained by DLS measurements of the Fe3O4@Au nanoparticles synthesized at different reaction temperatures, using a Au:Fe3O4 ratio of 7.
Figure 7. (a) Size distribution obtained by DLS of the Fe3O4@Au nanoparticles synthesized at different reaction temperatures. (b) UV–vis spectra of the Fe3O4@Au nanoparticles synthesized at different reaction temperatures.
Download figure:
Standard image High-resolution imageThese results showed that the reaction temperature does not influence the Au shell thickness between 190 and 210 °C. However, we found a size evolution of the Au shell thickness when the reaction temperature was above 230 °C: Between 230 and 250 °C, we observed a significant increase of the Au nanoshell from 3 to 10 nm, as demonstrated by DLS. The TEM micrographs are shown in the supporting information, figure S2. In addition, the SPR band of the nanoparticles was studied by UV–vis spectroscopy, see figure 7(b). Here we can observe that the maximum of the SPR band was at 521 nm when the nanoparticles were synthesized at a reaction temperature between 190 and 210 °C, and that the SPR maximum is red-shifted when the nanoparticles were synthesized above 230 °C. A picture taken from the cuvettes containing the as-synthesized nanoparticles is shown in the supporting information, figure S3. These results are in agreement with the DLS data, because the increment in size of spherical Au nanoparticles provokes red-shifts of their SPR bands.
4. Conclusions
In this work we have done a systematic study of the synthesis of core@shell Fe3O4@Au nanoparticles by a high temperature reaction using the Fe3O4 nanoparticles as seeds and gold (III) acetate as Au precursor. We have demonstrated that the Au:Fe3O4 ratio is a key parameter that should be optimized in order to obtain the maximum yield of Fe3O4@Au nanoparticles and to avoid tedious and time-consuming purification processes. In our study, for the Fe3O4 nanoparticles with a mean diameter of 7.5 nm, the optimized Au:Fe3O4 ratio was 3.7; when the reaction was performed with lower ratios we obtained an incomplete coating of the Fe3O4 nanoparticles whereas when the reaction was performed with higher ratios we obtained a mixture of pure Au nanoparticles and Fe3O4@Au nanoparticles. Therefore, if the synthesis is performed with an excess of Au reagent, pure Au nanoparticles will be formed together with the desired Fe3O4@Au nanoparticles, and the purification procedures are tedious and time consuming. With these results, we suggested an addition to the reaction mechanism proposed by Zhong and coworkers, in which the deposition of Au nanocolloids onto the surface of the Fe3O4 nanoparticles could be the slowest and limiting step, whereas the heterogeneous coalescence to form the final Au shell could be fast and irreversible. Finally, we demonstrated that the reaction temperature affects the Au shell thickness. When the reaction temperature increased above 230 °C, we could observe a significant increase in the Au shell.
Acknowledgments
The authors acknowledge financial support from the Spanish Science and Innovation Ministry (Grant MAT2010-15349 and MAT2014-55065) and from COST Action CM1101. Paulino Alonso-Cristobal acknowledges the Spanish Education Ministry for an FPU fellowship to perform this work. We want also to thank Agustin Fernandez from UCM-Electron-Microscopy CAI for his help with TEM micrographs and fruitful discussion.