Abstract
Microfluidic organs and organoids-on-a-chip models of human gastrointestinal systems have been established to recreate adequate microenvironments to study physiology and pathophysiology. In the effort to find more emulating systems and less costly models for drugs screening or fundamental studies, gastrointestinal system organoids-on-a-chip have arisen as promising pre-clinical in vitro model. This progress has been built on the latest developments of several technologies such as bioprinting, microfluidics, and organoid research. In this review, we will focus on healthy and disease models of: human microbiome-on-a-chip and its rising correlation with gastro pathophysiology; stomach-on-a-chip; liver-on-a-chip; pancreas-on-a-chip; inflammation models, small intestine, colon and colorectal cancer organoids-on-a-chip and multi-organoids-on-a-chip. The current developments related to the design, ability to hold one or more 'organs' and its challenges, microfluidic features, cell sources and whether they are used to test drugs are overviewed herein. Importantly, their contribution in terms of drug development and eminent clinical translation in precision medicine field, Food and Drug Administration approved models, and the impact of organoid-on-chip technology in terms of pharmaceutical research and development costs are also discussed by the authors.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
List of abbreviations
| CF | Cystic fibrosis |
| CFTR | Cystic fibrosis transmembrane conductance regulator |
| CRC | Colorectal cancer |
| CTC | Circulating tumor cell |
| DILI | Drug-induced liver injury |
| EGFR | Epidermal growth factor receptor |
| EBs | Embryoid bodies |
| GOoC | Gastrointestinal system organoids-on-a-chip |
| GI | Gastrointestinal |
| HE | Hematoxylin and eosin staining |
| FDA | Food and Drug Administration |
| H. pylori | Helicobacter pylori |
| IL-6 | Interleukin 6 |
| IL-8 | Interleukin 8 |
| IL-1β | Interleukin β |
| iPSCs | Induced pluripotent stem cells |
| MPOC | Metabolic patterning-on-a-chip |
| N/A | Non applicable |
| PDECs | Pancreatic ductal epithelial cells |
| PDMS | Dimethylpolysiloxane |
| PDGOoC | Patient-derived gastrointestinal system organoids-on-a-chip |
| R&D | Research and development |
| TNF-α | Tumor necrosis factor-α |
| VLSLL | Very large-scale liver-lobule |
1. Introduction
The human gastrointestinal system is of vital importance in the individual well-being, and has been the subject of extensive scientific research due to its role in physiological aspects such as metabolism and digestion, and it is strongly interconnected immune system [1–3].
The key objective of the gastrointestinal system is to transform food into nutrients to be absorbed into the body [4]. When food is ingested, proteins, fats and carbohydrates are processed in the stomach and small intestine, where smaller molecules are then absorbed through the epithelium of the small intestine, entering the circulation. The large intestine reabsorbs the surplus water. Lastly, undigested food and waste secretions are eliminated from the body in the form of feces [4]. Equally important, the digestive organ liver helps digesting food by producing bile and eliminating toxins. The pancreas yields enzymes with the function to break down proteins, fats and carbohydrates [5].
In practical terms, the gastrointestinal tract can be divided into four stratums: mucosa, submucosa, muscularis propria, and the serosa [6]. An ever-important part of the gastrointestinal system is the presence of a nervous system known as enteric nervous system, present with the function to control muscular and epithelial cells.
Given the importance of such an vital group of organs, there is an enormous interest in studying gastrointestinal system homeostasis and diseases [7]. Gastrointestinal diseases disturb a particular part of the gastrointestinal tract or auxiliary digestive organs—liver, gall bladder and pancreas, and includes a widespread variety of diseases, such as Crohn's disease, with a huge burden on society [8].
The physiological gap between laboratory animals and humans, the inconsistency between flat in vitro systems and the human body, and the limitations of in silico modeling have created the necessity for new solutions to the ever-increasing demand for safety screening and speed of new potential pharmaceuticals.
The current challenge affecting the current drug development routes relies in the attainment of high reliability/predictability in the results of drug treatment, with the aim of ensuring minimal or no unanticipated side effects [9]. This has been quite challenging with only 21% of in vitro hits reaching phase III trials. The paradigm of using animal models in drug development and evaluation being considered key in medicine needs to change, or be complemented at least. Regardless of the evidences, models such as these are still required to be drug approval agencies, although the success rate of the drugs in clinical trials being stunningly small [10].
Organoids can result from human tissue or from pluripotent stem cells [11]. Human tissue has the evident limitation of biopsies rates and availability in a timely manner. Organoid cultures derived from pluripotent stem cells appear to have some benefits when compared to the first method [12]. With the ability to virtually differentiate into every cell type of the human body, human iPSCs can be used as an unique tool for developmental studies, regenerative medicine, drug screening and personalized medicine, since they can realistically mimic the in vivo situation [13].
However, the generation of a tissue-specific organoids from human iPSCs is also time-consuming and often does not complete full maturation levels. Most importantly, in cases of drug testing, patient-derived organoids are the natural choice, since they keep the patients genetic signature [14]. Nevertheless, organoids can be grown to resemble a wide variety of organs, exhibiting remarkable similarity to their in vivo counterparts.
Good research depends on reliable model systems, however, the lack of consistent and reliable methods that recreate one or several aspects of the in vivo complexity is real, and needs to be addressed. Therefore, translational research in the clinical setting ought to be more concentrated on complex models mimicking human conditions [11]. One of these models are organoids-on-chips. Organoids-on-chips are microfluidic systems that recapitulate aspects such as the 3D structure, cell–cell and cell–materials interplay, present in pathology and healthy tissues in vitro [15]. One feature that is common to all organoids is that they are generated from pluripotent stem cells or adult stem cells, also known as tissue stem cells by mirroring human organ development or regeneration in vitro. The analysis of organoid development can thus deliver valued information about the mechanisms underlying human development and organ regeneration. Therefore, their value for basic biological research in addition to their potential application in pharmaceutical drug testing and molecular medicine is immense.
