Abstract
In this study we report the facile synthesis of environmentally benignant copper nanoparticles (CuNPs) using Cuminum cyminum (Cumin) seed extract. Bio-molecules present in the seed extracts can be used to reduce the metal ions to nanoparticles in a single-step green synthesis process. The synthesized CuNPs have been characterized by studying the structural, morphological, optical and antimicrobial properties. The x-ray diffraction (XRD) pattern was used to analyze the phase and crystal structure of the nanoparticles formed. The morphology and shape of CuNPs were studied by field emission scanning electron microscopy (FESEM) and transmission electron microscopy (TEM) techniques. The existence of elemental copper (Cu) was revealed by energy dispersive spectroscopy (EDS) analysis. The presence of an absorption peak at 590 nm using ultraviolet-visible (UV-Vis) spectroscopy confirms the formation of CuNPs. Fourier transform infrared spectroscopy (FTIR) spectrum ascertains the reduction and capping nature of phytoconstituents of seed extract in CuNPs synthesis. Antimicrobial activity of CuNPs showed the better inhibitory activity towards Pseudomonas spp. and Penicillium spp. compared to other test pathogens through the standard Kirby-Bauer's disc-diffusion method.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Nanotechnology is an attractive area of research related to the synthesis of nanoparticles of varying sizes, shapes and chemical compositions and their possible bio-medical applications for human benefits [1–3]. Nanoparticles exhibit a high surface to volume ratio with decreasing size of samples. Specific surface area is relevant for catalytic activity and other related properties such as antimicrobial activity in nanoparticles [4–8]. The synthesis and use of metal nanoparticles have gained consideration due to their unique optical, magnetic, electrical, catalytic and antimicrobial properties [9, 10]. Nanoparticles can be synthesized using various physical, chemical and biological methods. Chemical synthesis method is found to be easy and cost effective but some of them use toxic raw materials and are not environmental friendly. The biosynthesis of nanoparticles has attracted attention of many researchers owing to their physical and chemical processes being expensive and drastic reaction conditions. Biological methods on the other hand neither require use of toxic solvents nor synthesis of hazardous by products. It has been reported by many researchers that as bio-synthesis of nanoparticles is free from toxic chemicals it is more suitable for the biological application of nanoparticles [11, 12].
The exploration of novel and low-cost routes for the synthesis of nanoparticles is being discovered by researchers with the aid of microorganisms, plant and seed extracts [13–16]. The biosynthetic pathway of nanoparticles preparation potentially eliminates the toxicity and making the nanoparticles more biocompatible. Among the various biosynthetic approaches, the use of seed extracts has advantages such as being easily available and safe to handle, and being easily scaled up for the large-scale synthesis of nanoparticles through the green route [17, 18]. The seed extracts provide a better way for nanoparticles synthesis as they are free from toxic chemicals and are natural reducing and capping agents [19, 20]. Cuminum cyminum (Cumin) is an herbaceous plant in the family of Apiaceae and grown in Mediterranean climate and is known for its stimulant, antispasmodic and carminative properties [21]. The major compounds in Cumin are reported as cuminaldehyde,  and
and  pinene, limonene,
pinene, limonene,  and
and  cymene, 1,8-cineole,
cymene, 1,8-cineole,  and
and  terpinene, linanool and safranal [22]. Cumin was also found to be highly effective against all the isolates of tested pathogenic fungi as it completely inhibited mycelia growth of all fungi when added to solid medium and have been found to possess antidiabetic, anticancerous, antioxidant and immunomodulatory properties [23, 24].
terpinene, linanool and safranal [22]. Cumin was also found to be highly effective against all the isolates of tested pathogenic fungi as it completely inhibited mycelia growth of all fungi when added to solid medium and have been found to possess antidiabetic, anticancerous, antioxidant and immunomodulatory properties [23, 24].
