Abstract
The black coatings were fabricated via electroless NiP deposits on Ti-6Al-4V alloy substrate with heat-treatment and blackening process. Two types of coatings were obtained: coatings with heat-treatment after blackening and coatings with blackening after haet-treatment. After parameter optimization, the temperature and pH of the solution were adjusted to 85 °C–95 °C and 4.3–4.7, respectively. The substrate was coated after the addition of modifying components to the solution. After refluxing the samples, the blackening process was done for 40 s in nitric acid (9molar). An SEM-EDS analyze used to assess the morphology and chemical composition of the coating. Moreover, adhesion strength and optical absorptance of the coating were measured by Pull Of test and Shimadzu UV-3100 analysis, respectively. The optical absorptance was measured by 0.89 and 0.95, respectively, for Oxalic acid and Nitric/Flouridric acid pretreated samples. Results showed that heat-treatment after blackening causes coating with higher micro-hardness and optical absorption. The blackened electroless NiP coating on Ti substrate with the heat-treatment of 4 h after the blackening process provided high solar absorption of 0.99, which is exceptionally suitable as a solar absorber coating for space and allied applications.
Export citation and abstract BibTeX RIS
1. Introduction
Titanium alloys are the great interest in electro-optic systems because of its unique properties [1]. The components of optical devices are always designed to help increase the light beams entry and reduce the intruder factors. Therefore, due to minimizing the pesky rays of the system and optimize the performance, the components of the systems should have the minimum reflection and maximum absorption [2]. So, the applied coatings should have a matte black color. Coatings should have appropriate corrosion resistance, color stability against the UV rays, high hardness, and excellent adhesion to the matrix [3]. Black coatings are used in many applications due to superior properties, such as optical instruments, absorbing materials, black decorative coatings, and aerospace industries. The leakage and dispersion of free particles from the coating caused to disturb the optical system performance [4]. The NiP electroless process is used to obtain continuous and uniform metal coatings. Nowadays, the electroless coating method is widely used for nickel-base coatings that have excellent properties such as high corrosion resistance, excellent wear resistance, high hardness, and non-magnetic properties [5]. Besides, surface treatment is an essential part of the surface coating. The surface treatment process causes the anticorrosion properties to result in the improvement of the fatigue behavior of the substrate [6]. There are two main factors for forming an excellent mechanical metal band and adhesion between coatings and substrates: (1) the primary replacement reaction on the active surface of metals, and (2) the bath ability to separate the fine particles. The electroplating rate and the coating thickness of electroless are the same in all sections of the solution [7, 8]. Black electroless NiP deposition on titanium alloy is the major challenge due to the presence of the oxide layer with the 50 to 200 Å thickness that prevents the adhesion of the precipitated metal coating to the substrate [9]. So, the preparation of the substrate is one of the most critical steps of the coating quality. The blackening coating was carried out by controlling and adjusting the parameters and immersed in different acidic solutions ratios [10]. Therefore, black nickel oxide is formed on the surface of the coating.
Vishal and et al [11] studied the influence of different parameters on the physical-optical properties of the black coating. They indicated that the coating process described herein is exceptionally suitable as a solar absorber coating for space and allied applications. Xing et al [12] investigated the tunable fabrication of the ultra-black NiP film. The blacking mechanism showed that this work provides a facile approach for the tunable fabrication of the ultra-black NiP film based on the anodization method in a non-oxidizing acid electrolyte, which can be practically applied in fields of black coating. Apachitei et al [13] investigated the effect of heat treatment on the structure and abrasive wear resistance of autocatalytic NiP and NiP–SiC coatings. They reported that the formation of a metastable equilibrium phase and precipitation of Ni3P compound caused to improve the abrasive wear behavior of the nanocrystalline coatings by heat treatment temperature up to 400 °C.
