Abstract
2-aminopurine (2AP) is a responsive fluorescent base analogue that is used widely as a probe of the local molecular environment in DNA. The ability of 2AP to report changes in local conformation and base-stacking interactions arises from the efficient quenching of its fluorescence by the natural DNA bases. However, the mechanism of this inter-base quenching remains imperfectly understood. Two previous studies of the collisional quenching of 2AP by the natural bases, in different buffer solutions, showed that dynamic quenching efficiency depends on the identity of the natural base, but disagreed on the relative quenching efficiencies of the bases. We report a comprehensive investigation of inter-base quenching of 2AP by the natural nucleoside monophosphates (NMPs), replicating the buffer conditions used in the previous studies. Using time-resolved fluorescence measurements to distinguish between dynamic and static quenching, we find that the dynamic quenching rate constants of the different bases show a consistent trend across both buffers, and this is in line with a charge-transfer mechanism. Time-resolved measurements also provide insight into static quenching, revealing formation of 2AP-NMP ground-state complexes in which 2AP displays a very short fluorescence lifetime, comparable to that seen in oligonucleotides. In these complexes, the dependence of the rate of quenching on the partner base also supports a charge-transfer mechanism.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
DNA is increasingly recognised as playing new and unforeseen roles in biochemical processes, not just as a store of genetic information, but also undergoing repair and epigenetic modification, suggesting a more complex web of interactions rather than the traditional linear transcription-translation model. Understanding these processes in more detail is often achieved using fluorescent probes inserted at specific sites in DNA strands, exploiting the high sensitivity and noninvasive nature of fluorescence spectroscopy. 2-aminopurine (2AP) (scheme
Scheme 1. Structures of 2-aminopurine (2AP) and the DNA nucleotides, cytidine-5'-monophosphate (CMP), deoxythymidine-5'-monophosphate (dTMP), guanosine-5'-monophosphate (GMP) and adenosine-5'-monophosphate (AMP).
Download figure:
Standard image High-resolution imageSeveral early studies of the photophysics of 2AP in DNA showed that the fluorescence is quenched by stacking with the natural bases and the quenching efficiency is sensitive to the identity of the neighbouring bases and the local duplex conformation [2–5]. Barton and co-workers established that 2AP fluorescence is quenched efficiently by photoinduced electron transfer from guanine and took advantage of this effect in their studies of the mechanism of charge transfer in DNA (see, for example, Kelley [6] and O'Neill and Barton [7–13]). Other fluorophores, including fluorescent dyes and Au nanoclusters, have been found to be quenched by photoinduced electron transfer with guanine, and this effect has been exploited in biosensing applications [14–17].
Further information on the quenching interaction between the natural bases and 2AP came from steady-state and time-resolved fluorescence studies of 2AP-containing deoxydinucleotides [18, 19]. These showed that the fluorescence quantum yield of 2AP depends on the identity of the natural base with which it is stacked, increasing in the order G ≪ T < A, C. An ultrafast spectroscopic study of 2AP-containing dinucleotides and non-covalent complexes, with G, A and 7-deaza guanine (Z), found that relative decay rates, Z > G > A, were consistent with non-radiative decay via a charge-transfer state [20]. More recent computational studies of π-stacked dimers of 2AP with the natural nucleobases (in the gas phase) [21, 22] confirmed the importance of charge-transfer character in the mechanism of fluorescence quenching and identified quenching pathways involving charge-transfer exciplex states or conical intersections with charge-transfer character.
In an early time-resolved fluorescence study of the effects of local environment on 2AP fluorescence, Rachofsky et al [23] investigated collisional (Stern-Volmer) quenching by the natural nucleosides in aqueous buffer solution. Dynamic quenching rate constants determined from fluorescence lifetime measurements were similar in magnitude for all four nucleosides (ranging from 1.7 × 109 to 2.2 × 109 M−1s−1), with the lowest value observed for guanosine and the highest for adenosine. This is the inverse of the order of quenching efficiencies found in dinucleotides (vide supra). In a more recent study, Narayanan et al [24] used steady-state fluorescence measurements, together with cyclic voltammetry, to show that photoinduced electron transfer is the most likely mechanism for quenching of 2AP fluorescence by the natural nucleosides. The reported dynamic quenching rate constants are similar in magnitude to those of Rachofsky et al [23], but vary more widely between the different nucleosides (ranging from 1.4 × 109 to 2.5 × 109 M−1s−1) and show the highest value for G, decreasing in the order G > T > A, C. This trend in quenching efficiency is in agreement with that reported for dinucleotides and is consistent with a charge-transfer mechanism.