Previously, some research groups have been reported on the synthesis of silver nanoparticles and their antimicrobial properties through the green synthesis route [7, 8, 12–14, 25, 26]. Whereas in this study, we are focusing on the synthesis of copper nanoparticles (CuNPs) through seed extract and their antimicrobial properties. Since, the availability and cost of Cu made it as a better choice comparing to the Ag metal for manifold applications. In addition, CuNPs have special properties, which have made them important for various applications such as: super strong materials, sensors [27], catalysts [28] and also shows the strong antibacterial activity [29]. Furthermore, they can also interact and react with other nanoparticles due to their high surface area-volume ratio. Recently, it has been reported that CuNPs possess superior antibacterial activity than AgNPs, for Escherichia coli and Bacillus subtilis [6, 4]. In the present report, biosynthesis of CuNPs using Cuminum Cyminum seed extract has been investigated. The synthesized nanoparticles were characterized by XRD, FESEM, EDS, TEM, UV-Vis spectroscopy and FTIR techniques. Finally, the bio-CuNPs were tested for their efficiency against different microbial pathogens.
2. Experimental
2.1. Materials
Cuminum cyminum seeds were collected from the supermarket, Tirupati, India. Copper acetate (monohydrate)  was purchased from Molychem and utilized as received without any further purification. In the present study all the aqueous solutions were prepared with milli-Q water.
was purchased from Molychem and utilized as received without any further purification. In the present study all the aqueous solutions were prepared with milli-Q water.
2.2. Aqueous Cuminum cyminum seed extract preparation
Freshly collected Cuminum cyminum seeds (15 g) were surface cleaned with milli-Q water to eliminate the dirt and dried in air to remove the moisture presence. Subsequently, a fine powder of Cuminum cyminum seeds was prepared through the kitchen blender. The seed powder (10 g) was weighted and added to 100 ml of milli-Q water followed by heating at  for 10 min. Then after, by using Whatman's no.1 filter paper the seed extract was filtered. Finally, the filtered extract was preserved at
for 10 min. Then after, by using Whatman's no.1 filter paper the seed extract was filtered. Finally, the filtered extract was preserved at  for further studies in order to store the sample from environmental conditions.
for further studies in order to store the sample from environmental conditions.
2.3. Synthesis of copper nanoparticles
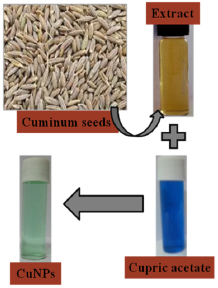
An aqueous 0.001 M copper acetate solution was prepared with 50 ml of milli-Q water at room temperature. Later, 10 ml of seed extract was added to the above solution at room temperature while stirring magnetically at 1000 rpm for 15 min. The blue color solution changes to pale bluish green within 5 min due to the fast bio-reduction of copper ions, confirming the formation of CuNPs and there was no colour change further. The obtained CuNPs were further purified by centrifugation at 10,000 rpm for 10 min with substantial redispersion of the pellet in Milli-Q water. The schematic synthesis procedure of bio-CuNPs using Cuminum Cyminum seed extract is depicted in figure 1. Finally, the synthesized CuNPs were stored in a clean amber bottle for further analysis.
Figure 1. Schematic synthesis procedure of bio-CuNPs using Cuminum cyminum seed extract.
Download figure:
Standard image High-resolution image2.4. Characterization of the synthesized CuNPs
The synthesized CuNPs were characterized comprehensively by using a variety of techniques. The crystalline structure of the prepared nanoparticles was analyzed by Seifert 3003TT, x-ray diffractometer with  radiation
radiation  . Chemical composition of the prepared samples were observed through energy dispersive spectroscopy (EDS) of Oxford Inca Penta FET x3 EDS instrument attached to Carl Zeiss EVO MA 15 scanning electron microscopy. The surface morphology of the as-synthesized CuNPs was obtained using a ZEISS, SUPRA 55 field emission scanning electron microscopy (FESEM) measurements. The particle size and structure confirmations were done by Phillips TECHNAI FE 12, transmission electron microscopy (TEM). UV-Vis absorption spectra were documented on a Perkin Elmer Lambda 950 UV-Vis-NIR spectrophotometer with the wavelength set in the range of 200–800 nm at a resolution of
. Chemical composition of the prepared samples were observed through energy dispersive spectroscopy (EDS) of Oxford Inca Penta FET x3 EDS instrument attached to Carl Zeiss EVO MA 15 scanning electron microscopy. The surface morphology of the as-synthesized CuNPs was obtained using a ZEISS, SUPRA 55 field emission scanning electron microscopy (FESEM) measurements. The particle size and structure confirmations were done by Phillips TECHNAI FE 12, transmission electron microscopy (TEM). UV-Vis absorption spectra were documented on a Perkin Elmer Lambda 950 UV-Vis-NIR spectrophotometer with the wavelength set in the range of 200–800 nm at a resolution of  . Fourier transform infrared spectra (FTIR) were recorded on ATR-FTIR Bruker Vettex-80 spectrometer. All the measurements were carried out at room temperature only.
