Abstract
In this study, in situ surface nanocomposites based on Mg-CuO and Mg-Cu systems were developed via six passes of friction stir processing (FSP) on the surface of AZ91 magnesium casting alloy. In-situ phase evolution during FSP with the addition of Cu and CuO micro-powders was studied by x-ray diffraction (XRD) analysis and microstructural investigations. Here, AZ91/Cu nanocomposite was reinforced by the in situ formation of Mg2Cu intermetallic compound. In AZ91/CuO nanocomposite, CuO particles were reduced and MgO and MgCu2 reinforcing particles alongside Mg2Cu intermetallic compounds were formed during FSP. Grain refinement and in situ formation of reinforcement particles with different sizes ranging from nano- to micro-scale significantly improved mechanical performance of the specimens. Dynamic recrystallization was found to be the main mechanism of grain refinement. After six passes of FSP, the hardness values of AZ91/Cu and AZ91/CuO nanocomposites were increased by 69.1% and 91%, respectively. Besides, AZ91/CuO nanocomposite exhibited the best tensile strength and wear resistance among all the samples. The dominant wear mechanisms were abrasive and delamination wear in AZ91 magnesium alloy, while nanocomposite specimens were worn mainly by abrasive mechanism.
Export citation and abstract BibTeX RIS
1. Introduction
Magnesium alloys are promising materials for aerospace, automotive and military industries as well as electronic components for portable devices. This is mainly due to their lower density than aluminum and steel [1, 2]. However, the potential applications of magnesium alloys have not been realized yet due to some inherent drawbacks such as low strength and creep resistance at high temperatures, high chemical reactivity, and low elastic modulus [2]. These drawbacks can be modified to some extent by the addition of alloying elements, grain refinement, and texture development to activate non-basal slip systems, and creating surface composites [3, 4]. The latter is typically fabricated using fusion or solid-state techniques. However, the fusion techniques suffer from several disadvantages such as melt segregation, lack of process control and non-uniform distribution of the reinforcement particles in the dendritic microstructure [5, 6]. Solid state techniques, on the other hand, strongly overcome these drawbacks. Friction stir processing (FSP) is an easy and affordable solid-state method to fabricate surface composites in metal alloys. The absence of a melting phase in FSP results in the omission of the imperfections in the casting structure, refines the microstructure and improves the mechanical properties. Moreover, the surface and bulk composites can be readily fabricated by FSP through the in situ formation of the reinforcing intermetallic compounds (ICs) [7, 8]. FSP induces severe plastic deformation in the processed materials, which results in significant microstructural refinements in the stir zone through dynamic recrystallization (DRX) and dynamic recovery (DRV) [9, 10]. In a research conducted by Chang et al [11], the grain size of AZ31 alloy was greatly reduced by two passes of FSP and a nano-sized structure with the average grain size of about 85 nm was fabricated on the alloy surface. Feng et al [12] also reported that FSP significantly refined microstructure and improved tensile properties of AZ91 alloy. Besides, aging treatment after FSP was found to be effective in improving mechanical properties of AZ91 alloy [12].
Besides grain refinement, reinforcement particles have been largely employed during FSP to further modify the microstructure and improve the mechanical properties of various magnesium alloys [13–17]. Reinforcing particles may be either directly added to the matrix or in situ formed through the reactions between alloying elements in the processed zone of FSP. The in situ formation of reinforcement particles benefits from the possibility of getting a uniform distribution of nanosized reinforcements having strong interfaces with the matrix. Lee et al [14] observed a significant grain refinement from 75 μm to 0.8 μm in AZ61 magnesium alloy processed by 4 passes of FSP and applying 5–10 vol.% of SiO2 nanoparticles. In addition, the SiO2 clusters became finer and the amount of Mg2Si and MgO secondary phases increased with increasing the number of FSP passes. Morisada et al [15] processed MWCNT/AZ31 nanocomposites with FSP and obtained a fine microstructure with a grain size less than 500 nm and a maximum microhardness value of about 78 Hv. Singh et al [16] also reported that the wear resistance of AZ91 alloy could be greatly increased by ex situ fabrication of AZ91/TiC composite using FSP. The fabrication of surface composites using FSP has been also widely published in other metal systems such as Ti–6Al–4V [18–21] and Al [22–24].