The discrepancy between the results of these two Stern-Volmer quenching studies is disturbing and casts doubt on the validity of this attractively simple approach as a means to investigate the susceptibility of fluorescent base analogues to inter-base quenching. The main difference between the studies is in the buffer that was used: 20 mM Tris-HCl (pH 7.5) in the earlier study and 0.1 M phosphate (pH 7.0) in the later one. This suggested that the solvent environment may affect the quenching process.
In an effort to resolve these conflicting reports, we have carried out a comprehensive investigation of quenching of 2AP by the natural nucleoside monophosphates (scheme
2. Experimental
2.1. Materials
Adenosine-5'-monophosphate disodium salt (AMP), 2-aminopurine (2AP) and cytidine-5'-monophosphate disodium salt (CMP) were purchased from Sigma. Deoxythymidine-5'-monophosphate disodium salt (dTMP) was purchased from Abcam. Guanosine-5'-monophosphate disodium salt (GMP) was purchased from Acros Organics. These were all used as received.
HPLC grade water (Fisher) was used as the solvent throughout. Measurements were made in phosphate buffer (0.1 M, pH 7) and Tris-HCl buffer (20 mM Tris-HCl, 60 mM NaCl, 0.1 mM Na2EDTA).
Samples of 2AP at a fixed concentration (∼3 μM) and varying concentrations of nucleotides were prepared in either phosphate or Tris-HCl buffer.
2.2. Fluorescence measurements
Steady-state fluorescence measurements were performed on a Horiba Fluoromax-P photon-counting spectrofluorimeter and the resulting spectra were analysed using Origin graphing software. An excitation wavelength of 325 nm was used and emission intensity was corrected for variation in the excitation lamp intensity. The detector response was constant over the emission wavelength range used. Emission intensities were determined by integration over a 180-nm bandwidth encompassing the emission maximum, and were averaged over five measurements.
Fluorescence lifetimes were measured using time-correlated single photon counting on an Edinburgh Instruments spectrometer equipped with TCC900 photon counting electronics. The excitation source was the third harmonic of the pulse-picked output of a mode-locked Ti-Sapphire laser (Coherent Mira pumped by Coherent Verdi), consisting of pulses of ∼150 fs duration at a repetition rate of 4.75 MHz and a wavelength of 310 nm. Fluorescence emission was detected orthogonal to the excitation beam through a polarizer set at the magic angle with respect to the vertically polarized excitation. A band-pass of 10 nm was used in the emission monochromator and photons were detected by a cooled microchannel plate detector (Hamamatsu R3809 series). The instrument response of the system was ∼80 ps full-width at half-maximum. Fluorescence decay curves were recorded on a time scale of 50 ns, resolved into 4096 channels, to a total of 10,000 counts in the peak channel. Decay curves were analyzed by iterative re-convolution, assuming a multi-exponential function, equation (1), using Edinburgh Instruments software FAST.
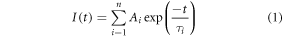
where I is the fluorescence intensity as a function of time, t, (normalised to the intensity at t = 0); τi is the fluorescence lifetime of the ith decay component and Ai is the fractional amplitude (A-factor) of that component.
2.3. Data analysis
The effect of a quencher, Q, on the fluorescence quantum yield of a fluorophore, M, can be expressed by the classical Stern-Volmer equation (2) [26].
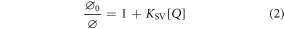
where  and
and  are the fluorescence quantum yields of M in the absence and presence of Q, respectively, [Q] is the molar concentration of Q, and
are the fluorescence quantum yields of M in the absence and presence of Q, respectively, [Q] is the molar concentration of Q, and  is the Stern-Volmer constant.
is the Stern-Volmer constant.
If quenching is purely dynamic, i.e. occurs by collision of Q with excited M,  is equal to the dynamic quenching constant, KD, as defined in equation (3).
is equal to the dynamic quenching constant, KD, as defined in equation (3).
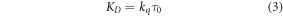
where kq is the bimolecular rate constant for collisional quenching and τ0 is the fluorescence lifetime of M in the absence of the quencher.
If quenching is purely static, i.e. occurs by interaction of M and Q in the ground state to form a complex, MQ, that has a fluorescence quantum yield less than that of free M, an average quantum yield is measured, as expressed by equation (4).