. Fourier transform infrared spectra (FTIR) were recorded on ATR-FTIR Bruker Vettex-80 spectrometer. All the measurements were carried out at room temperature only.
2.5. Antimicrobial properties of copper nanoparticles
The antibacterial activity of biosynthesized CuNPs was tested against different pathogenic microorganisms. For the determination of antibacterial activity of bio-reduced CuNPs, the gram-negative (Pseudomonas spp., Escherichia coli) and gram-positive (Staphylococcus spp., Bacillus spp.) bacteria pathogens were used. The pure cultures of all specified pathogens were collected from Microbiology research laboratory, SV University, Tirupati, India. To determine the antibacterial activity of biosynthesized CuNPs, a standard Kirby-Bauer disc diffusion assay was employed [30]. The bacterial test organisms were grown in nutrient broth for 24 h and made availed for further study. Nutrient agar plates were prepared, sterilized and solidified. After solidification, bacterial lawns were prepared by spreading  overnight culture of each organism on the petriplates using a sterile glass rod. Sterile discs (diameter of 3 mm) are placed on these plates and CuNPs were loaded at required volumes of
overnight culture of each organism on the petriplates using a sterile glass rod. Sterile discs (diameter of 3 mm) are placed on these plates and CuNPs were loaded at required volumes of  and
and  on respective discs and incubated at
on respective discs and incubated at  for 24 h. Next to the incubation period, zones of inhibitions were observed around the discs.
for 24 h. Next to the incubation period, zones of inhibitions were observed around the discs.
Anti-fungal activities of bio-CuNPs were determined by Kirby-Bauer disc diffusion method [30]. The selected fungal test organisms were Aspergillus niger, Aspergillus flavus, Penicillium spp. and Rhizopus spp. respectively. The fungal pathogens were grown in potato dextrose broth for 72 h and utilized for further work. Lawn culture of the respective organism was prepared by pour plate method with  of corresponding culture on potato dextrose agar (PDA) media by using a spreader. The discs were then kept on PDA plates and loaded with CuNPs solution at specific volumes of
of corresponding culture on potato dextrose agar (PDA) media by using a spreader. The discs were then kept on PDA plates and loaded with CuNPs solution at specific volumes of  ,
,  ,
,  and
and  on their respective discs and plates were incubated for 72 h at room temperature. Then fungicidal efficacy was proved by the zone of inhibition encircling the discs. The diameter of all such zones was calculated and mean values for each fungal pathogen were recorded and denoted in millimeters.
on their respective discs and plates were incubated for 72 h at room temperature. Then fungicidal efficacy was proved by the zone of inhibition encircling the discs. The diameter of all such zones was calculated and mean values for each fungal pathogen were recorded and denoted in millimeters.
