Abstract
Development of chemoresistance is a significant restriction on the success of cancer treatment. Combination chemotherapy and drug delivery nanosystem are two promising strategies to overcome this limitation. Administration of two or more anticancer drugs at the same time can promote synergistic effect and suppress drug resistance through distinct mechanisms of action. Drug delivery nanosystem, on the other hand, improves delivery, efficacy and safety of drugs, and also can escape from some mechanisms of drug resistance. In this study we prepared drug delivery nanosystems from copolymers of lactic acid (PLA) and d-α-tocopheryl polyethylene glycol 1000 succinate (TPGS). The nanosystems incorporated with folic acid as targeting agent were used to load curcumin (Cur) and paclitaxel (PTX) contemporaneously and denoted as (Cur + PTX)-PLA-TPGS-Fol. The results showed that (Cur + PTX)-PLA-TPGS-Fol nanoparticles has average size range of 100–200 nm depending on the ratio between PLA and TPGS. Loading efficacy of the two drugs was about 35%–83% with the highest encapsulation efficiency belonged to the system with the highest ratio of PLA. All of the prepared nanosystems with single drug or in combination exhibited strong cytotoxicity to cancer cells, but the combination was more effective in case of A549 cancer cell line. These results showed that our combination of Cur and PTX in our drug delivery nanosystem can be a promising candidate for cancer treatment.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Every year, there are numerous number of people die by cancer. Most common types of cancer therapy are surgery, chemical therapy and radiotherapy. However, the use of traditional drugs to treat cancer diseases causes many adverse effects by killing not only tumor cells but also healthy cells [1–3]. Moreover, over the time using chemotherapy, therapeutic efficacy of anticancer drugs is limited due to the resistance of cancer cells. Drug resistance is responsible for most of the failed cases in the treatment of cancer. There are many methods of drug resistance such as: epigenetics, drug efflux, DNA damage repair, cell death inhibition, epithelial–mesenchymal transition, drug target alteration and drug inactivation. These mechanisms can act independently or in combination and through various pathways [4]. To overcome this limitation for better therapeutic efficacy, combination chemotherapy can be considered as a promising cancer treatment. Administration of two or more anticancer drugs at the same time can promote synergistic effect and suppress drug resistance through distinct mechanisms of action.
Drug delivery nanosystem has been demonstrated to enhance therapeutic efficacy of drug while minimize unwanted effects on healthy tissues [5]. Many drug delivery systems have been well studied such as liposomes [6], polymer–drug conjugates [7] and copolymeric micelles [8]. Among them, copolymeric micelles have attached a great deal of attention thanks to its high bioavailability, high stability, small size, low cytotoxicity [9–12]. Polymeric micelles PLA-TPGS are based on a block copolymer of monomer L-LA and TPGS which is synthesized by ring opening polymerization method. Thanks to its core–shell structure, it can increase solubility of water poorly soluble drugs. This block-copolymer has been applying in drug delivery nanosystems due to its safety, stealth effect, biocompatibility, targeting possibility and easiness of preparation. Moreover, TPGS is absorbed easily into the gastrointestinal tracts and it also inhibits P-glycoprotein, the multidrug transporter, improves the cytotoxicity of anti-cancer compounds such as doxorubicin, vinblastine, PTX and Cur [13, 14].
Using drug delivery nanosystem in co-delivery of chemotherapy has been rapidly developed over the last two decades. Wang et al prepared PEG-PLGA nanoparticles loading doxorubicin and PTX and found that the combination at molar ratio of 2/1 showed the best synergistic therapeutic effect [15]. Some nanodrug codelivery systems on other drugs such as cisplatin in combination with PTX, doxorubicin or rapamycin also obtained very promising results on treatment of ovarian cancer and melanoma [16, 17]. The combination, moreover, can be applied for anticancer drugs with gene therapy (SiRNA, DNA, ...) [18, 19].
Besides, one of the way to improve the targeting capacity is modifying drug delivery systems with targeting ligands, such as folic acid, aptamers, peptides and antibodies [20]. Among them, folic acid is the most popular due to its binding faculty to folate receptors that are abundant on many human cancer cells and mostly absent in normal tissue [21].