In the majority of studies on FSP of magnesium alloys ex situ reinforcing particles have been used to fabricate surface composites. However, the feasibility of in situ reinforcing of pure magnesium [25] and AZ31 alloy [26] with the addition of Al powder to the stir zone has been recently demonstrated. To the best of our knowledge, the in situ fabrication of surface nanocomposite on the AZ91 magnesium alloy using FSP has not been reported so far. Considering the desirable effect of MgxCuy ICs on the microstructure and mechanical properties of magnesium matrix [13, 17], this study aims to evaluate the phase evolution pathway to in situ intermetallic formation during FSP of an AZ91 magnesium alloy using Cu and CuO elemental powders. The microstructure, mechanical properties, and wear behavior of the fabricated composites were thoroughly investigated and the relevant fracture and wear mechanisms were revealed.
2. Experimental
2.1. Materials and methods
Cast AZ91 alloy plates with dimensions of 11 cm × 4 cm × 1 cm and a chemical composition as in table 1 were prepared. In order to develop a surface nanocomposite in Mg/Cu and Mg/CuO systems, copper and copper oxide powders with a particle size of about 20 μm (>99% purity, Merck) were used. A groove with 2 mm depth and 1 mm width (about 12.5 vol. % powder) was prepared on the surface of each AZ91 alloy plate. The specimens were first degreased in acetone and then immediately underwent FSP. The grooves were first filled with reinforcing powders, and then closed using a pinless tool to prevent the flare out of the powders during subsequent FSP passes. Afterwards, each specimen was processed by up to 6 passes of FSP using a threaded cylindrical tool made of H13 hardened steel with a clockwise twist, as schematically represented in figure 1(a). Table 2 lists the processing parameters employed to fabricate the surface composites.
Table 1. Nominal composition of AZ91C alloy.
| Elements | Mg | Al | Zn | Mn | Fe |
|---|---|---|---|---|---|
| wt.% | 90.6 | 8.63 | 0.59 | 0.17 | <0.059 |
Figure 1. (a) Schematic representation of the FSP tool, (b) position and dimension of a standard sample for tensile test.
Download figure:
Standard image High-resolution imageTable 2. The processing parameters used in this study.
| Friction stir processing parameters | ||||
|---|---|---|---|---|
| Sample | Reinforcement | Rotational speed (rpm) | Traverse speed (mm min−1) | Tilt angle (deg.) |
| AZ91 | — | — | — | — |
| AZ91-FSPed | — | 1000 | 40 | 3 |
| AZ91/Cu | Cu | 1000 | 40 | 3 |
| AZ91/CuO | CuO | 1000 | 40 | 3 |
2.2. Microstructural characterization
An optical microscope (OM-Olympus) was used to study the microstructure and particle distribution in the matrix. The samples were mounted and given a shiny surface finish using emery papers (grit size: 5000) followed by polishing. For microstructural studies, the samples were etched in a solution consisted of ethanol (70 ml), distilled water (70 ml), acetic acid (20 ml), and picric acid (4.2 g). The linear intersection method was applied to calculate the grain size from the microscopic images. The microstructural features of the stir zone were studied by Scanning Electron Microscopy (SEM-Leo Philips) and Field Emission Scanning Electron Microscopy (FESEM-Mira 3-XMU).
2.3. Mechanical characterization
Hardness values of the specimens were measured by Micro Vickers hardness tester (MH3 - Koopa) with an applied force of 100 g for 10 s dwell time. Tensile tests were carried out using an Instron type Hounsfield H50KS tensile machine with a jaw speed of 1 mm min−1 at room temperature. Tensile specimens were cut from the stir zones according to ASTM E8 standard (figure 1(b)). The fracture surfaces of the specimens were investigated by SEM technique.
The wear behaviors of AZ91 alloy and the fabricated nanocomposites were examined by a reciprocating wear tester. The applied pins were made of 52 100 steel with a hardness of 64 HRC. The tests were carried out at room temperature with a constant load of 15 N over a distance of 1100 m under dry conditions. The linear speed of the device was fixed at about 0.1 m s−1. The wear surface and the wear debris were examined by SEM to reveal the underlying mechanisms. The damaged areas of the worn surfaces were calculated by Clemex software for different specimens.