3. Results and discussion
3.1. Crystallographic analysis of CuNPs
The x-ray diffraction (XRD) profile of biosynthesized CuNPs is depicted in figure 2. From the XRD profile, three distinct diffraction peaks at  ,
,  and
and  are perceived corresponding to (1 1 1), (2 0 0) and (2 2 0) lattice planes, respectively which are characteristic of face centered cubic (fcc) structure of metallic copper (JCPDS No. 04-0836). The peak positions are in good agreement with the literature values of metallic copper [31, 32]. The high intensity and broadened diffraction peaks evidently state that the CuNPs are highly crystalline in nature. XRD pattern elucidates that the peak analogous to (1 1 1) plane is more intense than other planes suggesting it as a predominant diffraction peak.
are perceived corresponding to (1 1 1), (2 0 0) and (2 2 0) lattice planes, respectively which are characteristic of face centered cubic (fcc) structure of metallic copper (JCPDS No. 04-0836). The peak positions are in good agreement with the literature values of metallic copper [31, 32]. The high intensity and broadened diffraction peaks evidently state that the CuNPs are highly crystalline in nature. XRD pattern elucidates that the peak analogous to (1 1 1) plane is more intense than other planes suggesting it as a predominant diffraction peak.
Figure 2. X-ray diffraction profile of bio-synthesized CuNPs using Cuminum cyminum seed extract.
Download figure:
Standard image High-resolution imageThe average crystallite size of the synthesized nanoparticles was calculated from the strong intensity peak of (1 1 1) plane by using Debye-Scherrer's equation

where  is the average crystallite size,
is the average crystallite size,  is the Scherrer's constant (
is the Scherrer's constant ( ),
),  is the wavelength of the
is the wavelength of the  radiation
radiation  ,
,  is the full width at half maximum (FWHM) of a Gaussian fit, and
is the full width at half maximum (FWHM) of a Gaussian fit, and  is the half diffraction angle. The crystallite size was calculated to be around 16 nm from high intensity of (1 1 1) orientation. The lattice constant was determined to 0.3625 nm from the (1 1 1) plane for the CuNPs and which is equivalence with standard data (
is the half diffraction angle. The crystallite size was calculated to be around 16 nm from high intensity of (1 1 1) orientation. The lattice constant was determined to 0.3625 nm from the (1 1 1) plane for the CuNPs and which is equivalence with standard data ( , JCPDS No: 04-0836) [33].
, JCPDS No: 04-0836) [33].
3.2. Surface morphology of CuNPs
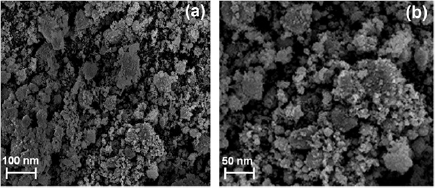
In order to study the morphology and size of the bio-synthesized CuNPs, FESEM images were recorded at different magnifications (figure 3). The formation of CuNPs as well as their morphological dimensions through the FESEM study demonstrated that the average size was around 25 nm with the shape of spherical nature. The FESEM image further confirms the production of a high density of CuNPs synthesized through the Cuminum cyminum seed extract.
Figure 3. FESEM images of bio-synthesized CuNPs at different magnifications.
Download figure:
Standard image High-resolution imageTransmission electron microscopy (TEM) has been employed to characterize the shape, size and morphology of bio-CuNPs. Figures 4(a) and (b) depict the TEM micrograph of CuNPs at different locations of loaded sample on a copper grid. TEM analysis proved the formation of nanocrystalline copper particles and average particle size was estimated to be around 18 nm. The crystallinity of CuNPs was observed through the selected area electron diffraction (SAED) pattern and it was recorded by pointing the electron beam upright to nanoparticles and displayed in figure 4(c). The corresponding fringe array represents (1 1 1), (2 0 0) and (2 2 0) planes of the face centered cubic (fcc) lattice structure commonly found for copper crystal, which is also evident from XRD results.
Figure 4. (a) and (b) TEM images at different locations, (c) SAED pattern of copper nanoparticles.
Download figure:
Standard image High-resolution image3.3. Elemental analysis by EDS
EDS analysis was used to identify the elemental composition of the synthesized nanoparticles. The EDS spectrum of bio-synthesized CuNPs is shown in figure 5. It shows a high intense major peak of elemental Cu with the atomic percentage of 74.5%, which is typical for the absorption of metallic copper due to surface plasmon resonance. The occurrence of weak signals of O, Cl, C and S together with strong copper peak may be owing to the presence of bio-molecules that are bound to the surface of CuNPs. The elemental analysis (inset of figure 5) provides the atomic% of all other elements that are present in the bio-CuNPs due to the interaction of bio-molecules of cumin extract in CuNPs formation.