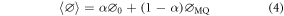
where  is the average quantum yield in the presence of quencher,
is the average quantum yield in the presence of quencher,  is the quantum yield of MQ, and
is the quantum yield of MQ, and  is the fractional population of free M, as defined by equation (5).
is the fractional population of free M, as defined by equation (5).
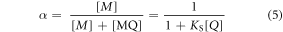
where KS, is the equilibrium constant for formation of MQ (equation (6)), and is known as the static quenching constant.
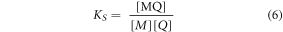
If  ≪
≪  0, equation (4) reduces to equation (7), which is identical in form to the classical Stern-Volmer expression (equation (2)), with KSV equal to the static quenching constant, KS. This corresponds to the commonly expressed assumption that complexes MQ are 'non-fluorescent' [26].
0, equation (4) reduces to equation (7), which is identical in form to the classical Stern-Volmer expression (equation (2)), with KSV equal to the static quenching constant, KS. This corresponds to the commonly expressed assumption that complexes MQ are 'non-fluorescent' [26].
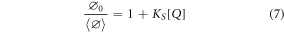
If both static and dynamic quenching occur, their combined effects on the quantum yield are expressed by equation (8).
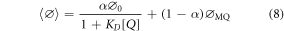
If  MQ ≪
MQ ≪  0, this becomes the modified Stern-Volmer equation (9).
0, this becomes the modified Stern-Volmer equation (9).
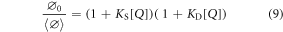
In steady-state fluorescence-quenching measurements, it is assumed that the ratio of the fluorescence intensity (I0) of M in the absence of Q to that (I) in the presence of Q is equal to the corresponding quantum yield ratio. This assumption is valid provided that the intensity of excitation light absorbed by M is constant throughout the measurements.
Time-resolved fluorescence measurements permit the effects of dynamic and static quenching to be separated. Dynamic quenching is manifested by a decrease in the fluorescence lifetime of M with increasing concentration of Q, according to equation (10).
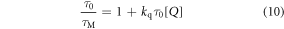
where τ0 and τM are the fluorescence lifetimes of M in the absence and presence of Q, respectively.
In the presence of static quenching, if the fluorescence lifetime of MQ is too short to be measurable (i.e. MQ is effectively non-fluorescent), then a mono-exponential decay will be observed with lifetime, τΜ. However, if the lifetime of MQ, τMQ, is measurable, an additional component will be seen in the fluorescence decay, as given by equation (11).
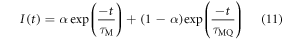
where I is the fluorescence intensity as a function of time, t, (normalised to the intensity at t = 0); both α (defined in equation (4)) and τM (equation (10)) depend on [Q], but τMQ is independent of [Q].
The modified Stern-Volmer equation may then be expressed in terms of the number-average lifetime, 
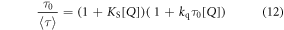
where 
3. Results and discussion
3.1. Measurements in Tris-HCl buffer
These measurements were made under the same buffer conditions as those used by Rachofsky et al [23], but we chose to use the natural bases in the form of the NMPs, rather than the nucleosides, because of their increased solubility. Furthermore, we used an excitation wavelength of 325 nm for our steady-state measurements, to minimise the inner filter effect that might occur due to absorption by the NMPs at high concentrations. Rachofsky et al [23] used an excitation wavelength of 309 nm, assuming negligible absorbance by the natural bases at this wavelength. However, although the molar absorption coefficients of the natural bases at this wavelength are very small, there is sufficient absorbance at concentrations of 10's of mM over a path-length of 0.5 cm to significantly attenuate the excitation intensity. This effect is greatest for GMP and TMP (see figures S1 and S2 of supplementary information, available online at stacks.iop.org/MAF/8/025002/mmedia), resulting in a decrease in intensity with increasing NMP concentration equivalent to KSV values of ∼14 M−1 and ∼7 M−1, respectively, and deviation from linearity at concentrations above 0.03 M.