Furthermore, integrating multiple organs on one chip is progressively more refined in terms of absorption, distribution, metabolism, excretion and toxicity representation. Also, it can be used for a better understanding in drug interaction and fate mechanisms in the body. One of the main advantages of microfluidics is the possibility to introduce vascularization. The microvascular system is crucial to transport nutrients and oxygen and remove the byproducts and CO2 in its inter-organ connections [16]. Regulation of blood flow in the intestine is a highly dynamic and precise process. During feeding, partially digested food (chyme) is generated in the stomach and flows to the small intestine. As chyme passes through the small intestine, blood flow increases to that segment of the gut. Once nutrient absorption has occurred, the blood flow in that area returns to baseline. Interestingly, this change in blood flow is influenced by the nutrient composition of the chyme and not simply gut distension. At steady state, the intestinal epithelial barrier and the gut vascular barrier control systemic microbial dissemination and the recruitment of circulatory cells to maintain a tolerogenic environment. During the tissue-invasive stages of diverse pathologies, a presumed loss of epithelial and endothelial barrier integrity results in a rapid and robust accumulation of inflammatory cells and tissue damage [17]. Since this circulatory system plays an integral part in human metabolism and is immensely associated with pathophysiology, it is crucial to reconstitute this organization in pre-clinical models [18].
The advances in terms of patient-derived gastrointestinal organoids-on-a-chip (GOoC) models and also stem cell-derived models are overviewed herein. The studies that use this technology to test drugs, and some other that seized the next step to clinical trials are highlighted. In addition, the future perspectives regarding clinical translation of GOoC are also provided from author's perspective.
Interestingly, in the case of organoids, several definitions over the years were used, as follows:
- (1)Thus, we would like to define an organoid as containing several cell types that develop from stem cells or organ progenitors and self-organize through cell sorting and spatially restricted lineage commitment, similar to the process in vivo' [19];
- (2)An organoid is now defined as a '3D structure grown from stem cells and consisting of organ-specific cell types that self-organizes through cell sorting and spatially restricted lineage commitment' [20];
- (3)Herein, an organoid is defined as an 'in vitro 3D cellular cluster derived exclusively from primary tissue, embryonic stem cells, or iPSCs, that are capable of self-renewal and self-organization, and exhibiting similar primary organ functionality' [21].
In order to avoid misconceptions, the terminologies organs-on-chip and organoids-on-chip may need a formal definition for a better understanding throughout the manuscript (box 1).
Box 1. Different concepts: organs-on-chip vs. organoids-on-a-chip
Organ-on-a-chip
An organ-on-a-chip is a micro-scale system used for mimicking the human body microenvironment. The goal of an organ-on-a-chip is to develop simple human tissue models for disease modeling and drug testing using cell lines or primary cells, to imitate the physiological and mechanical conditions experienced in the body. They can control the movement and behavior of materials and cells by using channels, chambers, membranes, and usually a hydrogel to mimic the extracellular matrix (ECM). The devices are manufactured using soft lithography or photolithography [22].
Organoid-on-a-chip
Organoid-on-a-chip: 'It derives from the concept of organoids described before. One can combine them with microfluidics technology: systems made of biocompatible materials to enable organoid studies and to reproduce in vivo conditions due to the use of a network of microchambers and microchannels and a laminar flow. The practical difference between organs on-a-chip and organoids-on-a-chip is the cellular part. Organs are composed of mixtures of cell lines or primary, while organoids use embryonic stem cells, iPSCs, or the final organ-like structure' [23, 24].
2. Gastrointestinal organoids-on-a-chip (GOoC) models
Organoids have undoubtedly become an advantageous tool in gastrointestinal research, either patient-derived or stem cell derived. However, the methodologies used to grow these organoids in different three-dimensional backgrounds often end developing stochastically, with limited lifespan and homeostasis [25].
A microfluidic chip can be fabricated to mimic various chemical, physical and/or biological cues, such as the ones shown in table 1. These platforms also offer the possibility of hosting patient' cells—or sometimes simply a piece of patient's explanted tissue, opening up the opportunity for the desired personalized medicine, i.e. by means of reflecting the genetic characteristics of cells of each patient [26, 27].
Table 1. Main organ and organoids-on-a-chip models used for drugs testing.
| Organoid model | Schematic representation/design | Example drug screening | Advantages | Limitations | Reference |
|---|---|---|---|---|---|
| Gut-on-a-chip PDGOoC | Human gut/microbiome—on-a-chip 
| N/A | Integrates oxygen sensors; electrical resistance (TEER) on a chip; extraction of DNA, RNA, proteins and metabolites | Shah et al [30] | |
| Gut-on-a-chip PDGOoC | Gastrointestinal human–microbe interface 
| Hypoxia effects. | Allows O2 gradients; working with human stool samples | Uses Caco-2 cells isolated from human CRC tumor, but show features of normal small intestine. | Jalili-Firoozinezhad et al [31] |
| 3D stomach-on-a-chip (human pluripotent stem cells) (hPSCs) | Stomach-on-a-chip 
| Non-applicable. | Mimicking peristaltic luminal flow in vitro; | Matrigel dependent. | Lee et al [32] |
| Very large-scale liver-lobule (VLSLL)-on-a-chip | Liver-on-a-chip 
| Non-applicable. | Each chamber contains a central outlet mimicking the central vein in the liver. | Short-term cultures | Banaeiyan et al [33] |
| In vitro liver zonation | Liver-on-a-chip 
| Acetaminophen. | Recreates the 'zonation' of the liver. | Shot time-frame experiments. | Kang et al [34] |
| Human liver microphysiology platform (HLMP) | Liver-on-a-chip 
| Troglitazone and nimesulide. | Include fluorescent protein biosensors. | Does not include vascularization. | Vernetti et al [35] |
| PDGOoC pancreas on-a-chip | Pancreas-on-a-chip 
| Non-applicable. | In situ pancreatic differentiation; generation of heterogeneous islet organoids. | Non-applicable. | Tao et al [36] |
| PDGOoC pancreas-on-a-chip | Pancreas-on-a-chip 
| Non-applicable. | Directly derived from micro dissect patient-derived human pancreatic ducts. | Hydrophobic PDMS absorbs hydrophobic small molecules. | Shik Mun et al [37] |
| Islet-on-a-chip | Pancreas-on-a-chip 
| Non-applicable. | Scalable manufacturing materials, on-line real time results readings. Insulin sensing. | Difficult of use. Calibrations needed. | Glieberman et al [38] |
| Mini-intestines-on-a-chip | Small intestine-on-a-chip 
| Non-applicable. | Long time culture without having to pass organoids. | Villi-like projections formed in the intestine chip do not exhibit the strict villus/crypt compartmentalization. | Nikolaev et al [39] |
| Human colon-on-a-chip | Colon-on-a-chip 
| PGE2 | Allows the study of mucus role in human intestinal homeostasis/disease. | Non-applicable. | Sontheimer-Phelps et al [40] |
| PDGOoC 2D stomach model | Inflammation models 
| Non-applicable. | Formation of antrum-derived gastric glands; study the cross talk of H. pylori and stroma. | Non-applicable. | Boccellato et al [41] |