Titanium is high strength and light metal, which coatings on lightweight substrates can cause a mass reduction. Mass reduction as an essential factor in the design of a spacecraft reduces the production cost. Black coating with the mass reduction property and extremely high absorptance are beneficial for earthly and space-borne optical instruments design, sensors for measurements in spectral regions of UV–vis and infrared, and the absorptance improvement of thermal detectors [14–16].
Significantly, determining the optimal parameters of the electroless process to achieve maximum optical properties and maintain the stability of the coating are the most critical challenges in the optical industry. The optimization process was performed by investigating the effect of different experimental conditions such as the pH of an electroless nickel solution, the thickness of the electroless nickel deposit, and blackening time and temperature on the black coating's optical properties. Then, the effect of pre-heating and heat treatment on the mechanical properties of coatings was investigated.
2. Materials and methods
In this study, Ti-6Al-4V alloy samples with 50 × 30 × 2 mm dimensions were used as substrates for NiP black electroless nickel coatings. Samples were degreased with ultrasound waves in acetone medium for 10 min and washed by deionized water. The surface oxide film was removed in two steps. At first, Samples were attacked with 45 ml glycerol, 15 ml nitric acid, and 30 ml hydrochloric acid. Then, to remove the surface oxide film, at first, the samples were immersed in 10% oxalic acid solution at 90 °C for 1 h, afterward immersed in nitric acid and hydrogen fluoride solution with 3 to 1 ratio, 30 s at 30 °C. Then samples were washed with deionized water and immersed in the bath. The SLOTONIP70 commercial bath was made by the Schlüter German Company used for coating. This solution especially uses for metals and non-conductive material, according to the instructions provided by the manufacturer. It can use for long periods and can be reused 8 to 10 times after each bathroom correction.
The sedimentation rate was 15–18 μ per hour, which was reduced after modifying and replacing the bath to about 10 to 12 μ per hour [17]. The characterization of the bath is presented in table 1. A resistance laboratory heater with a maximum temperature of 350 °C was used to heat the electroless solution. The bath temperature was controlled at the range of 75 °C–85 °C by a mercury thermometer. The pH was between 4.3–4.7 during the electroless process. The ammonia solution was added every 10 min to prevent the solution pH reduction. Also, the digital pH meter by the precision of 0.01 was used to measure the pH of solutions.
Table 1. Characterization of bath (1 Lit).
| Material | Density | Measure (ml) |
|---|---|---|
| starter Slotonip71-1 | 1.20 | 166 |
| Ni solution Slotonip 72 | 1.27 | 70 |
| stabilizer Slotonip 76 | 1.02 | 7 |
| Distilled water | — | 757 |
Blackening coating was performed by immersing the samples in 9 M nitric acid solution for 15 s at 34 °C. After the blackening process, to stabilize the black color, the samples were immersed in hot water (70 °C) for 20 min. The present study aims to find two processes of heat treatment (hardening) and blackening. One sample was heat-treated following the blackening process, and the other sample was blackening with the following heat-treatment process. The heat treatment of the samples was carried out at 400 °C for four different holding times of 1, 2, 3, and 4 h. The Vickers microhardness test was performed according to ASTM E-92 standard at the load of 200 g for 10 s duration time using the Shimadzu micro-indention device. The microhardness test was performed on the surface of the samples.
The microhardness test was performed on the surface of the samples.
A scanning electron microscope (SEM) was used to study surface morphology and the thickness of the coatings of the samples. Energy Dispersive x-ray Spectroscopy (EDS) analysis was used to determine the chemical composition of coatings. Also, to measure the absorption coefficients of the samples, reflectance spectra obtained by a Shimadzu UV-3100 device and equation (1) were used. α in the equation was calculated by MATLAB software:
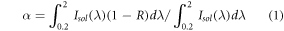
In which R is the reflectance spectra, λ(nm) is the wavelength, Isol (W/m−2) solar spectrum on air mass 0 [18].