In our previous works [22–25], we have successfully synthesized copolymer PLA-TPGS and used it in encapsulation of anticancer drugs with very promising results. In this study we synthesized five different copolymers with different ratio between PLA and TPGS. All of the copolymers were used to co-encapsulate PTX and Cur in a nanosystem conjugated with folic acid as a targeting agent. The nanosystems were characterized by morphology, size, and efficacy of drug loading, cytotoxicity essays on HepG2, HeLa, LU-1 and Vero cell lines and found the best copolymer for encapsulation purpose. The best system was further studied in vitro compared to the system with only one drug.
2. Materials and methods
2.1. Materials
Lactide momomer (C6H8O4), stannous octoate (Sn(OOCC7H15)2), folic acid, N-hydroxysuccinimide (NHS), 1-[3-dimethylamino propyl]-3-ethylcarbodiimide hydrochloride (EDC), aspartic acid, folic acid (Fol), ethanol (>99.5%), dichloromethane, methanol, toluene were purchased from Sigma-Aldrich. TPGS and PTX were purchased from Merck. Cur (>95% purity) was provided by Institute of Chemistry, Vietnam. In vitro cytotoxicity experiments were performed on HepG2, HeLa, LU-1 and Vero cells. Moreover, other cytotoxicity assays on A549 (human alveolar adenocarcinoma), MCF7 (human breast adenocarcinoma), HepG2 and HacaT (aneuploid immortal keratinocyte) cell lines were carried out by scientist at Faculty of Biology, University of Science in Hanoi.
All chemicals were used as received without further purification.
2.2. Methods
2.2.1. Synthesis of copolymers.
Ring-opening polymerization method was used to synthesize 5 formulations of PLA-TPGS as described in the previous work [25]. Briefly, exact amount of the lactide monomer and TPGS was weighted and dissolved in toluene. Lactide monomer, TPGS (in toluene) and stannous octoate were added in an ampoule and allowed to react at 130 °C in nitrogen atmosphere. After reacting for 7 h, the solvent was removed to obtain raw product. Then this mixture was purified by dissolving in dichloromethan followed by precipitating in cold methanol for 3 times. Afterthat, copolymer PLA-TPGS was obtained by centrifugation and dried in oven at 45 °C for 48 h.
In order to optimize the structure of this delivery system, we carried out modifying ratios between PLA and TPGS as 1:3, 1:2, 1:1, 2:1, 3:1 (w/w) to find out which ratio has the best Cur and PTX loading and biological effect.
2.2.2. Preparation of activated folate solution.
Initially, the hydroxyl groups of TPGS were reacted with carboxyl groups of aspartic acid. The esterification was performed at room temperature for 24 h with the catalystic activity of EDC and NHS (molar ratio of 1.2:1.2). Next, folic acid (Fol) was also activated with EDC and NHS in NH4OH 2 M for 6 h at 50 °C to establish an identified mixture of Fol-NHS. The activated TPGS-COOH solution above was dropped into the Fol-NHS solution, and then the obtained mixture was adjusted pH to 8, before being stirred at 37 °C for 4 h.
2.2.3. Preparation of folate modified, CUR and PTX coloaded PLA-TPGS nanoparticles.
First of all, each 10 mg PLA-TPGS copolymer with different ratios of monomer was dissolved in 10 ml dichloromethan and magnetically stirring for 2 h. Then 10 ml H2O was added and the mixture was continuously stirred until all the copolymer transferred to the aqueous phase. Then this aqueous phase was collected. Simultaneously, a solution of Cur and PTX was also prepared by dissolving completely 4 mg Cur and 4 mg PTX in 10 ml ethanol. In the next step, the solution of Cur and PTX was dropped into water layer separated from the copolymer mixture above. The obtained solution was stirred for 24 h at room temperature. Finally, 1 ml activated folate solution was added and repeatedly stirred for 2 h to form (Cur + PTX)-PLA-TPGS-Fol NPs. After centrifugation, the nanosystem was obtained from the supernatant while the pellet was used to determine the nonencapsulated amount of PTX and CUR.