| Colon-intestine-on-a-chip for inflammatory diseases | Inflammation models 
| IL-22 | High-throughput human emulation system called Zoë (Emulate, Inc®)—ease of manipulation. | Requires long experimental times. | Apostolou et al [42] |
| Small intestine-on-a-chip | Small intestine-organoid-on-a-chip 
| Non-applicable | Continuous collection of fluid samples, able to determined nutrient digestion and mucus digestion. | Requires training and handling of many cell types, organoids, microfluidics, bacteria. | Kasendra et al [43] |
| Colorectal-liver–lung organoids-on-a-chip (metastasis) | Multi-organoids-on-a-chip 
| Non-applicable. | Allows for increasing organ complexity. | Simplistic versions of actual in vivo tissue. | Aleman and Skardal [44] |
| Liver–heart organoids-on-a-chip | Multi-organoids-on-a-chip 
| Clomipramine (anti-depressant drug cardiac safety). | Able to assess cardiac beating and calcium flux. Real-time imaging of organoids-on-chip. | Complex use | Yin et al [45] |
| Vascularized, 2-channel, organ chips (e.g. intestine, liver, kidney, heart, lung, skin, blood–brain barrier and brain) | Multi-organs-on-a-chip 
| Inulin and nicotin. | Prediction of human drug pharmacokinetic responses. | Complex system of robotics, computer mathematical models and know how in several organs. | Novak et al [46] |
A physiologically relevant tumor microenvironment is also essential for the modeling of every healthy or disease models. This is especially important when working with organoids. Regarding the ECM used in most models, Matrigel®, a mouse tumor ECM protein mixture, is a crucial part of most organoid tissue culture, namely in Gastrointestinal organoids [28]. For example, synthetic poly(ethylene glycol) (PEG) hydrogels modified with ECM peptides (e.g. RGD) and protease-degradable peptides (e.g., GPQGIWGQ) or natural hydrogels, such as alginate hydrogel or fibrin gel mixed with ECM proteins (e.g. laminin-111), have been tested as artificial 3D environments for GI organoids. Kim et al have proven that gastrointestinal tissue decellularization can be a reliable option to culture GI organoid [28]. Another approach to this issue was described by Kim et al [29], which explored developed a 3D cell printing-based gastric cancer model, in combination with gastric tissue-specific bioinks and cellulose nanoparticles to provide adequate stiffness to gastric cancer cells. Although the field of organoids-on-a-chip is still growing, there is space for optimization of the ECM materials that are used, as seen by the previous examples.
Different models of gastrointestinal organs and organoids-on-a-chip have been innovatively explored in drug testing, some of which are already GOoC models, summarized in table 1 [28–44].
2.1. Human gut/microbiome–on-a-chip
The bacterial cells living in the human gastrointestinal tract are known as the gut microbiome which are far superior to the number of cells in the human body [47]. The gut microbiome and its possible relationship in health/disease conditions has been the focus of many studies [47]. There is a large amount of unknown information regarding the complexity of the gut microbiome and how it affects the overall gastrointestinal system of the human host, which prevents the advance of drug discovery for disease states [48].
Jalili-Firoozinezhad et al [31] co-cultured living human intestinal epithelium (using primary epithelial cells derived from surgical biopsied of human ileum) together with aerobic and anaerobic human gut microbiota. It is noteworthy that the authors worked with gut microbiota isolated from human feces, to maintain the inoculum at the genera and species levels. The team used a microfluidic platform made of PDMS with two-channels. Sensors were placed directly on the surface of both the vascular and epithelial channels of the Organ Chips that allows for the control and real-time assessment of oxygen gradients [31]. The chips were placed in an anaerobic environment to achieve a physiological oxygen gradient over the intestinal epithelium. The authors successfully achieved a co-culture of highly complex communities of anaerobic and aerobic human commensal gut bacteria in the same channel as mucus-producing human villus intestinal epithelium. At the same time, the platform allowed for the simultaneously monitoring oxygen levels and intestinal barrier function for at least 5 d in vitro [31]. This example of intestine-on-a-chip might aid as a tool for the development of microbiome-related therapeutics, probiotics and nutraceuticals. In another example, Shah et al [30] designed a different system and introduced an host–microbiota interaction module, which interfaces with the in vitro simulator of the human intestinal microbial ecosystem model, incorporates a semi-permeable membrane between to co-culture human enterocytes and bacteria. The device is composed of a modular stacked assembly of elastomeric gaskets (thickness: 700 μm) sandwiched between two polycarbonate (PC) enclosures, and each gasket defines a distinct spiral-shaped microchannel with the following characteristics: length of 200 mm, width of 4 mm and height of 0.5 mm (figure 1), amounting to a total volume of 400 μl per channel. CD4+ T were added in the perfusion, in the presence and absence of Lactobacillus rhamnosus GG. Its presence did not exhibit any significant differences in terms of cell viability. HuMiX platform was validated for transcriptomic, metabolomics and immunological analyses [30].
Figure 1. (A) Conceptual diagram of the HuMiX model for the representative co-culture of human epithelial cells with gastrointestinal microbiota; and (B) annotated exploded view of the HuMiX device. Reproduced from [30]. CC BY 4.0.
Download figure:
Standard image High-resolution image2.2. Stomach-on-a-chip
Nowadays, many health claims have been made involving the study of certain food impact and human digestion [49]. The transformation of food structures during digestion, as well as the hydrodynamics of digestion and the absorption process for nutrients needs to be further studied. The mechanical forces of stomach are the most complex parameter to mimic in the in vitro models to increase its realism. The gastric mucosa comprises distinct cell types with various functions. However, is has a rather simple structure comprising the columnar epithelium, which produces mucus, and tissue stroma cells that produce growth factors responsible for supporting the maintenance of gastric stem cells, muscularis externa and serosa [50].
The most commonly used organoid culture implemented in microfluidic devices consists in a 'sandwich' structure comprising two micro-sized channels divided by a porous membrane [50]. This is also applied in stomach-on-a-chip models.
For advanced biomedical usage, novel eligible approaches should reflect the ordered interplay and interdependence between the different cell types in the tissue.
Jeong et al described the first human stomach microphysiological system (hsMPS)-on-a-chip with a greatly enhanced gastric mucosal barrier based on the combination of MPS technology with organoids. The hsMPS where epithelial cells derived from human antral organoids and primary gMSCs extracted from stomach tissue are cultivated under controlled flow. The ECM mimic that was used in this model was Matrigel©.
However, it has been reported that because of the simple gastric structure, it could be advantageous to work with 2D monolayer gastric organoids, where homogenous confluent monolayers generate a near physiological model for studies in drug development and infection diseases [50].