3. Results and discussion
3.1. Morphology and chemical composition of NiP coating
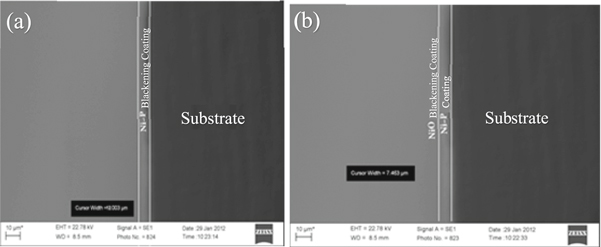
Figure 1 shows the SEM image and EDS analysis of black NiP coatings. Also, figure 1(b) shows the SEM image of the surface zone of the black NiP coatings. As observed in figure 1(a), the fine and uniform cauliflower-like morphology was formed on the surface in most grains. Fiad et al [19] reported the cauliflower-like morphology in NiP coatings. Due to immersing the coated samples into the nitric acid and performing the blackening process, the black NiP coating phase was formed on the coating surface. Also, figure 1(b) shows the SEM image of the edge zone of the black NiP coatings. As shown in figure 1(b), the image of the edge of the coated sample is seen to have been lost due to the grinding effect during the preparation process. The chemical composition of the sample edge is shown in figure 1(b), which shows the presence of titanium and carbon (due to preparation).
Figure 1. SEM image of black NiP coatings (a) surface, and (b) edge, EDS analysis of (c) point A (d) point B.
Download figure:
Standard image High-resolution imageFigures 1(c) and (d) shows the SEM image point A and point B of black NiP coatings, respectively. According to figure 1(c), the percentage of nickel, phosphorus, and oxygen elements are the highest due to the formation of phases with the mentioned elements on the coating surface. Also, due to the low thickness of the coating, the effect of the substrate elements, and contact of the coating surface and acidic solution and water, the impact of the element of calcium is evident.
Generally, the absorption coefficients of these coatings are attributed to their specific morphology and nickel oxides formed during the blackening process. The high roughness and the shape of the cavities created on the surface caused to continuous reflections of the coating surface and trapping light. The absorption coefficients of the coating are related to the blackened surface layer that formed after blackening [20]. The thickness of coatings affects solar absorption. The absorption coefficient increases for thicker coatings; this means that the minimum thickness of coating should be as thick as the depth of light penetration [21]. Due to the higher sediment rate and the greater thickness of the coating with preparation operation, the absorption coefficient of the sample was prepared with the nitric acid, and the fluoric acid solution was about 0.95.
3.2. Thickness of NiP coating
Figure 2(a) shows the SEM image of a cross-sectional coated sample after electroless plating for 45 min Owning to the coatings thickness and precipitin rate in the bath were predictable. According to figure 2(b), the blackening process is caused to surface corrosion of about 5 μm and forms a black layer on the NiP coating surface. The thickness of this black layer is generally between 200 to 2000 nm [22].
Figure 2. The cross-section images of samples: (a) before blackening, (b) after blackening.
Download figure:
Standard image High-resolution image3.3. The effect of blackening time on the absorption coefficient
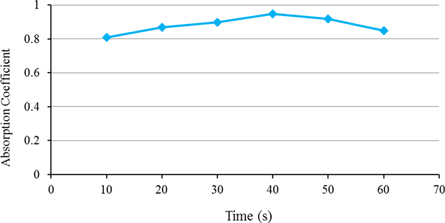
The blackening time has an essential role in obtaining the absorption coefficient and quality of electroless nickel blackening due to the use of concentrated acid in the blacking process. The blacking time has a high effect on the uniformity and absorption coefficient, and appearance of the coating. As observed in figure 3, the absorption coefficients were low at short blacking time, but the absorption coefficients were increased by increasing the blacking time. The undesirable absorption coefficient was obtained in the short times, and the non-uniformity coatings were formed, especially at the edges of the samples, by enhancing the etching time [23]. Figure 3 shows the plot of blackening time at nitric acid versus time of 10–60 s. As can be seen, by increasing the immersion time up to 40 s, the absorption coefficient increases to 0.95, then decreased to 0.85, which is due to the excess reaction of the acid with the surface. The coatings were divorced from the edges, so the total absorption coefficient of the sample was reduced. The maximum absorption coefficient was observed at a time between 40 to 50 s.