The encapsulation efficiency ( ) of PTX and Cur in micelle was calculated by the following equation:
) of PTX and Cur in micelle was calculated by the following equation:

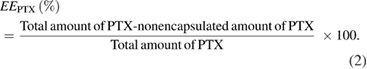
2.2.4. Characterization.
FTIR spectra of PLA-TPGS copolymer, Cur, PTX, folic acid and nanoparticles were determined by using compressed KBr pellet method in Perkin Elmer FTIR spectrophotometer (Perkin Elmer Spectrum Two, Waltham, Massachusetts, USA). Size and morphology of nanoparticles was obtained by FESEM images. Dynamic size, polydispersity and zeta potential of (Cur + PTX)-PLA-TPGS-Fol were performed using a Zetasizer version 7.03, Malvern instruments Ltd, Malvern, UK [16].
2.2.5. Cytotoxicity assay.
In the first cytotoxicity assays, five (Cur + PTX)-PLA-TPGS-Fol nanosystems based on different PLA-TPGS copolymers were studied for their anticancer activity on 4 cell lines: Hep-G2, HeLa, LU-1 and Vero. This assay was carried out as described by Skehan et al and Likhitwitayawuid et al [26, 27]. In brief, cells were cultured in Dulbecco's modified eagle medium DMEM, a medium consisting of 1% penicillin-streptomycine and 10% bovine calf serum, at 37 °C under 95% air and 5% CO2 atmosphere for 24 h. Next, the medium containing (Cur + PTX)-PLA-TPGS-Fol nanosystems was replaced the initial medium. After 48 h of incubation, trichloroacetic acid was added to stop the reaction and incubated for 1 h. After being washing with distilled water, the plates were dyed with SRB for 30 min. The results were read by ELISA. The cell survival value (CS) was calculated according to the following formula:
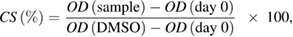
in which OD(sample) is the optical density of the plates incubated with (Cur + PTX)-PLA-TPGS-Fol; OD (day 0) is the optical density of the plates before incubation; OD(DMSO) is the optical density of the plates incubated with dimethyl sulfoxide (DMSO, control sample).
In the second cytotoxicity, the best nanosystem chosen from these above results was used to test its activity on A549, MCF7, Hep-G2 and HacaT cell lines in comparison with free drugs and nanosystem encapsulating single drug (Cur or PTX). This assay was carried out by MTS method. In brief, each cancer cell line was cultured in a 96 well cell plate and then was added with accurate concentration of drug solution or nanosystems, incubated for 48 h. After that, the cells were incubated with MTS in 3 h and the results were read by ELISA. Half maximal inhibitory concentration ( ) was calculated as the concentration of drug or drug delivery nanosystems that was required to inhibit 50% of cancer cells.
) was calculated as the concentration of drug or drug delivery nanosystems that was required to inhibit 50% of cancer cells.
3. Results and discussion
3.1. Characterization of nanoparticles
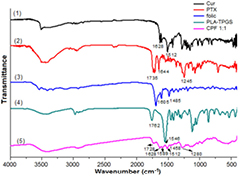
There is no significant difference between FTIR spectra of nanoparticles with 5 distinctive ratios of PLA and TPGS (unpublished results), therefore, we choose the copolymer with ratio of PLA-TPGS of 1:1 to discuss for further information. Figure 1 shows the FTIR spectra of PLA-TPGS, pure Cur, pure PTX, acid folic and (Cur + PTX)-PLA-TPGS-Fol. The FTIR spectrum of (Cur + PTX)-PLA-TPGS-Fol shows clear diferences with those of the starting materials. The stretching of carbonyl (C=O) groups appearing at strong  in PLA-TPGS shifted to
in PLA-TPGS shifted to  in the nanosystems. PTX is attributed by C=O and C–C stretchings at 1735 and
in the nanosystems. PTX is attributed by C=O and C–C stretchings at 1735 and  , respectively, but they might be covered by C=O stretchings of Cur and PLA-TPGS. C=C olefienic stretching of Cur and characteristic absorption band of phenyl ring of folic appear at 1512 and
, respectively, but they might be covered by C=O stretchings of Cur and PLA-TPGS. C=C olefienic stretching of Cur and characteristic absorption band of phenyl ring of folic appear at 1512 and  (
( in standard spectrum), respectively. The C=C band of PLA-TPGS (
in standard spectrum), respectively. The C=C band of PLA-TPGS ( ) is overlapped by C=O olefienic of Cur so that it is not shown up in the spectrum of (Cur + PTX)-PLA-TPGS-Fol. Finally, characteristic peak of C–N bond was shifted from 1245 to 1280 cm−1, which is referred to PTX spectrum. The aforementioned data proved that Cur and PTX were successfully encapsulated in folate modified PLA-TPGS micelles.