3. Results
3.1. Microstructural evaluation
The microstructures of AZ91 alloy before and after 6 passes of FSP are presented in figures 2(a) and (b). The heterogeneous coarse structure of the cast AZ91 alloy with a grain size of about 100 μm (figure 2(a)) entirely transformed to a homogenous equiaxed structure with a grain size of about 30 μm after 6 passes of FSP. The matrix phase (α) and the discontinuous precipitates of eutectic reaction (β) in a coarse and elongated form can be observed at the grain boundaries (figure 2(a)). Micro-scaled cavities and β-phase precipitates that exist at the grain boundaries of as-cast AZ91 alloy (figure 2(a)) act as stress concentration locations and result in crack initiation and subsequently poor tensile properties of AZ91 magnesium alloy. Microstructure refinement during FSP is the direct consequence of the shoulder-rubbing action, pin stirring action and dynamic recrystallization, which are simultaneously activated during successive passes. The XRD spectra of AZ91 alloy confirmed that the microstructure was composed of α solid solution (Mg-Al) and β (Mg17Al12) precipitates (figure 3). The XRD peaks corresponding to β (Mg17Al12) precipitates almost disappeared after 6 passes of FSP, which could be ascribed to the breakdown, redistribution and dissolution of these precipitates in α matrix phase [11].
Figure 2. Optical microscopy images of: (a) base alloy and (b) AZ91-FSPed.
Download figure:
Standard image High-resolution imageFigure 3. X-ray diffraction patterns of: (a) base alloy and (b) AZ91-FSPed.
Download figure:
Standard image High-resolution imageFigure 4 shows the traverse cross-sectional microstructure of SZ in AZ91/Cu nanocomposite processed by 2, 4, and 6 passes of FSP. Large agglomerated Cu particles can be clearly observed in the specimen after 2 passes of FSP (figure 4(a)). Further FSP passes broke down the agglomerated particles and refined the microstructure. Some agglomerated particles were presented in the microstructure of specimens even after 4 passes of FSP, which indicated by arrows in figure 4(b). Besides, fine particles (indicated by a circle in figure 4(b)) were also revealed in the microstructure of 4-pass FSPed specimens. These fine reinforcement particles may be refined Cu fragments and some Mg2Cu ICs that in situ formed by reactions between Cu particles and the Mg of the AZ91 matrix alloy. The grain size also significantly decreased to about 10 μm after 4 passes of FSP. A homogeneous microstructure consisting of the uniform distribution of nano-sized reinforcement particles in an ultrafine microstructure was obtained after 6 passes of FSP (figure 4(c)).
Figure 4. OM images of SZ (traverse section) in AZ91/Cu composite after (a) 2 passes, (b) 4 passes, and (c) 6 passes of FSP.
Download figure:
Standard image High-resolution imageTo gain a better understanding of the microstructural properties, OM images of the interface at different zones of AZ91/Cu nanocomposite are shown in figure 5. Successive structural evolution during FSP forms distinct stir zone (SZ), thermo-mechanically affected zone (TMAZ) and heat affected zone (HAZ). The boundaries between different zones are quite distinguishable in figures 5(a), (b). FSP involves high temperature severe plastic deformation which results in dynamic recovery and dynamic recrystallization and subsequently structural refinement at SZ. Besides, Well-distributed Cu particles and the in situ-formed fine ICs can effectively suppress dislocations slip and grain boundaries migration through Zener pinning effect [27]. The synergetic operation of dynamic recrystallization and the pinning effect of the secondary phase provides the nanocomposites with an ultrafine microstructure with a grain size of about 1 μm in SZ. The transition zones could be clearly detected due to the grain-size difference between SZ, TMAZ and HAZ. While dynamic recrystallization resulted in fine grains with new boundaries at TMAZ, the microstructure of HAZ slightly altered because of heat gradient and thermal effects. Same microstructural properties were also observed in AZ91/CuO nanocomposite, which are not shown here.
Figure 5. Optical microscope images of the boundaries between HAZ and TMAZ (a), and TMAZ and SZ (b) in AZ91/Cu nanocomposite.