2.3. Liver-on-a-chip
Liver is the largest internal organ in the body and comprises vital functions such as synthesis and metabolism of carbohydrates, proteins, and hormones. It also promotes bile secretion and plays a significant part in inflammatory responses [51, 52]. In more detail, in a non-diseased liver, metabolic and tissue remodeling functions require elements of inflammation. This inflammation, in combination with regular exposure to dietary and microbial products likely creates excessive immune activation. In this complex microenvironment, the hepatic immune system tolerates harmless molecules while at the same time remaining alert to possible infectious agents, malignant cells or tissue damage. Upon suitable immune activation by tissue damage, mechanisms to the resolution of inflammation are essential to maintain liver homeostasis [53].
Liver is the major organ involved in phase I and phase II drug metabolic processes [54]. Abnormal drug metabolism profile could lead to life-threatening complications. Both phase I (mainly CYP450s) and phase II (mainly UDP-glucuronosyltransferase (UGTs)) enzymes play a significant role in drug metabolism. Although metabolites in general are expected to be not active and not toxic, certain metabolites can cause hepatotoxicity. However, metabolism could be affected by a myriad of factors such as pregnancy, age, transplantation, concomitant drugs, among others [54].
DILI is shared by many classes of medicines, therefore leading to drugs withdrawing from the market, resulting in loss of enormous amounts of money, time, and effort invested in the drug development pipeline [55].
The establishment of liver organoids-on-a-chip will therefore deliver promising platforms that can help to advance liver research with applications in regenerative medicine, disease modeling and drug testing, studying adverse reactions of new drugs in an in vivo-like scenario, which is of the upmost importance.
Banaeiyan et al [33] designed and fabricated a large-scale liver-lobule (VLSLL)-on chip device, describing a microphysiological niche for hepatocytes. The device has a network of liver-lobule-hexagonal shaped tissue-culture chambers and universal feeding ports allows for the flow circulation between the layers, mimicking the function of the central vein in a classic liver lobule. HePG2 carcinoma cells were cultured for 5 and 14 d and hiPSC-derived hepatocytes cultured for 21 d [33]. In the case of long-term experiments cells were mixed in a 1:1 ratio with 20% Geltrex® as ECM, instead of the traditionally used Matrigel®. Functionality testes were done: albumin excretion and urea synthesis were tested, as well as cells morphology and, which were kept. Moreover, 3D tissue-like structures and bile-canaliculi features were observed [33].
In another study, a MPOC platform was established using a tree shape-like liver-on-a-chip for the creation of co-gradients and to mimic the liver zonation [34]. As ECM matrix, rat tail collagen type I coating was used. In normal physiology, a glucagon/insulin gradient exists along the liver sinusoid [34]. This microfluidic tissue patterning using opposing gradients in insulin and glucagon, is similar to the in vivo gradients in carbohydrate/glucose metabolism in the liver sinusoid. When testing Acetaminophen, a gradient of cell viability/mitochondrial activity in both primary rat and primary human hepatocytes was observed [34].
Vernetti et al [35] described a platform to test drug safety in a liver model of disease that includes a 3D microfluidic model with sequentially layered cell types (four: primary human hepatocytes along with human endothelial (EA.hy926), immune (U937) and stellate (LX-2) cells); It also contains protein biosensors for mechanistic readouts, as well as a system database to explore obtained data [35]. As a result, exposure to a wide range of drugs produced the expected liver effects (acute or delayed toxicity), confirming that this model can be integrated into a wider database with information regarding chemical and bioactivity, pre-clinical and clinical information [35].
2.4. Pancreas-on-a-chip
The pancreas is a glandular organ involved in the functioning of the whole body. The emerging pancreatic inefficiency is the inability of the pancreas to biosynthesize and/or secrete digestive enzymes enough to digest and absorb food components in the intestines [56]. The pancreas is made up of two types of glands: (i) the exocrine gland secretes digestive enzymes; and (ii) the endocrine gland which consists of the islets of Langerhans. It secretes hormones into the blood [56]. Most examples of pancreas-on-a-chip focus on study and model Type 1 diabetes, study islet function, and pre-clinical drug testing [57]. Human pluripotent stem cell derived islet cells have lately provided an auspicious resource for the study of diabetes. Recently, hPSC-derived organoids have embodied a new whole type of models. Tao et al [36] set the goal to engineer human islet organoids using an organ-on-a-chip platform. The system comprises a multi-layer microfluidic device that permits EB aggregation, pancreatic differentiation and generation of heterogeneous islet organoids under a dynamic 3D culture. Results show that the microfluidic platform provided continuous media and nutrients support, maintain the cell viability of islet organoids during the prolonged perfused 3D culture [36].
CF is a genetic condition triggered by defective CFTR function. Increasing the complexity of organoids microfluidic platforms, Shik Mun et al [37] engineered PDMS-based microfluidic devices (coated with collagen to increase cell adhesion) to grow pancreas-on-a-chip, an in vitro model system with the goal of mimicking the functional link between PDECs and pancreatic islets. The pancreas-on-a-chip allows us to understand cell's interaction efficiently, by means of using a rather low number of patient-derived cells [37]. Efficaciously, co-cultured patient-derived PDECs and islet attenuated CFTR, reducing insulin secretion in about 50%. The above-mentioned pancreas-on-a-chip acts as a pancreatic function monitoring tool and is another good example of an approach for personalized medicine.
The β cells function in the pancreas is impaired in diabetes, being usually evaluated by measuring insulin levels throughout glucose stimulus. Glieberman et al [38] designed and built a thermoplastic microfluidic-based islet-on-a-chip. That platform is automated in islet loading, stimulation and insulin sensing [38]. Achieving flowing suspensions of human cadaveric derived islets inside the platform, the team was able to check capture of islets, fluorescent glucose tracking and quantification of insulin secretion by on-a-chip immunoassay and fluorescence anisotropy [38].
2.5. Inflammation models
Intestinal inflammation has an increasing incidence in the Western world [58].
Inflammation is a normal part of the body's defense to injury or infection, and, therefore, it is beneficial. But inflammation is damaging when it occurs in healthy tissues or lasts too long. Known as chronic inflammation, it may persist for months or years. Some of the reasons that can cause inflammation are environmental chemicals, nutrition, microbiome, among many others [59].