) is overlapped by C=O olefienic of Cur so that it is not shown up in the spectrum of (Cur + PTX)-PLA-TPGS-Fol. Finally, characteristic peak of C–N bond was shifted from 1245 to 1280 cm−1, which is referred to PTX spectrum. The aforementioned data proved that Cur and PTX were successfully encapsulated in folate modified PLA-TPGS micelles.
Figure 1. FTIR spectra of (1) Cur, (2) PTX, (3) acid folic, (4) PLA-TPGS and (5) (Cur + PTX)-PLA-TPGS-Fol.
Download figure:
Standard image High-resolution image3.2. Size and morphology
From field emission scanning electron microscope (FESEM) images in figure 2 we can obtain that all of the drug delivery nanosystems had spherical shape and homogenous size of about 40–100 nm with less aggregation. We can also see that the copolymers with higher ratio of PLA had larger size (but the changes were not very clear).
Figure 2. FESEM images of (Cur + PTX)-PLA-TPGS-Fol NPs based on 5 different ratios between PLA and TPGS of 1:3 (a), 1:2 (b), 1:1 (c), 2:1 (d) and 3:1 (e).
Download figure:
Standard image High-resolution imageResults of dynamic light scattering (DLS) in figure 3 also tell us that the nanoparticles had small size of less than 200 nm and homogenous with small polydispersity index (PDI) value. Moreover, DLS results confirm the judgement: the higher the ratio of PLA, the bigger the size of nanoparticles. This can be the result of the core–shell architecture with size-controlling PLA hydrophobic core of the nanoparticles [28]. The larger of the DLS size compared to the FESEM size on the same nanosystem may be due to the fact that FESEM is performed on dried particles but DLS is the dynamic size which is also be affected by the water molecule interacting with the particles.
Figure 3. Size distribution and zeta potential of (Cur +PTX)-PLA-TPGS-Fol NPs at different ratios of PLA-TPGS of 1:3 (a1, a2), 1:2 (b1, b2), 1:1 (c1, c2), 2:1 (d1, d2), 3:1 (e1, e2). Dz is the Z-average value for particle size.
Download figure:
Standard image High-resolution image200 nm or smaller nanoparticles was reported to prolong the circulation time of the particles in the blood. They were also able to concentrate in tumors through the Enhanced Permeability and Retention effect [3]. Thus, a drug delivery system is required to be in appropriate size in order to avoid being removed by phagocytes and obtain high treatment efficiency. Besides, with the larger core of the micelle, the amount of drugs loaded into the micelle can be also increase.
With the small size less than 200 nm, the cellular or tumorous uptake of nanoparticles can be improved, leading to enhancement in the therapeutic efficiency.
Stability of nanosystems can be predicted through zeta potential. All of the nanosystems had negative charge of from −21.5 to −28.4 mV (figure 3). Therefore, our nanosystems can be very stable.
3.3. Encapsulation efficiency
According to results in table 1, the highest encapsulation efficiency (EE (%)) of both Cur and PTX was seen at the ratio of PLA:TPGS = 3:1 with 80.1% and 82.6%, respectively, while the lowest one of both them was seen at the ratio of 1:3 about 35% for 2 drugs. This could be explained as follows: Cur and PTX are such hydrophobic drugs that it would interplay strongly with the PLA core of nanoparticles. Therefore, the decline in the ratio of PLA:TPGS (from 3:1 to 1:3) results in a similar decrease in drug EE. EE of the formulation at three ratios of 1:1, 1:2 and 1:3 were nearly equal, which might be due to they had the same initial amounts of PLA.