Download figure:
Standard image High-resolution imageFESEM images of the stir zone of AZ91/Cu and AZ91/CuO nanocomposites are shown in figures 6(a) and (b), respectively. It was expected that Cu and CuO, as well as the in situ formed intermetallic reinforcement particles locked the grain boundaries and prevented grain growth during FSP process. This is characterized by a heterogeneous deformation and restoration of the primary grains to form a fine-grained microstructure during FSP passes [28, 29]. The grain-size of AZ91 magnesium alloy was decreased from 100 μm to about 30 μm for AZ91-FSPed specimen (figure 2(b)) and to about 1 μm for both AZ91/Cu and AZ91/CuO nanocomposites (figure S1 is available online at stacks.iop.org/STMP/8/045002/mmedia, supplementary information), which highlighted the significant role of the secondary particles to suppress grain growth and retain the fine recrystallized microstructure. Hierarchical particles with sizes ranging from nanometer to micrometer were revealed during microstructural observations (figures 6 and S1). Extensive EDS analysis was performed to determine the chemical composition of these particles, as shown in figure S1. Considering the electron interaction diameter of ∼1 μm in EDS [30] and the particle size of below 1 μm in our nanocomposites, the EDS results are better to be evaluated qualitatively. However, we used EDS results coupled with the XRD data (figure 7) to identify the reinforcement particles. We mainly identified these nano- and micro-dimension particles as Cu and Mg2Cu for AZ91/Cu nanocomposite, and Mg2Cu, MgCu2, and MgO for AZ91/CuO nanocomposite. EDS and XRD analysis also revealed that the amount of unreacted Cu in the stir zone of AZ91/CuO is lower than that of the stir zone of AZ91/Cu nanocomposite. One reason for this could be the in situ reaction between CuO and Mg to form MgO-reinforcement particles. This exothermic reaction also facilitated the formation of Mg2Cu and MgCu2 ICs in AZ91/CuO nanocomposite, so that the amount of these intermetallic phases increased in the matrix of AZ91/CuO nanocomposite. On the other hand, the XRD spectra of AZ91/Cu nanocomposite after 6 passes of FSP was characterized mainly by the in situ formation of Mg2Cu phase and the remained Cu particles (figure 7).
Figure 6. FESEM images of (a) AZ91/Cu and (b) AZ91/CuO nanocomposites showing nanoparticle dispersion with different sizes.
Download figure:
Standard image High-resolution imageFigure 7. XRD patterns of AZ91/Cu and AZ91/CuO nanocomposites processed by different passes of FSP.
Download figure:
Standard image High-resolution image3.2. Mechanical properties
The microhardness profiles of the AZ91 alloys in different processing conditions are shown in figure 8. The microhardness of AZ91 alloy was measured to be 68.3 ± 3 Hv, which was increased up to 82 Hv after 6 passes of FSP mainly due to the formation of a fine-grained structure in the processed region. On the other hand, the synergetic effect of grain refinement and second particles hardening significantly increased the microhardness of AZ91/Cu and AZ91/CuO nanocomposites by about 69.1% and 91%, respectively. The highest microhardness values were obtained for AZ91/CuO nanocomposite in the entire processing region. This may be ascribed to the higher content of the equiaxed reinforcement particles in AZ91/CuO nanocomposites, as confirmed by FSEM and XRD analysis. Besides, it can be clearly observed that the hardness of HAZ in AZ91/CuO nanocomposite is significantly higher than that of AZ91/Cu nanocomposite. We attributed this to the higher heat gradient and thermal effect experienced by HAZ due to the exothermic reduction of copper oxide by magnesium in AZ91/CuO nanocomposite.
Figure 8. Microhardness profiles of the AZ91 base alloy and the FSPed specimens in different conditions.
Download figure:
Standard image High-resolution imageFigure 9 shows the tensile properties of AZ91 alloy at different processing conditions. The obtained mechanical properties for AZ91 magnesium alloy were similar to those reported for this alloy in the literatures [31]. Mechanical properties of AZ91 magnesium alloy were greatly enhanced after 6 passes of FSP. A large elongation of 12.5% (∼267% increases) was realized in AZ91 alloy after 6 passes of FSP without the addition of secondary particles. This may be attributed to the breakdown and dissolution of β precipitates, elimination of structural defects, and refined microstructure through DRX (figure 2(b)). AZ91/Cu and AZ91/CuO nanocomposites exhibited an outstanding tensile strength of 258.5 MPa and 290.8 MPa, respectively. Our nanocomposites, especially AZ91/CuO, exhibited significantly higher strength values than those reported by Aatthisugan et al [32] for AZ91/B4C/Gr hybrid composite, and those reported by Chung et al [33] for equal channel angular pressed AZ91 alloy. The tensile strength of 290.8 MPa and the yield strength of 170.2 MPa obtained for AZ91/CuO nanocomposite were close to those reported by Nie et al [31] for AZ91 alloy processed by multidirectional forging. These improved mechanical properties could be directly ascribed to the grain refinement and the strengthening effect of secondary particles, as detailed in microstructural analysis.