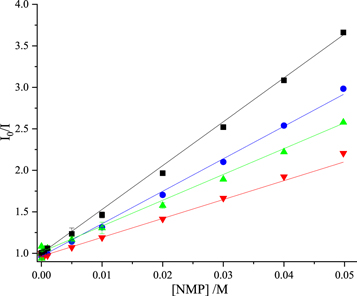
The dependence of the fluorescence intensity of 2AP on the concentration of each of the NMPs is shown in figure 1, in the form of a classic Stern-Volmer plot. The data could be fitted adequately by equation (2) yielding the values given in table 1 for the Stern-Volmer constant, KSV. However, there may be small deviations from linearity, suggesting the occurrence of static quenching. Rachofsky et al [23] reported only dynamic quenching constants obtained from time-resolved fluorescence measurements. They indicated that they were unable to obtain static quenching constants because of the low solubility of the ribosides, but commented that upward curvature in Stern-Volmer plots of steady-state data was observed.
Figure 1. Stern-Volmer plots of fluorescence intensity data for quenching of 2AP fluorescence by the nucleoside monophosphates in Tris-HCl buffer. GMP (blue), dTMP (green), AMP (black), CMP (red). Experimental data are shown as points and fitted function (equation (2)) as solid line. In all cases, R2 values were greater than 0.99.
Download figure:
Standard image High-resolution imageTable 1. Stern-volmer constants, KSV, determined from the steady-state fluorescence data shown in figures 1 and 3, in Tris-HCl and phosphate buffers.
| KSV (±σ)/M−1 | |||
|---|---|---|---|
| Nucleoside | Tris-HCl | Phosphate | Phosphate (Lit.)a |
| GMP | 52.1 (0.5) | 28.8 (0.3) | 31.8 |
| AMP | 38.4 (0.7) | 26.2 (0.3) | 26.4 |
| CMP | 23.1 (0.6) | 15.0 (0.2) | 16.5 |
| dTMP | 30.8 (0.5) | 19.2 (0.3) | 23.4 |
aValues of KSV in phosphate buffer derived from the results reported by Narayanan et al [24] are given, for comparison.
To obtain definitive dynamic quenching constants, we carried out time-resolved fluorescence measurements. An excitation wavelength of 310 nm (near the maximum of the 2AP absorption spectrum) was used, since the inner filter effect does not affect the fluorescence lifetime, only the fluorescence intensity. The decay parameters of 2AP measured as a function of GMP concentration are given in table 2.
Table 2. Fluorescence decay parameters for 2AP in Tris-HCl buffer as a function of concentration of GMP. 3-component decays for concentrations 5–50 mM were fitted globally with τ2 and τ3 as common lifetimes.
| [GMP]/mM | τ1/ns | τ2/ns | τ3/ns | A1 | A2 | A3 | τ/ns |
|---|---|---|---|---|---|---|---|
| 0 | 11.38 | — | — | 1.0 | — | — | 11.38 |
| 0.5 | 11.28 | — | — | 1.0 | — | — | 11.28 |
| 1.0 | 11.11 | — | — | 1.0 | — | — | 11.11 |
| 5.0 | 10.00 | 2.75 | 0.06 | 0.86 | 0.02 | 0.12 | 8.64 |
| 10 | 8.86 | 2.75 | 0.06 | 0.84 | 0.02 | 0.14 | 7.46 |
| 20 | 7.35 | 2.75 | 0.06 | 0.79 | 0.03 | 0.18 | 5.93 |
| 30 | 6.36 | 2.75 | 0.06 | 0.74 | 0.04 | 0.22 | 4.80 |
| 40 | 5.61 | 2.75 | 0.06 | 0.67 | 0.04 | 0.28 | 3.90 |
| 50 | 5.04 | 2.75 | 0.06 | 0.60 | 0.05 | 0.35 | 3.19 |
For concentrations of GMP up to 1 mM, single exponential decays were observed, with the lifetime decreasing with increasing quencher concentration, as expected for dynamic quenching (equation (10)). However, above this concentration, an additional two, shorter, decay components (τ2 and τ3) appeared, which can be attributed to the onset of static quenching. The 3-component decays for GMP concentrations from 5 to 50 mM could be fitted globally with τ2 and τ3 as common (i.e. concentration-independent) lifetimes; the value of τ1 decreases steadily with increasing GMP concentration. The fractional populations (A factors) of components τ2 and τ3 increase with increasing GMP concentration. This is consistent with a model in which τ1 is due to free 2AP undergoing dynamic quenching by GMP, while τ2 and τ3 are due to two photophysically distinct 2AP-GMP complexes, in which 2AP is statically quenched by GMP. The nature of the complexes (as reflected by their lifetimes) is independent of GMP concentration, but their populations increase as GMP concentration increases. In the τ3 complex, 2AP is highly quenched by interaction with GMP to give a lifetime of only 60 ps. This is comparable to the very short lifetimes observed for 2AP stacked with G in DNA duplexes [1] and for the 2AP-G dinucleotide [19, 27], implying that there is intimate stacking of 2AP and G in the τ3 complex. The much longer lifetime of the τ2 complex suggests a structure in which there is much weaker interaction between 2AP and G, but still significant quenching. The fractional population of the τ3 complex is much greater than that of the τ2 complex, indicating that a well-stacked structure is favoured. This is in line with computational prediction of highly stacked structures for non-covalent complexes of 2AP and the natural bases [21, 22, 28].