Table 1. The encapsulation efficiencies of Cur and PTX.
| Ratio of PLA:TPGS (w/w) | Cur loaded (mg ml−1) | PTX loaded (mg ml−1) | EE (%) | |
|---|---|---|---|---|
| Cur | PTX | |||
| 1:3 | 0.14 | 0.14 | 35.0 | 34.7 |
| 1:2 | 0.15 | 0.16 | 37.5 | 39.8 |
| 1:1 | 0.17 | 0.18 | 42.5 | 44.5 |
| 2:1 | 0.27 | 0.26 | 67.5 | 65.3 |
| 3:1 | 0.32 | 0.33 | 80.1 | 82.6 |
3.4. Cytotoxicity assays
3.4.1. Cytotoxicity assay of nanosystems based on different copolymers.
The efficacy of 5 drug delivery nanosystems based on 5 copolymers with different ratio of PLA and TPGS were tested on 3 cancer cell lines (HepG2, HeLa, LU-1) and Vero cell line. The results were presented in table 2.
Table 2. Results of cytotoxicity assay on 5 selected cell lines of (Cur + PTX)-PLA-TPGS-Fol at different ratio of PLA:TPGS.
| Label | Final drug concentration (µg ml−1) | Cell lines CS value (%) | Comment | |||
|---|---|---|---|---|---|---|
| HepG2 | HeLa | LU-1 | Vero | |||
| Solvent | — | 100 | 100 | 100 | 100 | |
| Control (+) | 0 | 2.5 ± 0.4 | 1.25 ± 0.2 | 29.15 ± 1.2 | Positive | |
| PLA:TPGS = 1:3 | 0.5 | 0 | 0 | 60.77 ± 1.9 | 0 | Positive on 3 cell lines |
| 0.25 | 3.54 ± 0.2 | 0 | 81.5 ± 2.2 | 50.2 ± 0.9 | ||
| 0.125 | 22.4 ± 0.7 | 0 | 85.2 ± 0.7 | 54.8 ± 1.1 | ||
| 0.0625 | 29.5 ± 0.5 | 0 | 96.5 ± 1.3 | 70.97 ± 1.2 | ||
| PLA:TPGS = (1:2) | 0.5 | 0 | 0 | 54.65 ± 1.3 | 6.3 ± 0.5 | Positive on 3 cell lines |
| 0.25 | 5.4 ± 0.6 | 13.6 ± 0.4 | 79.26 ± 0.9 | 48.5 ± 0.7 | ||
| 0.125 | 11.86 ± 0.8 | 16.56 ± 0.8 | 88.2 ± 0.8 | 69.9 ± 1.2 | ||
| 0.0625 | 25.76 ± 0.1 | 20.89 ± 0.2 | 92.22 ± 0.4 | 82.4 ± 1.4 | ||
| PLA:TPGS = (1:1) | 0.5 | 0 | 0 | 0 | 0 | Positive on 4 cell lines |
| 0.25 | 0 | 0 | 75.7 ± 0.6 | 9.8 ± 1.2 | ||
| 0.125 | 9.04 ± 1.3 | 11.33 ± 0.4 | 93.38 ± 1.9 | 58.1 ± 0.6 | ||
| 0.0625 | 12.1 ± 0.7 | 35.92 ± 1.5 | 98.5 ± 0.4 | 71.2 ± 0.9 | ||
| PLA:TPGS = (2:1) | 0.5 | 1.6 ± 0.5 | 3.2 ± 0.8 | 79.3 ± 1.2 | 27.48 ± 1.7 | Positive on 3 cell lines |
| 0.25 | 12.68 ± 0.9 | 28.95 ± 1.4 | 86.97 ± 0.3 | 57.83 ± 0.8 | ||
| 0.125 | 25.82 ± 0.7 | 55.04 ± 0.6 | 91.66 ± 1.7 | 68.34 ± 1.3 | ||
| 0.0625 | 30.6 ± 1.5 | 76.5 ± 1.3 | 98.25 ± 0.6 | 87.31 ± 0.3 | ||
| PLA:TPGS = (3:1) | 0.5 | 0 | 0 | 13.2 ± 1.8 | 0 | Positive on 4 cell lines |
| 0.25 | 2.34 ± 0.2 | 4.29 ± 0.3 | 79.11 ± 1.9 | 52.8 ± 1.7 | ||
| 0.125 | 17.06 ± 1.8 | 38.58 ± 1.2 | 85.4 ± 1.6 | 61.65 ± 0.6 | ||
| 0.0625 | 20.8 ± 0.3 | 66.5 ± 0.4 | 92.5 ± 0.7 | 78.25 ± 1.9 | ||
From table 2 we can see that different formulations had different effects on cancer cell lines. All of them were cytotoxic to HepG2 cells with very high effect at low concentration of  . Besides, all of the formulation had positively effect on HeLa cells but with different levels of action: the nanosystems based on copolymer with higher ratio of TPGS (1:3, 1:2, 1:1) performed a very strong effect on HeLa cells when that of formulation with high ratio of PLA (2:1, 3:1) still existed but weaker. LU-1 cancer cells seem hard to be influenced by the nanosystems except the formulation of PLA-TPGS (3:1) and (1:1) at high drug concentration of