Figure 3. The effect of time on the absorption coefficient.
Download figure:
Standard image High-resolution image3.4. The effect of pH on the absorption coefficient
The pH of the solution is one of the most critical parameters in the electroless process that should be adjusted, however, to obtain the best conditions. Figure 4 shows the effect of pH on the absorption coefficient. As observed in figure 4, the phosphorous contents of electroless coatings are depended on the pH of the bath. The high phosphorus coatings due to high resistance to etching solutions need to use a higher concentration of acid. Also, increasing the phosphorus percentage of more than 11% reduced the absorption coefficients of the coatings. Mainly, by increasing the pH, the phosphorus content of the coating decreased, which caused an improvement in the absorption coefficient [24, 25]. It may be caused to the bath decomposition and lack of precipitate coating at pH greater than 5 [26]. According to figure 4, the appropriate pH range for the maximum absorption coefficient and the stability of the coating was 4.3 to 4.7, with the absorption coefficients of 0.92 and 0.95, respectively.
Figure 4. The effect of pH on the absorption coefficient.
Download figure:
Standard image High-resolution image3.5. The effect of blackening temperature on the absorption coefficient
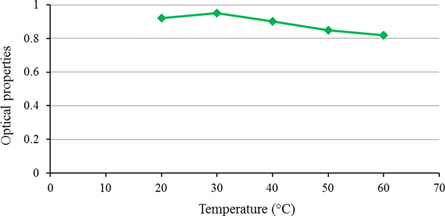
The effect of temperature on the absorption coefficient is presented in figure 5. As shown in this figure, the absorption coefficients were decreased by increasing the blackening temperature, and the optimum temperature was obtained close to the environment temperature. Because by increasing the temperature, the maximum optical absorption coefficients were reduced, and process control became difficult. The results show that the etching rate of the surface was decreased after blackening coating at ambient temperature. So, controlling of the blackening coating process gets easier. Therefore, the optimum operating temperatures for obtaining the high absorption coefficients was the ambient temperature. The blackening temperature of 30 s resulted in the absorption coefficient of 0.95.
Figure 5. The effect of temperature on the absorption coefficient .
Download figure:
Standard image High-resolution imageFigure 6 shows that the electroless black coating samples were prepared using oxalic acid, nitric acid, and fluoride solution. A titanium oxide layer (TiO2) was dissolved in solution due to the surface reaction with the acid solution. Due to removing the titanium oxide layer after immersing the surface into the bath, the nickel buds were deposited on them by the elemental displacement process. Thus, the process of filtration continues. Nickel refinery was deposited on the substrate by the elemental displacement process [27].
Figure 6. Image of NiP coated samples (a) before and (b) after blacking.
Download figure:
Standard image High-resolution imageThe blackened electroless NiP coatings, like the coatings that are fabricated in this research, include a composite of metal/metal and oxide/phosphate. This composite structure makes these coatings extremely suitable for space applications. These coatings are stable in the space environment, mainly when a lightweight substrate is used [2].