In the stomach, H. pylori is the primary pathogen causing life-long colonization of the gastric mucosa, sometimes causing other diseases such as H. pylori induced chronic inflammation [41]. Boccellato et al [41] established polarized epithelial monolayers from antrum-derived gastric glands (patient derived surgery samples) to study cross-talk with H. pylori and the stroma [41]. Maintenance of these stem cells in vitro via the niche signaling factors Wnt and R-spondin enables long-lived cultures of gastric organoids. Results show that these polarized epithelial layers actually discharge mucus at the surface. Also, they tend to differentiate to a MUC5AC-producing phenotype [41]. Clinically, the role of this mucus secretion can confidently contribute to our understanding and possible drug targeting strategies.
In the gastrointestinal system, it comprises a range of diseases, from the less defined irritable bowel syndrome to full impairment of intestinal function in inflammatory bowel disease (IBD) [60]. A leaky barrier is gradually being appointed as part of these inflammatory and infectious diseases [42]. Apostolou et al [42] used the proprietary Human Emulation System Organ-on-a-chip by Emulate® to develop their system: Colon Intestine-Chip. The Colon Intestine-Chip consists of 2 parallel channels separated by a porous PDMS membrane (diameter, 7 μm; spacing, 40 μm; thickness, 50 μm), and 2 lateral channels where vacuum is applied. The platform is based on human organoid-derived epithelial cells (culture in Matrigel®) and human primary microvascular endothelial cells. The fragmented organoids were introduced in the top channel of the chip and seeded on a membrane uniformly coated with ECM. Their model and data strongly suggest that IL-22 targets the human colonic epithelial cells and disrupts the barrier function, acting as an injurious, rather than a homeostatic or anti-inflammatory factor [42]. A key benefit of organoids is the fairly possibility to uncover the disease phenotypes by direct comparisons between patients' samples [42]. Beaurivage et al [61] developed a model to study IBD (figure 2), which is multifactorial, and therefore difficult to model in vitro. Mechanistically, IBD patients may port genetic mutations in genes involved in the integrity of the intestinal epithelium, which allows microbiota to translocate in the intestinal tissue and initiate an inflammatory reaction. IBD patients lack the required regulatory mechanisms or have abnormal activation of these immune cells, leading to a continuous inflammatory state [61]. The developed Gut-on-a-chip model is composed exclusively of patient-derived human primary material. Monocyte-derived macrophages and human intestinal organoids (HIOs) from different donors were integrated in the model and therefore epithelial cell types derived from the intestinal stem cells recapitulate the cellular diversity of the intestinal in vivo epithelium. The ECM gel used in this study was Matrigel®. Importantly, the transcriptome of the microfluidic gut-on-a-chip resembles that of adult human normal colon in vivo. The team also induced IBD hallmarks by triggering the gut-on-a-chip with lipopolysaccharide and interferon-gamma, leading to the activation and increase cytokine production of both HIO and macrophages (IL-12p70 and IL-6, and the polarized secretion of CXCL10, IL-8 and CCL-20).
Figure 2. Intestinal epithelial cells cultured under microfluidic conditions show higher expression of polarization and differentiation markers. (A) Schematic representation of the model. The top view shows the two perfusion channels flanking the middle channel where an extracellular matrix (ECM) gel is seeded. The transversal view shows the IEC forming an epithelial tubule in the upper channel against the ECM gel. (B) Representative 20X stitched photographs of zonula adherens marker E-CADHERIN, apical polarization marker EZRIN, polarization marker ACTIN and goblet cell marker MUC2 in HIO grown in microfluidic conditions for 10 d. Blue depicts nuclei by DAPI staining. Reproduced from [61]. CC BY 4.0.
Download figure:
Standard image High-resolution image2.6. Small intestine, colon and CRC organoids-on-a-chip
Cancer is still one of the highest causes of death worldwide, despite huge efforts to cure the disease [62, 63]. It is no longer arguable that a better understanding of the tumor microenvironment, as well as a more effective means of screening anti-cancer drug leads are urgently necessary [64–66].
Personalized cancer medicine is an emerging methodology to tailor anti-cancer therapeutic strategies for every patient according to their tumor's genomic portrayal [67]. Hence, there is a pressing demand for research in personalized tumor modeling to confirm its response predictions in the preclinical setting, which is not being achieved with current models [14]. Tumor organoids have emerged as a promising platform since cellular and molecular heterogeneity of tumor cells is conserved due to their stem cell content [14]. Tumor organoids of CRC are essentially 3D cultures resultant from tumor tissues, but can also be derived from differentiation of pluripotent stem cells.
The most comprehensive application in the attempt of personalized cancer therapy using tumor organoids was reported by van de Wetering et al [68], who organized an extensive organoid biobank of colon cancers. Exome examination has shown that colon tumor organoids actually conserve the cancer subtypes detected in the tumor sections. Although this work does not comprise microfluidic platforms, it is a useful base for future applications on chip.
Sontheimer-Phelps et al [40] isolated epithelial cells from colon resections, developed them as colon organoids in Matrigel® [40] and placed them on a chip, to develop an in vitro model of colon mucus physiology. The chips are composed of PDMS, with 2 parallel microchannels (apical channel and basal channel, separated by a 50 μm thick PDMS porous membrane). In the chip, the mucous layers presented characteristics similar to in vivo, such as thickness and the presence of proliferative epithelial cells. In this case, the organoid-on-a-chip presented benefits when compared to their intestinal organoid counterpart, including the ability to continuously collect samples from both the luminal and albuminal sections. Moreover, PGE2-induced mucus layer swelling on-a-chip, as happens in vivo.
Nikolaev et al [39] developed a 3D animal organoid-on-a-chip, which is based on a hydrogel (mix of Matrigel and collagen type I). The microdevice aimed at development of mini-guts because of its mini-gut-like geometry. The hydrogels are in a perfusable platform to generate a hybrid microchip system that consists of an elastomeric device with a central chamber for hydrogel loading and subsequent organoid culture. The team was able to maintain tubular intestinal epithelia for several weeks without passaging, eliminating the dead cells by perfusion.
One of these examples was developed by Lee et al [32] that established a 3D human gastric organoid platform through hiPSCs [32]. This platform was composed of two perfusable microchambers (upper and lower) separated by a porous PDMS membrane (>10 μm thickness), and was used to evaluate absorption of orally administrated drugs. The flexible PDMS membrane was coated with ECM and lined by human Caco-2 intestinal epithelial cells. This system allowed for the evaluation of orally administrated drugs absorption, as well as to rhythmically stretch and contract the organoid, as it happens in vivo. This was achieved by applying suction to both microchambers, in an attempt to mimic the mechanically active microenvironment of the stomach and small intestine [69]. Moreover, this 3D organoids-on-a-chip presented the capabilities for long-term time-course optical imaging.