Figure 5. EDS spectrum of bio-synthesized CuNPs (inset: atomic% of various elements).
Download figure:
Standard image High-resolution image3.4. Optical properties of CuNPs
UV-Vis absorbance spectroscopy is a valuable tool to observe the size and shape controlled nanoparticles in aqueous suspensions [34]. In general, the metal particles of small size ranging from 2 nm to 100 nm can display the strong surface plasmon resonance (SPR) [35]. Figure 6 shows the UV-Vis absorption spectrum of bio-synthesized CuNPs and a strong absorption peek was observed at ~590 nm. This band can be attributed to the surface plasmon resonance due to collective oscillations of surface electrons. The only single maximum curve of SPR ascribes to the spherical nanoparticles on endorsement with FESEM and TEM images [36]. In addition, no absorption band was observed in case of pure cumin seed extract solution due to the absence of metallic SPR.
Figure 6. UV-Vis absorbance spectrum of C. cyminum seed extract and bio-CuNPs.
Download figure:
Standard image High-resolution image3.5. FTIR spectroscopic analysis
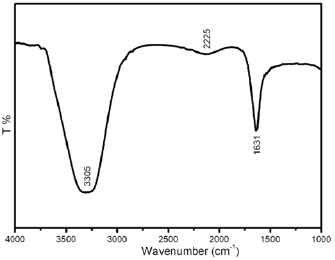
FTIR spectroscopy is a useful technique to study the probable interactions of CuNPs with various functional groups [37]. FTIR spectrum evinces the existence of different functional groups at various positions (figure 7). The intense band at  corresponds to the carbonyl group involved in the creation of nanoparticles. Also, a minor broad peak at
corresponds to the carbonyl group involved in the creation of nanoparticles. Also, a minor broad peak at  assigned to the O–C stretching mode. Another strong, intense band at
assigned to the O–C stretching mode. Another strong, intense band at  is attributed to both
is attributed to both  in primary aromatic amines and –OH groups in alcohols [38]. It is well known that biological components interact with metal salts and mediate reduction process with the above said functional groups. FTIR spectrum was clearly confirmed the presence of functional groups present in the Cuminum cyminum seed extract and its ability to perform both reducing and capping actions in the formation of CuNPs.
in primary aromatic amines and –OH groups in alcohols [38]. It is well known that biological components interact with metal salts and mediate reduction process with the above said functional groups. FTIR spectrum was clearly confirmed the presence of functional groups present in the Cuminum cyminum seed extract and its ability to perform both reducing and capping actions in the formation of CuNPs.
Figure 7. FTIR spectrum of bio-synthesized copper nanoparticles.
Download figure:
Standard image High-resolution image3.6. Antimicrobial studies
Synthesized CuNPs have shown significant antimicrobial activity against all selected bacterial and fungal test pathogens and subsequent zone of inhibition (ZOI) in mm of corresponding discs was measured and mentioned in table 1. The difference of zone of inhibitions at selected pathogenic bacteria and fungi with respect to bio-copper volumes of  and
and  are shown in figure 8. The bactericidal property of CuNPs is mainly due to the release of copper cations from CuNPs that acts as reservoir for them [39]. Bacterial membrane permeability increases with copper cations interaction results in severe changes in the membrane structure of bacteria. In general, copper cations from CuNPs attach to the bacterial cell wall due to electrostatic attraction and rupture it which leads to denaturation of protein and dissipation of the proton motive force and finally cell lysis [40, 41].
are shown in figure 8. The bactericidal property of CuNPs is mainly due to the release of copper cations from CuNPs that acts as reservoir for them [39]. Bacterial membrane permeability increases with copper cations interaction results in severe changes in the membrane structure of bacteria. In general, copper cations from CuNPs attach to the bacterial cell wall due to electrostatic attraction and rupture it which leads to denaturation of protein and dissipation of the proton motive force and finally cell lysis [40, 41].