3.6. The effect of heat treatment on the blackening process
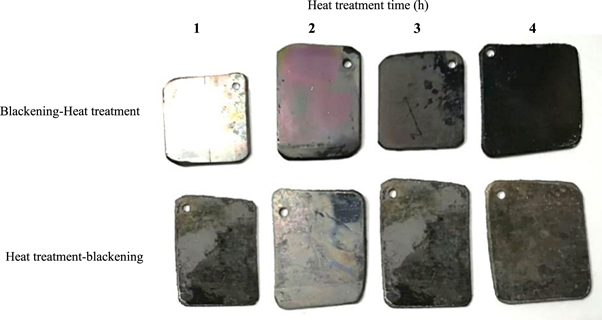
The effect of effective parameters (such as time, temperature, and PH) on the blackening was investigated, and the optimum parameters of this process were selected. In this section, the effects of heat treatment and its time on the blackening process were investigated. So, the samples were exposed to heat treatment at 400 °C for 1, 2, 3, and 4 h before and after the blackening process. Figure 7 shows the images of the sample was prepared in different condition. As observed in figure 7, in the heat-treated samples before the blackening process, the surface reaction did not occur, and the NiO black coating was not formed. This can be attributed to the reaction of nickel and phosphorus on the surface, resulting in hard phases formation after the heat treatment process. According to figure 7, the samples, which were heat-treated after the blackening process, had more excellent color stability. The NiP black coating is caused by the formation of oxide alloys, including nickel oxide (NiO, Ni3P, and Ni2P) and nickel-phosphorus (NiP) on the NiP black surface. The same sediments have been reported in similar studies [28]. The results show that with increasing the heat treatment time, the black color stability was higher on the sample, and the sample, which was heat treatment for 4 h, was the darkest sample. Previous studies reported that the highest hardness was obtained after heat treatment in the temperature range of 300 °C–400 °C. Because the NiP amorphous alloy converts to nickel crystalline and nickel phosphate phases, hardness increases, but corrosion resistance decreases. The results show that the hardness value depends on the phosphorus content, temperature, and heat treatment time. Sodagar et al [29] showed that the hardening value depends on the phosphorus content, temperature, and heat treatment time. As explained in the introduction, the NiP electroless coatings with high phosphorus content had an utterly amorphous structure. The heat treatment of these coatings at temperatures above 200 °C caused them to get crystallized.
Figure 7. Images of the sample was prepared in different condition.
Download figure:
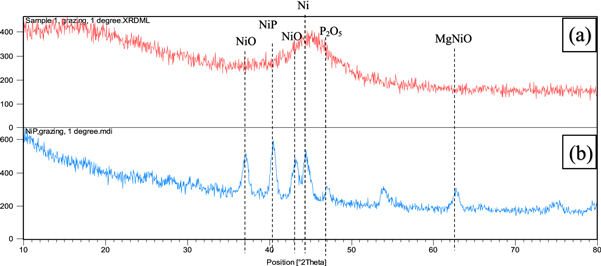
Standard image High-resolution imageXRD analysis was used to study the phases formed on the surface. According to figure 8, the Ni phase was created before the heat treatment, but the Ni, NiP, P2O3, NiO, and MgNiO phases were formed after heat treatment at 400 °C for 2 h. Figure 8 shows the X-ray diffraction pattern of NiP coatings before and after heat treatment at 400 °C for 2 h. According to figure 9(a), the broad matrix peak was observed in the range of 2θ of the amorphous phase. Heat treatment of the black coating leads to the coating crystallization and deposition of Ni3P hard coating in the nickel matrix [16]. Figure 9 shows the effect of heat treatment time on the absorption coefficient.
Figure 8. X-ray diffraction pattern of the electroless nickel-phosphorous coating (a) before heat treatment, (b) after heat treatment.
Download figure:
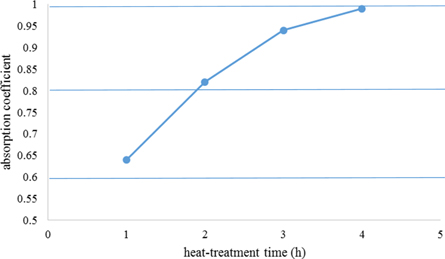
Standard image High-resolution imageFigure 9. The effect of heat treatment time on the absorption coefficient.