Similar decay behaviour was observed for 2AP in the presence of the other NMPs, as shown in tables S1–S3 in the supplementary information. In all cases, two statically-quenched complex populations are observed at concentrations above 1 mM, a minor population with a lifetime (τ2) of ∼3 ns, and the major component with a much shorter lifetime (τ3). The value of τ3 depends on the identity of the natural base, and decreases in the order A > C > G ∼ T, indicating a decrease in quenching efficiency in that order. In all cases, at a NMP concentration of 50 mM, the τ3 complex accounts for ∼30% of the emitting population, while τ2 constitutes less than 10%. Although the statically quenched complexes show measurable fluorescence, their average quantum yield is no more than 3% that of unquenched 2AP.
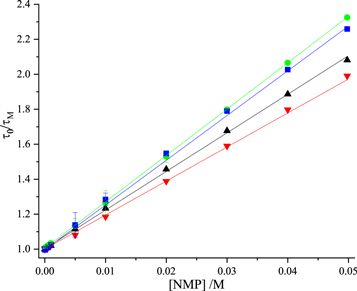
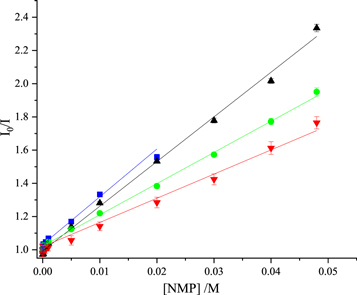
The value of KD, and hence kq, (equation (3)) for each NMP was determined from a plot of  versus [NMP] (equation (10)), where τM is the lifetime component τ1. The linear fits are shown in figure 2 and the quenching constants are given in table 3.
versus [NMP] (equation (10)), where τM is the lifetime component τ1. The linear fits are shown in figure 2 and the quenching constants are given in table 3.
Figure 2. Stern-Volmer plots of fluorescence lifetime data for dynamic quenching of 2AP fluorescence by the nucleoside monophosphates in Tris-HCl buffer. GMP (blue), dTMP (green), AMP (black), CMP (red). Experimental data are shown as points and fitted function (equation (10)) as solid line. In all cases, R2 values were greater than 0.99
Download figure:
Standard image High-resolution imageTable 3. Dynamic quenching constants obtained from fluorescence lifetime data for 2AP in the presence of the nucleoside monophosphates in Tris-HCl buffer.
| .Nucleoside | KD (±σ) /M−1 | kq/109 M−1 s−1 | kq (Lit.)a/109 M−1 s−1 |
|---|---|---|---|
| GMP | 25.7 (0.3) | 2.25 (0.05) | 1.70 (0.13) |
| AMP | 22.0 (0.2) | 1.85 (0.04) | 2.21 (0.10) |
| CMP | 19.8 (0.2) | 1.70 (0.04) | 1.90 (0.03) |
| dTMP | 26.5 (0.1) | 2.22 (0.05) | 2.14 (0.04) |
aLiterature values reported by Rachofsky et al [23] for the nucleosides are shown for comparison.
We find that the rate constant for collisional quenching, kq, is greater for GMP and dTMP than for AMP and CMP. Our kq values for GMP and AMP differ significantly from those of Rachofsky et al [23]; we find that GMP has the greatest value of kq, whereas they report that guanosine has the lowest kq value and adenosine the highest. Rachofsky et al [23] do not present the fluorescence decay data that they used to obtain kq values; however, it is evident that they observed multi-exponential decays at higher nucleoside concentrations, and we note that they incorrectly employed the intensity-average lifetime for Stern-Volmer analysis.