. Besides, all of the formulation had positively effect on HeLa cells but with different levels of action: the nanosystems based on copolymer with higher ratio of TPGS (1:3, 1:2, 1:1) performed a very strong effect on HeLa cells when that of formulation with high ratio of PLA (2:1, 3:1) still existed but weaker. LU-1 cancer cells seem hard to be influenced by the nanosystems except the formulation of PLA-TPGS (3:1) and (1:1) at high drug concentration of  . Surprisingly, the highest loading content of Cur and PTX was achieved on the 3:1 nanosystem, however, the nanocarrier-based formulation with the ratio 1:1 of PLA/TPGS was the most effective system on all cancer cell lines, especially on HepG2 and HeLa. This observation could be explained by 2 reasons. One reason is the smaller size helps 1:1 formulation easier cellular uptake. Another reason is the faster rate of Cur or PTX release resulted from high percent of hydrophilic TPGS in 1:1 formulation cause higher toxicity on cancer cells than 2:1 or 3:1 formulation [28]. Therefore we chose the copolymer PLA-TPGS 1:1 for further study.
. Surprisingly, the highest loading content of Cur and PTX was achieved on the 3:1 nanosystem, however, the nanocarrier-based formulation with the ratio 1:1 of PLA/TPGS was the most effective system on all cancer cell lines, especially on HepG2 and HeLa. This observation could be explained by 2 reasons. One reason is the smaller size helps 1:1 formulation easier cellular uptake. Another reason is the faster rate of Cur or PTX release resulted from high percent of hydrophilic TPGS in 1:1 formulation cause higher toxicity on cancer cells than 2:1 or 3:1 formulation [28]. Therefore we chose the copolymer PLA-TPGS 1:1 for further study.
3.4.2. Cytotoxicity assay with nanosystems based on PLA-TPGS (1:1 w/w) copolymer.
From these above results, we chose copolymer PLA-TPGS (1:1 w/w) to study the cytotoxicity of the drug delivery nanosystem encapsulating both Cur and PTX compared to the nanosystems with only one drug (PLA-TPGS-Cur-Fol or PLA-TPGS-PTX-Fol). The outcomes were expressed in table 3, figures 4 and 5.
Table 3.  of the drug delivery nanosystems on A549, MCF7, HepG2 and HacaT cell line.
of the drug delivery nanosystems on A549, MCF7, HepG2 and HacaT cell line.
| Cell | IC50 ± SD | |||
|---|---|---|---|---|
| A549 | MCF7 | HepG2 | HacaT | |
| PLA-TPGS-Cur-Fol | 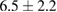 |
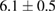 |
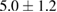 |
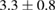 |
| PLA-TPGS-PTX-Fol | 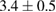 |
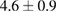 |
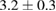 |
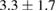 |
| (Cur + PTX)-PLA-TPGS-Fol | 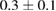 |
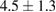 |
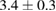 |
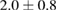 |
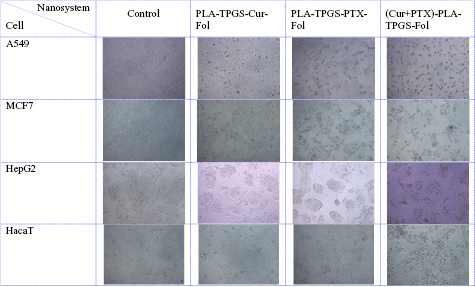
Figure 4. Images of A549, MCF7, HepG2 and HacaT cell line (control and treated with  drug delivery nanosystems).
drug delivery nanosystems).