Download figure:
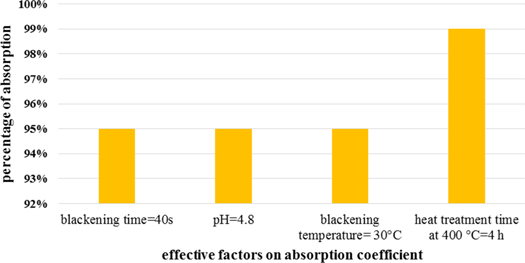
Standard image High-resolution imageIn summary, as shown in figure 10, the parameters that influence the absorption coefficient was optimized as blackening time for 40 s, pH of 4.8, blackening temperature of 30 °C, and finally, the process of heat treatment after blackening process at 400 °C for 4 h. these stages, with optimization, the factors resulted in a high absorption coefficient of 0.99.
Figure 10. The parameters that influence the absorption coefficient was optimized.
Download figure:
Standard image High-resolution image3.6.1. Hardness testing of coatings
Hardness is one of the most common tests used for investigating the influence of surface treatments on the mechanical properties of metallic materials. Figure 11 shows the microhardness of samples heat-treated after and before the blackening process for 1, 2, 3, and 4 h at 400 °C. The results show that the microhardness of samples heat-treated after the blackening process for 1, 2, 3, and 4 h was 854, 887, 909, and 950 Hv, respectively. Also, the microhardness of the samples heat-treated before the blackening process for 1, 2, 3, and 4 h were 489, 502, 534, and 580 Hv, respectively. The arrangement of the blackening process and the heat treatment had a considerable effect on the microhardness. As shown in figure 11, samples heat-treated after the blackening process had higher microhardness than samples heat-treated before the blackening process, which, according to the phases formed on the surface, is justified. As observed in figure 11, the hardness was enhanced by increasing the heat treatment time. After the heat treatment process, samples with different heat-treatment times have increased due to the formation of Ni3P hard phase. The results show that the sample heat-treated after the blackening process for 4 h was considered the best condition to achieve the optimum microhardness. According to previous research results, the featured composition and structures caused to increase the hardness. So, the phosphorus content of NiP coating is proved to be appropriate to achieve a high hardness of the coating [30]. As mentioned above, the heat-treated sample after blackening for 4 h, has the highest microhardness. The high hardness, stable black color, and the titanium substrate make this sample very useful for space applications compared to black paints with organic binders, which have a high total mass loss. The black paints with organic binders contain considerable amounts of volatile material can weaken the equipment performance. The fabricated electroless nickel coating in this research results in weight loss in space applications, and with the stable black color can be used as an absorber coating [2, 4].
Figure 11. Plot of Microhardness of samples after 1, 2, 3, and 4 h at 400 °C.
Download figure:
Standard image High-resolution image4. Conclusion
In this research, black electroless NiP deposits on Ti-6Al-4V titanium alloy and investigation of optical and adhesion properties. The purpose of this paper was to report the effect of heat treatment on the blackening coating, and the following results were obtained:
- (1)Nickel phosphorus electroless coating of an acid-type NiP electroless bath was successfully deposited on the titanium substrate.
- (2)The optimum conditions in terms of temperature and pH for deposition of NiP coatings on titanium were obtained in the range of 85 °C–95 °C and pH range of 4.3 to 4.7
- (3)The optimum conditions of blackening coating were obtained at ambient temperature and etching time of 40–50 s.
- (4)The results show that the microhardness of samples heats treated after the blackening process for 1, 2, 3, and 4 h were 854, 887, 909, and 950 Hv, respectively.
- (5)After the blackening process for 4 h, the sample heat-treated was considered the best condition to achieve the optimum microhardness of 950 Hv and an absorption coefficient of 0.99.
- (6)Samples prepared in two 10% oxalic acid solutions at 90 °C for one hour and 3: 1 ratio of nitric acid and hydrofluoric acid at 30 °C for 30 s after coating with adhesion. They were the same, but the optical absorption coefficient was 0.89 for the first sample and 0.95 for the second sample.
- (7)The prepared black coating with high absorption of 95% and titanium as alow weight substrate is beneficial for use as a solar absorber coating for space and allied applications.