2.7. Multi organoids-on-a-chip
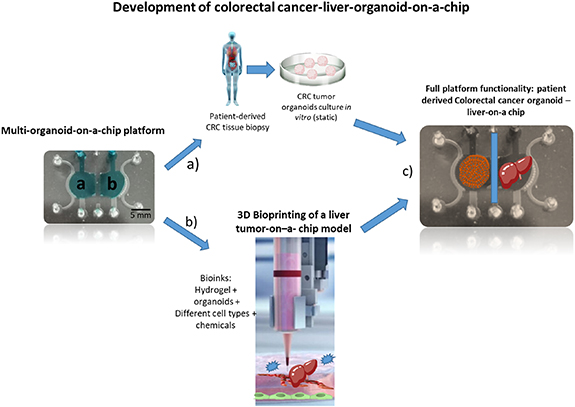
Gastrointestinal organs have a vital importance, and as such, can be used to study a wide array of phenomena, especially in combination with other organs. These progresses have similarly exposed new challenges and opportunities to fully appreciate and concretize the promise of multi organoids-on-a chip for fundamental studies and translational applications [70].
There are many ways in which multi-organoids can be designed and engineered in order to best tackle a specific research question. The microfluidic design can change, being more or less complex, having a place for endothelial channels, and even mix organoids-on-a-chip with organs-on-a-chip, depending on the complexity of what is envisioned to be achieved, as schematically depicted in figure 3.
Figure 3. Original schematics representing multi-organoid-on-a-chip. This example (non-published data) comprises two large chambers separated by a microchannel (to hold vascularized tissue). The chambers will hold: (a) patient-derived colorectal tumor organoids; (b) a 3D bioprinted liver tissue; and (c) after assembly, full functionality of colorectal cancer-liver organoid/central endothelial channel functionality will be assessed. Observation of the natural tendency of migrating cells (metastasis) from colorectal cancer organoid to the liver organoid, drug testing (personalized medicine) will be performed in this model.
Download figure:
Standard image High-resolution imageEsch et al [71] established an expandable body-on-a-chip system featuring gastrointestinal tract and liver. Fluidic flow through the organoids-chips is driven via gravity and controlled passively via hydraulic resistances of the microfluidic channel network. The resistance through the GI tract cell layer was successfully measured using the Ag/AgCl electrodes. The transepithelial resistance reached values between 250 to 650 Ω cm2 during the co-culturing (14 d) [71]. The developed two-tissue body-on-a-chip platform that combines GI tract tissue, liver tissue constructed from primary cell sources, and blood surrogate in a near physiologic relationship with each other. Results show that measurements of urea and albumin production, and of CYP enzyme induction point out that the liver tissue maintained high levels of metabolic activity [60].
Another relevant example was performed by Yin et al [45], who engineered a multi-organoid-on-a-chip system derived from hiPSCs, allowing the study of cardiac toxicity of an antidepressant drug after liver metabolism [45]. The aforementioned platform comprises liver and heart organoids-on-a-chip platform and is made of compartmentalized chambers separated by a porous membrane, which consists of a top layer of PDMS, through-hole PDMS layer, polycarbonate porous membrane and PDMS bottom layer (figure 4(i). The co-culture of 3D human liver organoids takes place in the upper chamber and cardiac organoids culture happens in the bottom chamber (figure 4(ii)) [45]. In the platform, when exposing the liver organoids to the antidepressant clomipramine, the heart organoids started to present signs of decreased cell viability, as well as compromised functions of cardiac beating. That study mirrors human organ's particular roles and their intricate and interdependent reactions. Although it is just a proof of concept, it provided drug safety assessment in a physiologically relevant platform [45].
Figure 4. Multi-organoids-on-a-chip. (i) Modular device design. Every chip is seeded with cells that characterize the desired organ: Caco-2 cells were seeded for the GI tract; and human primary liver cells seeded for liver organoids. Reproduced from [71] with permission from the Royal Society of Chemistry. (ii) (a) Schematic diagram of antidepressant drug clomipramine and its metabolites; (b) design of multi-organoids-on-a-chip device, which consists of a top layer, through-hole PDMS layer, polycarbonate porous membrane and bottom layer; and (c) Heart organoids were formed by in situ differentiation and generation from hiPSCs on the bottom layer, and the day-20 hiPSC-derived liver organoids were then seeded into the top layer. Reproduced from [45] with permission from the Royal Society of Chemistry.
Download figure:
Standard image High-resolution imagePerhaps one of the most complex multi-organoid-on-a-chip was developed by Novak et al [46]. The team described a system that employs liquid-handling robotics, custom software and an integrated mobile microscope for the automated culture, perfusion, medium addition, fluidic linking, sample collection and in situ microscopy imaging. The system maintained its viability and organ-specific functions of intestine, liver, kidney, heart, lung, skin, blood–brain barrier and brain for 3 weeks in culture. After functionality was assessed in all organs, two small size drugs were tested in the system: cisplatin and nicotine. This microphysiological system has been developed to create a model that incorporates drug transport and metabolism with subsequent in vitro-in vivo translation [46]. It composed of PDMS or polycarbonate and contains two parallel, continuously perfusable microchannels separated by a porous membrane, which are seeded with living human organ-specific parenchymal cells and vascular endothelial cells on either side of the membrane to create a tissue-tissue interface (supports vascularization). Results show that the system has capability of evaluating drug pharmacokinetics (PK)/pharmacodynamics (PD) in a first-pass and was able to recapitulate clinical drug profiles and PK parameters for the two drugs (nicotine and cisplatin) [46].
3. Impact of organoids-on-a-chip technology on drugs validation and pharmaceutical R&D costs
The cost of R&D burdens society by limiting innovation and keeping drug prices high, being a matter of discussion worldwide between doctors, Pharma and scientists. Being a relatively new field, the R&D cost on organs and organoids-on-a-chip and efficiency data are rather sparse but relevant for all stakeholders [72].
It is well described in the literature that the vast majority of pharmaceutical 'hits' are not successful between its course down the pipeline between animal testing and clinical trials. The one successful drug, since it is considered a promising 'hit' until it is launched commercially takes between 12 and 15 years and can often exceed 1 billion euros in costs [73]. Importantly, even those that enter the market can face several problems afterwards.
An example of this is that happened in Merck, a prominent pharmaceutical company which failed to accurately evaluate the drug's effect and safety of Vioxx (Rofecoxib), a COX-2 selective inhibitor NSAID, resulting in large number of adverse reactions in patients [74]. As a result, Merck was forced to pay $4.85 billion to resolve 27 000 law suit cases from patients suffering from adverse reactions [74].