Figure 9. Tensile properties of AZ91 magnesium alloy at different processing conditions.
Download figure:
Standard image High-resolution imageFigure 10 shows the fracture surface of AZ91 magnesium alloy, AZ91-FSPed, AZ91/Cu, and AZ91/CuO specimens. The AZ91 cast alloy represented mainly a brittle fracture nature characterized by cleavage facets, sharp edges and no detectable deep cup and cone shapes (figure 10(a)). It should be noted that deformation in magnesium alloys is limited to their confined slip systems, and the formation of stepped faces on the fracture surface is due to the slip of (0001) planes in the hexagonal structure of magnesium [34]. After 6 passes of FSP, the brittle cleavage fracture completely disappeared and ductile fracture mode with a river-like pattern and deep and uniform dimples dominated (figure 10(b)). This brittle to ductile failure mode transition may be ascribed to the microstructural changes and the activation of non-basal slip systems in FSPed AZ91 alloy. Fracture surface of AZ91/Cu (figure 10(c)) and AZ91/CuO (figure 10(d)) nanocomposites resembled a mixed mode brittle-ductile failure that characterized by voids, cracks and small and shallow dimples.
Figure 10. SEM images of the fracture surface of AZ91 base alloy (a), FSPed AZ91 (b), AZ91/Cu (c), and AZ91/CuO (d) specimens.
Download figure:
Standard image High-resolution image3.3. Wear properties
The volumetric wear rate of the specimens measured over a total sliding distance of 1100 m is plotted in figure 11(a), and the variations of volume loss as a function of sliding distance are shown in figure 11(b). The volume wear rate was roughly estimated by mass loss using the density of AZ91 magnesium alloy. The calculated volumetric wear rate for AZ91 is close to that reported by Chen et al [35] at a sliding speed of 0.1 m s−1 and a wear load of 20 N.
Figure 11. (a) The volumetric wear rates calculated at the total sliding distance of 1100 m, and (b) The change of volume loss as a function of sliding distance.
Download figure:
Standard image High-resolution imageThe results clearly show that the wear resistance of all FSP processed specimens has been improved compared to the AZ91 base alloy. Especially, the overall wear rate of specimens decreased from 0.012 mm3/m for AZ91 alloy to about 0.002 mm3 m−1 for AZ91/CuO nanocomposite (about 87% decrease). The base alloy exhibited an unsteady wear behavior in which the ascending trend of volume loss was retained in the entire wear path (figure 11(b)). On the other hand, the slope of volume loss slowly declined in AZ91/Cu and AZ91/CuO nanocomposites, especially at higher sliding distances. The temperature in the friction surface increased with increasing sliding distance due to the excessive friction heat produced during dry sliding wear. This may increase the extend of adhesive wear on the surface of samples, and consequently rise the ascending trend of volume loss. The lower increase in volume loss of nanocomposites may be ascribed to the harmony of several strengthening mechanisms induced by the in situ formation of ICs with sizes ranging from nano to micro scales. These hierarchical reinforcement particles also reduced the effective contact area between the AZ91 matrix and the wearing tool due to their higher ability to bear loads. The higher hardness of nanocomposite samples is also contributed to their outstanding wear behavior. All these favored the wear properties of AZ91/Cu and AZ91/CuO nanocomposites. Besides, the OM images of the cross-sectional area of the worn surfaces (figure S2) indicated that the depth of the worn surface in AZ91 alloy was four times higher than that of the AZ91/CuO nanocomposite. This further confirms the outstanding wear resistance of AZ91/CuO nanocomposite.