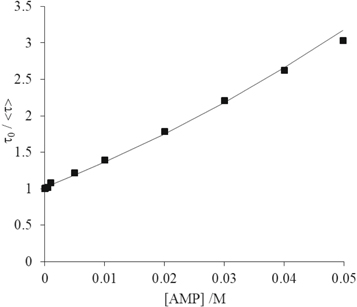
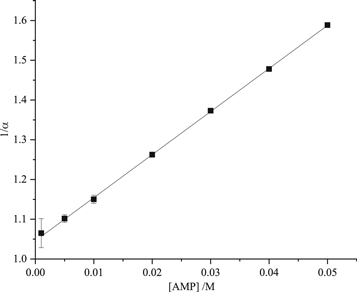
The considerable difference between the KD values obtained from lifetime data (table 3) and the Ksv values obtained from the classic Stern-Volmer plots (table 1, figure 1) confirms the occurrence of static quenching. We used three alternative methods to determine the static quenching constant for each of the NMPs: (i) fluorescence intensity data were fitted with the modified Stern-Volmer equation (equation (9)), with the value of KD fixed; (ii) average lifetime data (tables 2 and S1–S3 of supplementary information) were fitted with the modified Stern-Volmer equation (equation (12)) with the value of KD fixed; (iii) from linear plots of 1/α versus [NMP] (equation (5)), where α, the fractional population of free 2AP, is obtained from decay data as the value of A1 (table 2 and S1–S3 of supplementary information). Examples of (ii) and (iii) are shown for AMP in figures 3 and 4. The data and fits for the other NMPs are given in the supplementary information (figures S3 and S4). The values of Ks obtained are given in table 4. The values of KS obtained from the two decay-data-based methods (τ0/〈τ〉 and 1/α) are in good agreement and are similar for all the NMPs, indicating a similar propensity for stacking with 2AP in all cases.
Figure 3. Average fluorescence lifetime data for quenching of 2AP by AMP in Tris-HCl buffer, fitted by the modified Stern-Volmer equation (equation (11)) with the value of KD fixed. Experimental data are shown as points and fitted function as solid line. The R2 value of the fit was 0.997. (Error bars are smaller than the data points).
Download figure:
Standard image High-resolution imageFigure 4. Plot of the inverse of the fractional population (α) of free 2AP as a function of AMP concentration, in Tris-HCl buffer. Experimental data are shown as points and the fitted function (see equation (5)) as solid line. The R2 value of the fit was better than 0.99.
Download figure:
Standard image High-resolution imageTable 4. Static quenching constants for 2AP in the presence of the nucleoside monophosphates in Tris-HCl buffer, obtained from intensity data, average lifetime data and fractional population data.
| KS (±σ) /M−1 | |||
|---|---|---|---|
| Nucleoside | I0/I | τ0/〈τ〉 | 1/α |
| GMP | 7.7 (0.2) | 11.1 (0.4) | 10.7 (1.0) |
| AMP | 9.0 (0.2) | 9.2 (0.3) | 10.8 (0.1) |
| CMP | 2.4 (0.2) | 9.7 (0.2) | 10.0 (0.7) |
| dTMP | 3.7 (0.3) | 10.0 (0.6) | 8.9 (1.1) |
However, for CMP and dTMP, there is a significant discrepancy between these values and those obtained from intensity data. This suggests that I0/I is not a reliable measure of relative quantum yield in these cases; the quantum yield of the complexes relative to free 2AP is overestimated. This would be accounted for by a greater molar absorption coefficient of 2AP in complex with these NMPs than that of free 2AP, at the excitation wavelength of 325 nm. Since we are exciting on the red edge of the 2AP absorption spectrum, a small complexation-induced spectral shift could result in a signifcant increase in absorption coefficient compared with free 2AP. It has been observed previously that interbase stacking interactions lead to a red-shift of a few nm in the absoprtion or excitation spectrum of 2AP when it is inserted into oligonucleotides [29–31].
3.2. Measurements in phosphate buffer
To replicate the conditions used by Narayanan et al [24], the quenching of the fluorescence of the 2AP base by each of the natural nucleoside monophosphates was investigated in 0.1 M phosphate buffer.
The dependence of the fluorescence intensity of 2AP on the concentration of each of the NMPs is shown in the Stern-Volmer plots in figure 5, and could be fitted adequately by equation (2), yielding the values given in table 1 for the Stern-Volmer constant, KSV. For GMP in this buffer, we saw evidence of G-quartet formation [32] (increase in viscosity of the solution and scattering of excitation light) at concentrations above 20 mM. Consequently, the analysis of quenching was restricted to lower concentrations, and this prevented the analysis of static quenching by GMP in this buffer. The formation of G-quartets, a form of self-aggregation unique to guanosine, is enhanced in this buffer system by the high concentration of potassium ions [32].