More than financial costs, adverse drug reactions experienced in treated people are a major reason of hospitalization in the United States, with 5% estimated hospitalizations associated to these reactions [75]. The critical subject of quantifying the human life loss due to fatal adverse drug reactions is challenging, with reports suggesting that the adverse reactions are the 4th cause of death in USA, certainly generating considerable debate [76]. As adverse reactions and drug discovery and development budgets rocket as a result of inaccurate data generated by in vivo and in vitro preclinical models, more consistent and cheap drug screening tools become more critically needed. It is in this scenario that organs and organoids-on-a-chip come to life. A promising technology that aims at renovating and re-designing R&D, decreasing costs and potentially save lives. This new organoid-on-a-chip technology may speed up a paradigm-shift in drug development pipeline and as a consequence, personalized medicine (figure 5). Organoid-on-a-chip can model the system of interest to pinpoint drug targets in a more controllable and traceable way than animal models [77].
Figure 5. Example of application of organoids-on-a-chip for early drug discovery, pre-clinical testing and pre-clinical trial and translation. (a) Vascularization mechanisms; (b) tumor-immune-vascular interactions; (c) mechanical forces and their role in cancer; (d) prediction of the PK parameters; (e) adverse drug reactions studied in liver model. (f) A multi-organ platform to analyze multi-organ toxicity. Reprinted from [79], Copyright (2020), with permission from Elsevier.
Download figure:
Standard image High-resolution imageImportantly, in the drug validation pipeline, PK and PD studies follow the 'hit' identification. The drug concentrations at different vital organs during the metabolic processes, known as absorption, distribution, metabolism, and elimination is performed, as well the effects of the drug (e.g. the relationship between drug dose and pharmacological or toxicological response) on target organs or tissues in very meticulous and fail-prone methods. Multi-organoids-on-a-chip systems consist of major metabolically active organ compartments can be of particular benefit for systematic and in situ PK–PD studies across diverse human organ sites and under various drug administration (figure 5). This has been demonstrated in the Multi organoids-on-a-chips [46].
However, experts are divided to whether these models will replace or instead bridge the gap between pre-clinical testing and human trials, serving as better intermediate predictive model, and hopefully helping to decrease the late failure of drugs [78, 79].
A recent study combined experts' opinion to estimate this technology's impact on R&D cost in terms of costs-per-project, and success rate and cycle time and R&D phase, within a framework of 5 years [72]. Results suggest that 73% of experts believe in a significant positive influence on the cost-per-project, and 80% impact on the success rates, where the preclinical phase showed the highest applicability, followed by the lead optimization phase [72]. Scientists also believed an R&D cost reduction of 10%–26% was possible when applying these technologies [72].
Undoubtedly, challenges in terms of automation, parallelization, standardization and ease of use are crucial hurdles to be solved prior to the adoption at an industrial and clinical level.
4. FDA-approved models, clinical trials and future perspectives
Organoids (not organoids-on-a-chip, yet) are being recently used in parallel with clinical trials (called co-clinical trials). The aim of co-clinical trials is to study and relate drug responses in organoids to clinical outcomes in the corresponding patients. After a tumor biopsy is obtained, it is typically divided into four pieces. The pieces then serve different purposes: organoids formation, DNA sequencing and histological analysis. Sequencing and histological analysis are performed for both the patient tissue and its corresponding organoid, to confirm mutational and biomarker similarities [77].
It is noteworthy that this section does not focus only on organoids-on-a-chip, since there are not enough examples to make a section out of it. Nevertheless, it is possible to find many clinical trials using organoids to test personalized medicine against cancer, and a single one using organoid-on-a-chip [80].
Recent results have now stated the positive and effective use of organoids to foresee treatment response, namely in oncological patients [77, 81]. Moreover, organoids have been adopted to predict treatment response to radiotherapy and immunotherapy [77, 82]. The development of high throughput drug screening technology can advance the use of patient-derived organoids in clinical practice and facilitate therapeutic decision-making.
Moreover, the scholars have already described how patient derived organoids with cells' stem-like properties preserve the chemoresistance and genetic mutations observed in the original tissue [82].
Scientists check the usefulness of organoids by scrutinizing the in vitro to in vivo correlations [77]. A key factor supporting the clinical applicability of organoids is their efficiency and reproducibility, allied to the fact that the results correlate to patients outcome, making this technology a promising one for the direct application into the clinics, as well as a valuable in speeding the drug discovery process [81]. Besides adding to help to select the correct therapy for patients, organoid models can also avoid cancer patients from receiving ineffective treatments and consequently their often-severe side effects. An example of this was reported by Ooft and Weeber [83], which demonstrated the use of patient derived organoids in preventing patients from receiving ineffective irinotecan-based chemotherapy. These examples prove that the translation into the clinics (bench to bedside) is already a reality and must be pursued: patient-derived tumor organoids could facilitate personalized medicine.
By searching on ClinicalTrials.gov website, it becomes obvious that organoids are now widely used in clinical trials (table 2) [80, 83–87]. Gastrointestinal organoids are no exception, with clinical trials using patient-derived pancreatic, colorectal, esophageal and liver cancer organoids to investigate chemotherapy [88]. Regarding the gastrointestinal system, an ongoing clinical trial will use chemotherapy guided by organoid drug sensitivity tests for advanced pancreatic cancer patients [85]. Refractory CRC patients will have their organoid derived from biopsies subjected to a 10-drug panel screening [84]. Regarding gastric and esophageal carcinomas, after developing organoids, a correlation of in vitro response in the organoid model with histological regression in the resected tumor will be performed [86].