SEM morphologies of the worn surfaces and the wear debris of all specimens are shown in figure 12. It can be seen that the worn surface of AZ91 alloy is mainly characterized by severe plastic deformation, delamination, deep grooves and shallow scratches along the sliding direction (figures 12(a), (b)), suggesting severe delamination as the main wear mechanism for AZ91 alloy [36, 37]. The debris of AZ91 alloy exhibited large delaminated morphologies with a size of about 200 μm (figure 12(c)), further confirming the delamination wear mechanism. Generally, delamination wear is dominated with the increase of sliding distance due to the rise in temperature and plastic deformation as well as the occurrence of surface fatigue phenomenon [35]. The presence of large delamination wear debris and cracks perpendicular to the wear path are the main features of delamination wear mechanism that can significantly increase the wear rate. These observations are in good agreement with the higher wear rate of AZ91 base alloy as shown in figure 11. The worn surfaces of all FSPed specimens with and without the addition of secondary particles represented some extent of delamination along with a number of relatively deep grooves and fine ploughs. These grooves and ploughs are typical characteristics of the abrasive wear [38]. However, the depth of the grooves greatly decreased in AZ91/Cu and AZ91/CuO nanocomposite samples. The in situ reinforcement particles in the nanocomposite matrices reduced plastic deformation as well as the delamination of the worn surface. Besides, these particles impede dislocations movement and increase work hardening on the surface of nanocomposites. EDS analysis confirmed the presence of oxide particles on the worn surfaces of the nanocomposite samples (figure S3). In addition, it was found that the morphology of wear debris changed from completely large flakes for AZ91 alloy to remarkably fine debris for nanocomposite samples. Fine wear debris also represents abrasive wear with ploughing mechanisms. Therefore, it can be concluded that severe delamination, grooves-delamination and grooves are the main wear mechanisms in AZ91 alloy, FSPed AZ91, AZ91/Cu, and AZ91/CuO nanocomposite samples, respectively.
Figure 12. SEM micrographs of worn surfaces and wear debris for (a)–(c) AZ91 magnesium alloy, (d)–(f) AZ91-FSPed sample, (g)–(i) AZ91/Cu and (j)–(l) AZ91/CuO nanocomposites.
Download figure:
Standard image High-resolution image4. Discussion
4.1. Microstructural characteristics
The mechanism of grain refinement in metal alloys strongly depends on the crystal structure and stacking fault energy (SFE). Typically, dislocations slip, mechanical twinning and transformation-induced plasticity are the main deformation modes in alloys [39–41]. However, in magnesium alloys with HCP structure and low SFE (60–70 mJm−2) [39], twinning and DRX are the main deformation trends during severe plastic deformation [42]. It is reported that DRX prevails during high temperature deformation of Mg alloys due to low SFEs [39]. Three main DRX mechanisms have been proposed: continuous DRX (CDRX), discontinuous DRX (DDRX), and twin DRX (TDRX) [39, 42, 43]. CDRX occurs through transformation of low-angle grain boundaries (LAGBs) to high-angel grain boundaries (HAGBs) by continues absorption of dislocations. DDRX involves the two-step classical process of nucleation and growth of new grains. TDRX involves twins nucleation followed by twin boundaries transformation to HAGBs due to the formation of orientation misfit dislocations [44]. However, the activated mechanisms of DRX during FSP change with strain, temperature, and the initial grain size of the materials [39]. Therefore, there is no consensus among researchers whether CDRX, TDRX, or DDRX causes such substantial structure refinement. Large grains in magnesium alloys have been reported to be beneficial for deformation twinning during FSP [39]. Here, we found excessive twins and twinning-induced recrystallized grains in the TMAZ of all the specimens processed by six passes of FSP (figure 13). The twins gradually nucleated and propagated inside the grains during six passes of FSP. Interestingly, no twins could be detected in the SZ of the samples. It is well documented that temperature has a critical effect on the operative DRX mechanisms in magnesium alloys [39]. The contribution of TDRX to the recrystallization process decreases with increasing temperature, and CDRX predominates the recrystallization process at temperatures above 300 °C [45]. Here, it seems that TDRX is the main operative mechanism in TMAZ, while CDRX and DDRX are the prevailing DRX mechanisms in SZ.
Figure 13. OM image of TMAZ in AZ91/CuO nanocomposite showing TDRX as the main DRX mechanism.