Figure 5. Stern-Volmer plots of fluorescence intensity data for quenching of 2AP fluorescence by the nucleoside monophosphates in 0.1 M phosphate buffer. GMP (blue), AMP (black), dTMP (green), CMP (red). Experimental data are shown as points and fitted function (equation (2)) as solid line. In all cases, R2 values were ≥0.99. For GMP, data are limited to concentrations ≤0.02 M, as explained in the text.
Download figure:
Standard image High-resolution imageNarayanan et al [24] chose to fit their intensity data to the modified Stern-Volmer equation (equation (9)), with both KD and KS as variable parameters. They do not comment on the deviation from linearity of their data, and, since they present the data plotted on a logarithmic concentration scale, this cannot be discerned. However, we find that the functions fitted by Narayanan et al [24] can be very well approximated by linear Stern-Volmer functions (equation (2)) (R2 > 0.998) over the range of concentrations measured (as shown in figure S5 of the supplementary information). This suggests that their data could be fitted adequately by the linear equation; indeed the values of KS that they report are small (<5 M−1), with relatively high errors. The KSV values that we estimate from their results, on that basis, are given in table 1. These values are in good agreement with those of the present work, and follow the same trend.
To further investigate the quenching process, the fluorescence decay of 2AP was measured as a function of NMP concentration. We found that the fluorescence lifetime of 2AP in this buffer, in the absence of NMP, is much shorter than expected, only 5.7 ns, half that in Tris-HCl buffer (table 2). This suggested that the buffer itself (H2PO4−/HPO42−) was acting as a quencher. This was confirmed by measurement of the lifetime as a function of buffer concentration (figure S6 of supplementary information), which demonstrated dynamic quenching, with kq = 1.00 × 109 M−1 s−1. To our knowledge the quenching of 2AP fluorescence by phosphate buffer has not been reported previously, although phosphate anions have been observed to quench the fluorescence of tryptophan and tyrosine [33–35]. Narayanan et al [24] were unaware of the quenching effect of the buffer and erroneously used a literature value of 11.4 ns for τ0 in calculating kq from KD.
For AMP, CMP and dTMP, the effect of their concentration on the fluorescence decay parameters of 2AP was similar to that observed in Tris-HCl buffer, as shown in tables S4–S6 of supplementary information. Monoexponential decays at sub-mM NMP concentrations switch to 3-exponential decays at concentrations above 1 mM, with the onset of static quenching. The values of τ2 and τ3 are shorter than in Tris-HCl buffer, suggesting that the interaction between 2AP and the NMP in the statically quenched complexes is influenced by the composition of the buffer. Nevertheless, the trend in τ3, A > C > G, T, is the same as seen in Tris-HCl.
For GMP, the decay parameters (table S7 of supplementary information) are anomalous due to G-quartet formation; this is manifested mainly as very high amplitudes of the shortest decay component at concentrations above 20 mM. (This may be due to some type of enhanced quenching process, but could be an artefact due to intense light scattering by the guanosine aggregates.) Therefore, the use of these decay data was restricted to the determination of the dynamic quenching constant at concentrations ≤20 mM.
The values of KD and kq determined from the lifetime data are given in table 5. The Stern-Volmer plots are shown in figure 6. As a consequence of the much shorter τ0, the KD vaues are approximately one half those measured in Tris-HCl (table 3), but the kq values and their trend are very similar in both buffers. The values of kq reported by Narajanan et al [24] are in surprisingly good agreement, given that they were calculated on the assumption of a τ0 of 11.4 ns. However, this is merely a fortuitous consequence of their substantial overestimation of the KD values from fits of intensity data to the modified Stern-Volmer equation.
Table 5. Dynamic quenching constants obtained from fluorescence lifetime data for 2AP in the presence of the nucleoside monophosphates in 0.1 M phosphate buffer.
| Nucleoside | KD (±σ)/M−1 | kq/109 M−1 s−1 | kq (Lit.)a/109 M−1 s−1 |
|---|---|---|---|
| GMP | 14.1 (0.3) | 2.50 (0.05) | 2.52 (0.72) |
| AMP | 11.4 (0.2) | 1.99 (0.03) | 1.55 (0.42) |
| CMP | 11.6 (0.2) | 2.00 (0.02) | 1.43 (0.41) |
| dTMP | 14.2 (0.2) | 2.50 (0.02) | 1.71 (0.44) |
aLiterature values reported by Narayanan et al [16].