Table 2. Patient-derived organoids models for drugs testing and clinical trials.
| Model | Drugs tested | Outcomes of clinical trial | Reference and identifier |
|---|---|---|---|
| Patient-derived organoids of metastatic colorectal cancer. | Irinotecan-based therapies; 5-fluorouracil plus Oxaliplatin. | Successful translation of irotecan, not successful translation of 5-FU + OXO. | [83] NCT05304741 |
| Non-small cell lung cancer—CTCs in a chip. | N/A—if the EGFR mutation can be detected in CTCs | N/A | [87] NCT01734915 |
| Organoid-guided chemotherapy for advanced pancreatic cancer. | Gemcitabine, 5-fluorouracil, Paclitaxel, Oxaliplatin, Irinotecan. | 6-month disease control rate; overall survival time; concordance between drug sensitivity test results and patients' treatment response. | [85] NCT04931381 |
| Selecting chemotherapy with high-throughput drug screen assay using patient derived organoids in patients with refractory solid tumors (SCORE). | 5-fluorouracil, carboplatin, cyclophosphamide, docetaxel, doxorubicin, gemcitabine, irinotecan, oxaliplatin, paclitaxel and vinorelbine. A further 5 drugs (etoposide, ifosfamide, methotrexate, pemetrexed and topotecan) will be screened if sufficient organoids are grown from the biopsy samples within the screening period. | Drug panel screening in organoids; gene analysis and immunohistochemistry analysis of biomarkers. | [84] NCT04279509 |
| Outcome prediction of systemic treatment in esophagogastric carcinoma (OPPOSITE). | Non-applicable. | Correlation of in vitro response in the organoid model with histological regression in the resected tumor; correlation of in vitro response in the organoid model with overall survival. | [86] NCT03429816 |
| Organoids-on-a-chip for colorectal cancer. | 5-FU; Oxaliplatin; Irinotecan; 5-FU+ Oxaliplatin; 5-FU+ Irinotecan; 5-FU+ Oxaliplatin+ Irinotecan; 5-FU+ Cetuximab Cetuximab Regorafenib. | Validate tumor organoids and organoid chips by morphology (hematoxylin and eosin (HE), immunohistochemical staining) and gene sequencing (target-seq) methods; local recurrence rate, disease progression-free survival rate and overall survival rate. | [80] NCT04996355 |
It becomes clear from the previous referred FDA approved studies that using microchips for several applications (e.g. tumor markers measured by a lab-on-a-chip in cervical cancer; testing participants' blood to try and find CTCs with the EGFR mutation in breast cancer), will soon fully open the door for future trials using organoids-on-a-chip for drugs testing [87]. Although not yet available for gastrointestinal system.
In fact, the first clinical trial to ever study drug responses on organoid-on-a-chip started earlier 2021, namely using a CRC organoids. The goal is to test the accuracy, specificity and sensitivity of in vitro drug sensitivity screening based on organoids-on-a-chip to predict the effect of chemotherapy (traditional chemotherapy, targeted therapy) for advanced CRC [80]. Single or combination of drugs will be tested over two years and compared to the sample donor's outcome. If this clinical trial reveals successful, medical treatment will suffer changes, namely by the introduction of microfluidic chip platforms combined with patient derived organoids to predict drug response will become a reality in the near future.
Keeping up with this reality, companies specialized in microfluidics organs/organoids-on-a-chip such as Emulate Inc®, Mimetas® and StemoniX® have moved from the labs into the market to bridge the gap between the microfluidic systems and the pharmaceutical companies. Namely, Emulate Inc® has joined forces with Johnson & Johnson, Roche, Takeda, Merck and FDA for the effectiveness validation of their various products [89, 90]. Moreover, Emulate Inc® announced earlier 2021 an additional $82 million financing by existing investor Northpond Ventures with additional participation from Perceptive Advisors. With this investment, Emulate Inc® has raised nearly $225 million to date [91]. Takeda is currently using an Intestine-on-a-Chip to discover and evaluate gastrointestinal disease drugs and biomarkers, revealing the strategic importance of gastrointestinal organoids to better understand the physiology of organs involved in drug response, as well as its connection to the rest of the human body well-being [92].
5. Conclusions
The current advances in GOoC technology have allowed to expand our understandings of human organ development and have become a useful tool to model human diseases and test therapeutics in vitro. The complexity of the models is growing, combining different technologies (bioprinting, different ECM materials, going from a simple organoid-on-a-chip to a body-on-a-chip). There is an increasing number of scientific reports showing strong correlations between patient-derived organoids and organoids-on-a-chip responses and patient responses, namely regarding cancer treatments. These can facilitate the widespread use for drug discovery and personalized medicine. If we really want to achieve a successful translation, these platforms should be also designed to model tissue/organ properties and biological/physical cues, in the future. In a time where standardization requirements are a must, the science field needs to adopt fabrication techniques and standardization for these platforms, which will dictate their acceptance by end-users, medical staff and most importantly, by the regulatory bodies such as FDA. This will require the generation of reproducible organoids with respect to cell-type composition and arrangement, and overall organoid structure and organization (utilization of bioprinting and other technologies).
From the economic point of view, as it has been described by the authors, the financial cost of taking a drug through the traditional pipeline and then observe it fails or has dangerous secondary effects after approved, is overwhelming. That is why Pharma industry is partnering-up with commercial microfluidic companies, to include them in the pre-clinical validation. As for the clinical trials, the presence of organoids and the first organoid-on-a-chip co-clinical reveals that this combination of technologies will continue gaining relevance among the scientific/medical community, and have a real impact in patient's lives in a near future.
Data availability statement
The data cannot be made publicly available upon publication because no suitable repository exists for hosting data in this field of study. The data that support the findings of this study are available upon reasonable request from the authors.
Grant support
Mariana Carvalho acknowledges her post-doctoral contract TERM RES Hub—Scientific infrastructure for Tissue Engineering and Regenerative Medicine Ref Norte-01-0145-FEDER-02219015. C H Z thanks The Special Sustainable Development Science and Technology Project of Shenzhen (KCXFZ202002011010593). Y L H thanks Regional Joint Key Project of National Natural Science Foundation of China (U20A20379) and support from the Guangdong Provincial Key Laboratory of Digestive Cancer Research (No. 2021B1212040006). L P Y also thanks the Open Key Grant Project from the Guangdong Provincial Key Laboratory of Digestive Cancer Research (GPKLDCR202201Z). This work was partially supported by the IET A F Harvey Engineering Research Award 2018 (ENG ThE CANCER).
Conflict of interest
The authors declare no competing interests.
Consent for publication
Not applicable at this point of submission.
Important references
8- This paper offers an extensive review and discussion about the time and cost that traditional pipeline of drug-discovery takes, together with its advantages and disadvantages. Namely, the need to use models to complement and reduce drug approval and life-years saved is considered critical.
25- This paper offers novelty by generating human gastric organoids via directed differentiation of hPSCs and the possibility to rhythmically introduce stretch and contraction to the organoid inside the chip.
52- This paper is of the utmost importance, because although it is not about organoids-on-a-chip, it mentions the establishment of a living CRC organoid biobank based on cultures from 20 consecutive CRC patients and its genetic evaluation.
65- This paper discusses how the organ-on-a-chip technology can have critical roles in different preclinical stages of drug development and highlights the current challenges in translation and commercialization of this technology for the pharmacological and medical end-users.
66- This reference mentions the first clinical trial comprising an organoid-on-a-chip for drug testing, which is ongoing.