Download figure:
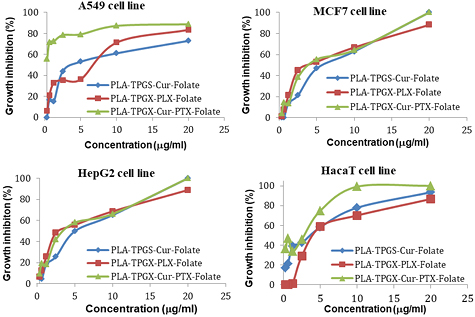
Standard image High-resolution imageFigure 5. Cell growth inhibition in function of concentration of drug delivery nanosystems on A549, MCF7, HepG2 and HacaT cell line.
Download figure:
Standard image High-resolution imageAs shown in figure 4, the cells of all the 4 cell lines were shrunk and distorted when treated with (Cur + PTX)-PLA-TPGS-Fol nanosystem. In addition, significant cell surface disruption and fragments of organelles were observed. Especially, at the same concentration of the drug, the co-formulation of (Cur + PTX)-PLA-TPGS-Fol show better effect on the cancer cells than monodrug formulation.
The free Cur, free PTX and PLA-TPGS had  of more than
of more than  , so they were considered as non-cytotoxic agents (data not shown). Meanwhile, the drug delivery nanosystems had significantly enhanced cytotoxicity on 4 cancer cell lines with very low
, so they were considered as non-cytotoxic agents (data not shown). Meanwhile, the drug delivery nanosystems had significantly enhanced cytotoxicity on 4 cancer cell lines with very low  values.
values.
The combination of Cur and PTX in the same drug delivery nanosystem showed a meaningful improvement in inhibiting A549 cancer cells when compared with the drug delivery systems with only Cur or PTX ( of
of  compared to 6.5 or
compared to 6.5 or  ). To explain for this result, besides anticancer efficacy, Cur assists to prevent PTX from turning inactive nuclear factor kappa B (
). To explain for this result, besides anticancer efficacy, Cur assists to prevent PTX from turning inactive nuclear factor kappa B ( ) into active form. To be more specific, this factor is inactive in healthy cells, ensuring apoptosis process happen in normal way. Almost anticancer agents including PTX activate
) into active form. To be more specific, this factor is inactive in healthy cells, ensuring apoptosis process happen in normal way. Almost anticancer agents including PTX activate  , which might lead to uncontrolled growth of tumor [29]. Moreover, Cur could down regulate the expression of permeability glycoprotein (P-gp) and multidrug resistance protein 1 (MDR1) in cancer cells [30], had reverse effect on enzyme system mediated tumor multidrug-resistance [31], reverse effect on apoptosis proteins mediated tumor multidrug-resistance [32], and reversal effect on DNA repair mechanisms mediated tumor multidrug-resistance [33].
, which might lead to uncontrolled growth of tumor [29]. Moreover, Cur could down regulate the expression of permeability glycoprotein (P-gp) and multidrug resistance protein 1 (MDR1) in cancer cells [30], had reverse effect on enzyme system mediated tumor multidrug-resistance [31], reverse effect on apoptosis proteins mediated tumor multidrug-resistance [32], and reversal effect on DNA repair mechanisms mediated tumor multidrug-resistance [33].
For other cancer cell lines, the combination did not improve the cytotoxicity of the nanosystems. HacaT cells also have folate receptors on their surface so it is still impacted by the folate-nanosystems.
The results point out that the loading of PTX and Cur by copolymer block enhances the cytotoxic effects of drugs. Besides, it can be implicated that sustained release of drug from nanocarriers along with the possibly higher cellular uptake could be major reason behind the enhanced inhibitory effect of nanoparticles.
4. Conclusion
In this study folate modified-, PTX and Cur coloaded-nanoparticles based on PLA-TPGS copolymer were successfully prepared. All the nanosytem had small size of about less than 200 nm and negative charge. The drug encapsulation effect of them was about 35%–83% with the highest encapsulation efficiency belonged to the system with the highest ratio of PLA. All of the (Cur + PTX) PLA-TPGS-Fol formulations had strong effect on tested cancer cell lines with the highest belong to the system based on PLA/TPGS of 1:1 (w/w). We also obtained that the combination of Cur and PTX in the sample nanoparticle significantly improved the cytotoxicity on A549 cancer cells. Therefore this combination on PLA-TPGS copolymer can be a promising candidate for cancer treatment.
Acknowledgment
This work was financially supported by Vietnam Academy of Science and Technology under Grant No. VAST03.04/16-17.