Download figure:
Standard image High-resolution image4.2. Thermodynamic analysis of phase evolution
4.2.1. AZ91/Cu Nanocomposite
Two phases of Mg2Cu and MgCu2 ICs exist in the equilibrium binary Mg-Cu phase diagram. Mg2Cu was the first IC that formed in AZ91/Cu composite after two passes of FSP, according to the XRD results (figure 7). Phase evolution in a binary system depends not only on the temperature but also on the chemical composition [46]. In order to shed light on the sequence of intermetallic phase formation in the binary Mg-Cu system, it is important to consider both thermodynamic and kinetic parameters. Ke et al [46] used the effective heat of formation to determine which phase is formed first in an Al-Ni system. However, it is more convenient to consider that the effective Gibbs free energy ( ) controls the thermodynamic preference of intermetallic phases in solid state reactions. In Mg-Cu binary system, the effective Gibbs free energy (
) controls the thermodynamic preference of intermetallic phases in solid state reactions. In Mg-Cu binary system, the effective Gibbs free energy ( ) for each phase can be calculated as bellow [47]:
) for each phase can be calculated as bellow [47]:
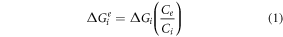
Where Ce
is the concentration of the limiting element at the interface, Ci
is the concentration of the limiting element in the intermetallic compound, and  is the Gibbs free energy of formation for Mg2Cu and MgCu2 phases [47]. These can be calculated using the following equations [48]:
is the Gibbs free energy of formation for Mg2Cu and MgCu2 phases [47]. These can be calculated using the following equations [48]:


The concentration of Mg and Cu in SZ were calculated to be about 79 at% and 21 at%, respectively. Therefore, Ce for the limiting element (Cu) was equal to 0.21. As well, the concentration of the limiting element (Cu) is 0.33 for Mg2Cu and 0.66 for MgCu2. The effective Gibbs free energy of formation for Mg2Cu and MgCu2 in Mg79Cu21 and two other systems (namely, Mg90Cu10 and Mg40Cu60) is presented in table 3. Similarly, the effective Gibbs free energy can be calculated for all Mg-Cu binary compositions (figure 14). It is obvious from figure 14 that in Mg-Cu binary system, for an Mg content of 0 to 48 at.%, MgCu2 is the first-precipitated phase at the interface of Mg-Cu, while Mg2Cu precipitates first if Mg content falls in the range of 48 to 100 at.%. Therefore, in the present study, Mg2Cu is the thermodynamically favorable phase.
Table 3. The calculated effective Gibbs free energy for different Mg-Cu systems.
| Alloying Systems | Mg90Cu10 | Mg78Cu18 | Mg40Cu60 | |||
|---|---|---|---|---|---|---|
| Intermetallic compound | limiting element |
 (543 K) Kj mol−1 (543 K) Kj mol−1
| limiting element |
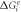 (543 K) Kj mol−1 (543 K) Kj mol−1
| limiting element |
 (543 K) Kj mol−1 (543 K) Kj mol−1
|
| Mg2Cu | Cu | −9.702 | Cu | −20.38 | Mg | −19.21 |
| MgCu2 | Cu | −4.32 | Cu | −9.16 | Cu | −25.90 |
Figure 14. The effective Gibbs free energy diagram for intermetallic compounds in Mg-Cu system.
Download figure:
Standard image High-resolution image4.2.2. AZ91/CuO
The XRD spectra of AZ91/CuO nanocomposite revealed the diffraction peaks of MgO phase beside those of ICs after six passes of FSP (figure 7). We assumed that these MgO particles were formed through direct reduction of CuO particles by the magnesium in the AZ91 matrix. Copper oxide reduction takes place according to the following reaction:

The Gibbs free energy of this reaction as a function of temperature is presented in equation (4) [49], and was calculated to be −436.893 KJ at 543 K.

The large negative value of ΔG for this reaction reveals the high driving force for CuO reduction during FSP. A schematic representation of phase evolution during FSP of AZ91/CuO nanocomposite is shown in figure 15. In Ellingham diagram, the oxidation line of CuO is located under that of MgO, so Mg can thermodynamically reduce CuO to form Cu and MgO. As a result, MgO nucleates firstly at the boundaries of copper oxide and magnesium matrix, and gradually grows as a shell around the CuO particles (figure 15). These MgO shells are shattered and distributed in the AZ91 matrix during the next FSP pass. Therefore, magnesium comes into direct contact with copper oxide and reduces it to form another MgO shell around the remaining CuO particles. This reduction-shattering cycle is continued repeatedly until the entire CuO is reduced to form Cu and MgO products. On the other hand, the reduced Cu particles may further react with Mg to form Mg2Cu and MgCu2 ICs. The exothermic reduction of copper oxide increases temperature in the stir zone, and subsequently increases the formation kinetics of Mg2Cu and MgCu2 ICs. XRD spectra of AZ91/CuO composite (figure 7) also confirmed the formation of Mg2Cu and MgCu2 ICs. The proposed mechanism can be further validated by figure S4, where the SEM image of a large agglomerated CuO particle and the corresponding EDS spectra of several points are shown. Therefore, it can be concluded that solid-state reactions are highly improved during FSP and a hybrid nanocomposite with uniform distribution of reinforcement particles is obtained when using CuO particles as the alloying phase instead of Cu powder.