Table 1. The zone of inhibitions of different bacterial and fungal pathogens with respect to bio-CuNPs volumes.
CuNPs volume  |
Zone of inhibitions (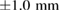 ) ) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Bacterial pathogens | Fungal pathogens | |||||||
| Bacillus spp. | Pseudomonas spp. | Staphylococcus spp. | E. coli | A. niger | Penicillium spp. | A. flavus | Rhizopus spp. | |
| 4 | 4 | 8 | 5 | 3 | 3 | 3 | 3 | 3 |
| 8 | 5 | 9 | 5 | 3 | 3 | 4 | 4 | 3 |
| 12 | 6 | 10 | 6 | 4 | 4 | 5 | 5 | 4 |
| 16 | 6 | 11 | 7 | 5 | 4 | 6 | 5 | 5 |
Figure 8. Disc-diffusion assay of different (a) bacteria, and (b) fungi activities at different volumes of bio-CuNPs.
Download figure:
Standard image High-resolution imageBio-synthesized CuNPs have shown prominent bactericidal activity against Bacillus spp., Pseudomonas spp., Staphylococcus spp. and Escherichia coli. Antibacterial studies reveal that Pseudomonas spp. is more susceptible to CuNPs and it has been eradicated possibly with the zone of inhibition of 11 mm at  of CuNPs volume, whereas Escherichia coli has shown least zone of inhibition of 5 mm at
of CuNPs volume, whereas Escherichia coli has shown least zone of inhibition of 5 mm at  of CuNPs volume. Antifungal property of spherical CuNPs against Aspergillus niger, Penicillium spp., Aspergillus flavus and Rhizopus spp. was evaluated. The obtained results attest that the Penicillium spp. are more sensitive to CuNPs and displayed zone of inhibition of 6 mm at
of CuNPs volume. Antifungal property of spherical CuNPs against Aspergillus niger, Penicillium spp., Aspergillus flavus and Rhizopus spp. was evaluated. The obtained results attest that the Penicillium spp. are more sensitive to CuNPs and displayed zone of inhibition of 6 mm at  of CuNPs volume and the zone of inhibition of other tested fungal pathogens are depicted in table 1. Finally, the present investigation clearly indicates that the Cuminum cyminum extract mediated CuNPs exhibited the excellent antimicrobial activity towards Pseudomonas spp. and Penicillium spp. bacterial and fungal pathogens, respectively.
of CuNPs volume and the zone of inhibition of other tested fungal pathogens are depicted in table 1. Finally, the present investigation clearly indicates that the Cuminum cyminum extract mediated CuNPs exhibited the excellent antimicrobial activity towards Pseudomonas spp. and Penicillium spp. bacterial and fungal pathogens, respectively.
4. Conclusion
In this investigation, copper nanoparticles were synthesized through an eco-friendly approach by using Cuminum cyminum seed extract. This approach towards the synthesis of CuNPs has many advantages such as simple and the process can be scaled up with economic viability. The XRD analysis showed that CuNPs crystallizes in fcc structure. The surface plasmon resonance band of the UV-Vis spectrum at 590 nm indicates the existence of copper nanoparticles. The shape and morphology of the bio-synthesized nanoparticles were investigated by FESEM and TEM analysis and found to be spherical shape. FTIR spectroscopy indicated the involvement of biomolecules present in Cuminum cyminum seeds in the synthesis process. Finally, the synthesized nanoparticles have shown significant antimicrobial activity on selected pathogenic bacteria and funguses. Therefore, the obtained eco-friendly bio-CuNPs can be served in a bio-medical field to exploit the existing antibiotics to an utmost level with cost effectiveness rather than spending the millions of dollars.
Acknowledgments
The authors would like to express their sincere gratitude for the financial support of University Grants Commission (UGC), New Delhi under Mid-Career Award (No. F-19 207/2017(BSR)) scheme and National Research Foundation of Korea (NRF-2015K1A4A3047100, NRF-2015M3A7B6027973).