Figure 6. Stern-Volmer plots of fluorescence lifetime data for dynamic quenching of 2AP fluorescence by the nucleoside monophosphates in 0.1 M phosphate buffer. dTMP (green), GMP (blue), AMP (black), CMP (red). Experimental data are shown as points and the fitted function (equation (10)) as solid line. In all cases, R2 values were greater than 0.99. For GMP, data are limited to concentrations ≤0.02 M, as explained in the text.
Download figure:
Standard image High-resolution imageStatic quenching constants for AMP, CMP and dTMP were determined as described above, yielding the values shown in table 6. Related plots are shown in figures S7 and S8 of supplementary information. The values of KS are comparable to those measured in Tris-HCl, although somewhat lower. Complexation appears to be less favourable in the phosphate buffer. A similar discrepancy between decay-derived and intensity-derived values is evident for CMP and dTMP. The values reported by Narayanan et al [24] are significantly less than our values and show much greater variability between the nucleosides. This, together with the overstimation of KD values (vide supra), is symptomatic of the correlation of variables inherent in unconstrained fitting of data with the modified Stern-Volmer equation.
Table 6. Static quenching constants for 2AP in the presence of the nucleoside monophosphates in 0.1 M phosphate buffer, obtained from intensity data, average lifetime data and fractional population data.
| KS (±σ) /M−1 | ||||
|---|---|---|---|---|
| Nucleoside | I0/I | τ0/〈τ〉 | 1/α | I0/I (Lit)a |
| AMP | 9.9 (0.4) | 6.5 (0.2) | 7.5 (0.3) | 5.0 (1.0) |
| CMP | 2.4 (0.2) | 5.0 (0.9) | 5.7 (0.5) | 0.2 (1.2) |
| dTMP | 3.6 (0.2) | 5.9 (0.2) | 6.0 (0.9) | 2.2 (0.4) |
aLiterature values reported by Narayanan et al [24].
4. Conclusions
Contrary to previous studies, we find that the dynamic quenching efficiencies of the NMPs follow the same trend in Tris-HCl and 0.1 M phosphate buffers. Rate constants for dynamic quenching of 2AP by GMP and dTMP are greater than those for quenching by AMP and CMP. This is consistent with a charge-transfer quenching mechanism. The nature of the buffer does, however, significantly affect the fluorescence lifetime of 2AP in the absence of the NMPs. In 0.1 M phosphate buffer the lifetime of 'unquenched' 2AP is one half that in Tris-HCl, as a result of dynamic quenching by phosphate anions, with a kq of 1.0 × 109 M−1 s−1. Surprisingly, this quenching process has not been reported previously and was not taken into account in the data analysis of Narayanan et al [24].
Time-resolved fluorescence measurements revealed the formation of highly quenched ground-state complexes between 2AP and the NMPs, with lifetimes comparable to those seen for 2AP in oligonucleotides. These solution-phase complexes evidently have a closely stacked structure, conducive to rapid quenching. The dependence of the rate of quenching on the NMP stacking partner mimics that seen in 2AP-containing dinucleotides and supports a charge-transfer mechanism. Static quenching constants indicate that all of the natural nucleotides have a similar propensity to stack with 2AP.
This work illustrates that time-resolved fluorescence measurements are not only important in the definitive analysis of dynamic quenching, but can also provide valuable insight into static quenching. In traditional Stern-Volmer analysis, statically quenched, ground-state complexes are treated as being 'non-fluorescent'. However, with ever-improving time-resolution in fluorescence lifetime measurements, species once deemed to be non-fluorescent can now readily be observed. Even with the relatively modest time-resolution of time-correlated single-photon counting, 2AP-NMP complexes with quantum yields a factor of a thousand less than unquenched 2AP have been detected and quantified, providing an alternative means of determining static quenching constants.
Finally, we conclude that, with rigorous execution, collisional quenching measurements can give useful insight into the mechanism of inter-base quenching and should be a valuable tool in the preliminary assessment of the responsiveness of new fluorescent base analogues to inter-base interactions.
Acknowledgments
This work was supported by the EPSRC and The University of Edinburgh, by the provision of a PhD studentship for KAP. Data associated with this paper are avaialable at https://doi.org/10.7488/ds/2717.
Conflicts of Interest
The authors have declared that no conflicting interests exist.