Figure 15. A schematic representation of the in situ reaction to form ICs in AZ91/CuO composite during six passes of FSP.
Download figure:
Standard image High-resolution image4.3. Mechanical behavior
A relatively large range of microhardness values and tensile properties were obtained due to the different microstructural features and intermetallic phase evolutions within the processed zone of the specimens, as shown in figures 8 and 9. It was revealed that the distinct in situ formation of ICs exerted a remarkable influence on the width of the reinforced region, indentation microhardness resistance, tensile strength and ductility, fracture mechanism, and wear behavior of the FSPed specimens in relation with the microstructure. The AZ91 alloy that was processed by six passes of FSP with the addition of CuO particles provided the maximum yield strength, tensile strength, microhardness and wear resistance, as shown in figures 8–11. The exothermic reduction of copper oxide in the processed zone was revealed to play a critical role in the in situ formation of Mg2Cu, MgCu2 and MgO reinforcement particles with sizes ranging from nano to micro scale. The difference between the thermal expansion coefficients of these particles and the matrix accompanied with the activation of Orowan mechanism may increase the density of dislocation in the nanocomposites. Besides, the simultaneous formation of MgO, Mg2Cu, and MgCu2 particles with different sizes not, only effectively impedes the movement of dislocations and provides further increase in hardness and strength, but also suppresses grain growth and retains the fine recrystallized grain structure in nanocomposites. According to the Hall-Patch equation, the strength of materials is augmented when the grain size decreases. In addition, the reinforcement particles in the microstructure may impose reciprocal stresses on dislocation sources through the Orowan mechanism. In this way, the next slip needs more shear stress. Thus, the rate of strain hardening increases in the presence of the reinforcement particles. This also improves the wear resistance of nanocomposites with increasing sliding distance [37]. The results suggest that by obtaining a nanocomposite with optimized strength, ductility, hardness, and wear resistance, phase evolutions during FSP must be accurately predicted and controlled through the proper choice of alloying powders. The overall implication of our results suggests that FSP is an effective route to fabricate in situ surface nanocomposite on magnesium alloys with tailor-able ICs reinforcement particles, which may bring magnesium alloys one step closer to the widespread applications.
5. Conclusions
In this study, the in situ formation of hybrid surface nanocomposite in AZ91 magnesium alloy during FSP with the addition of Cu and CuO micro powders was investigated. The main findings can be outlined as follows:
- 1.The grain size of AZ91 base alloy decreased from 100 μm to about 30 μm after six passes of FSP. Addition of Cu and CuO micro-powders further decreased the grain size to about 1 μm after six passes of FSP. The main DRX mechanisms were found to be TDRX in TMAZ and CDRX and DDRX in SZ of specimens.
- 2.In situ ICs were successfully evolved after six passes of FSP. Mg2Cu IC was formed in AZ91/Cu system, while a hybrid nanocomposite reinforced by Mg2Cu, MgCu2 and MgO particles with sizes ranging from nano to micro scale was evolved in AZ91/CuO specimen.
- 3.The indentation microhardness values significantly increased by about 69.1% and 91% for AZ91/Cu and AZ91/CuO nanocomposites, respectively. Outstanding mechanical properties with a yield strength of 170.2 MPa, a tensile strength of 290.8 MPa and an elongation of 7.33% were obtained in AZ91/CuO hybrid nanocomposite mainly due to the presence of hierarchical nano- and mico-scale reinforcement particles.
- 4.The wear rate of AZ91/CuO nanocomposite substantially decreased by a factor of about 8. The wear mechanism changed from mainly delamination wear in AZ91 alloy to mainly abrasive wear in the FSPed specimens.
















