Abstract
The Peppermint Initiative seeks to inform the standardisation of breath analysis methods. Five Peppermint Experiments with gas chromatography-ion mobility spectrometry (GC-IMS), operating in the positive mode with a tritium 3H 5.68 keV, 370 MBq ionisation source, were undertaken to provide benchmark Peppermint Washout data for this technique, to support its use in breath-testing, analysis, and research. Headspace analysis of a peppermint-oil capsule by GC-IMS with on-column injection (0.5 cm3) identified 12 IMS responsive compounds, of which the four most abundant were: eucalyptol; β-pinene; α-pinene; and limonene. Elevated concentrations of these four compounds were identified in exhaled-breath following ingestion of a peppermint-oil capsule. An unidentified compound attributed as a volatile catabolite of peppermint-oil was also observed. The most intense exhaled peppermint-oil component was eucalyptol, which was selected as a peppermint marker for benchmarking GC-IMS. Twenty-five washout experiments monitored levels of exhaled eucalyptol, by GC-IMS with on-column injection (0.5 cm3), at t = 0 min, and then at t + 60, t + 90, t + 165, t + 285 and t + 360 min from ingestion of a peppermint capsule resulting in 148 peppermint breath analyses. Additionally, the Peppermint Washout data was used to evaluate clinical deployments with a further five washout tests run in clinical settings generating an additional 35 breath samples. Regression analysis yielded an average extrapolated time taken for exhaled eucalyptol levels to return to baseline values to be 429 ± 62 min (±95% confidence-interval). The benchmark value was assigned to the lower 95% confidence-interval, 367 min. Further evaluation of the data indicated that the maximum number of volatile organic compounds discernible from a 0.5 cm3 breath sample was 69, while the use of an in-line biofilter appeared to reduce this to 34.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Volatile organic compounds (VOC) in exhaled breath have been reported as non-invasive indicators of health since 1970 [1, 2], and many exhaled compounds have been proposed as biomarkers [3–7]. However, the variation in reported values is large and in some cases as high as a factor of 1,000 [8, 9]. This arises from: the variability between, and within, individuals as well as from sampling and analysis methods. Standardisation is a crucial step for further development and translation of breath research into clinical and deeper-research applications. The International Association for Breath Research is addressing this challenge with the Peppermint Initiative; an international multi-centre benchmarking study seeking to provide a set of comparative data [10].
The Peppermint Experiment establishes a 'peppermint' background before inducing a measurable chemical change in the breath of participants with a standardised dose of ingested peppermint-oil, followed by the collection of breath-samples for a further 6 h. A detailed description of the washout experiment and rationale behind the Peppermint Experiment is given in an introductory paper [10]. This work presents the first results from running the Peppermint Experiment with gas chromatography-ion mobility spectrometry (GC-IMS) systems.
IMS is a gas-phase detection technique which distinguishes compounds based on the differences in ion mobilities in an electric-field under controlled conditions [11]. The technique was originally developed for military and security applications to detect traces of explosives, narcotics and chemical warfare agents [12]. Its applications have since been expanded to encompass use-cases requiring trace-detection of VOC [13–15], as well as an adjunct to mass spectrometry [11]. Coupling IMS with GC significantly increases the dimensionality of the analysis by combining the analytical-selectivity from high-resolution chromatographic separation with the analytical-sensitivity of IMS (limits of detection range from 0.2 µg m−3 to 2 mg m−3, with analyte ionisation chemistries and ionisation sources as important operational factors). GC-IMS has subsequently been used to study breath VOC in lung cancer [8], chronic obstructive pulmonary disease [16], asthma [17] sarcoidosis [18], and recently, COVID-19 [19]. This portable technique is a promising candidate for diagnostic and research applications undertaken at point-of-care and point-of-need settings.
This work seeks to provide GC-IMS benchmark values for the Peppermint Experiment.
2. Methods
2.1. Ethics
This work was undertaken in accordance with the Helsinki Declaration and was approved by: Loughborough University Independent Ethics Committee (Ethics No: G09-P5); Radboud University, Ethics Committee Science (ECS17012); University of British Columbia, Ethics Committee (H19 02114); Southeast Scotland Research Ethics Committee 01 (16/SS/0059); and Oslo Regional Committee for Medical and Health Research Ethics (2016/698/REK North).
Informed written consent was obtained from each participant.
2.2. The Peppermint experiment
Three laboratories ran the GC-IMS Peppermint Experiment where a reference breath sample was analysed by GC-IMS before ingestion of a peppermint oil capsule (Product no. 10115320, Boots UK Ltd) at t = 0 min, followed by a further five GC-IMS breath measurements at t + 60, t + 90, t + 165, t + 285, and t + 360 min, [10] (Studies 1–4, figure 1 and table S1 available online at stacks.iop.org/JBR/16/036004/mmedia). Additional peppermint washout data were acquired during proficiency-testing for breath analysis undertaken as part of the H2020 funded project TOXI-triage [20]. In these ancillary clinical studies, a limited group of participants provided peppermint GC-IMS washout data (Studies 5 and 6, figure 1 and table S1) with breath samples collected at t = 0 min, and then at t + 60 min followed by collection at every 30 and/or 60 min intervals, over a period of 5 h.
Figure 1. Summary of the GC-IMS preliminary Peppermint-Benchmark study. Note: N—number of recruited participants/the number of peppermint experiment washout tests they provided; n—number of samples collected.
Download figure:
Standard image High-resolution image2.3. Breath sampling
2.3.1. Method 1 (Studies 1, 4–6)
This method adopted a robust, easy-to-use single use disposable sampler for use in an acute or emergency care setting [19, 20]. A single breath was collected with a disposable Haldane tube breath-sampler (GAS Dortmund), made from two one-way mouthpieces (ACE Instruments) and a 10 cm3 Eppendorf tube drilled and cut to accept a 5 cm3 polypropylene syringe tip and fit the mouthpieces (figure 2 Top). Immediately before sampling, the breath-sampler was assembled, fitted with a 5 cm3 polypropylene syringe (Norm-Ject, DE) and handed to the participant to provide a breath-sample. The participant was coached in how to provide a sample by breathing out slowly through the tube and 'empty-their-lungs'. At the end of expiration, the plunger of the syringe was withdrawn to collect 5 cm3 of the end-tidal portion of the exhaled breath. The syringe was immediately removed from the sampler and fitted to a disposable 3-way polypropylene stopcock connected by 4 cm of 1/8'' stainless steel transfer line tubing to the injection port of the instrument with a 1/8'' stainless steel Swaglok® fitting. The polypropylene stopcock protected the inlet of the GC-IMS from possible atmospheric contaminants and aerosols. It was also possible to fit a luer lock syringe body filled with adsorbent to the third port to prevent possible vapour ingress while enabling the sample inlet pump to continue to run and ventilate the inlet lines with purified air, The inlet port was tubing was fitted into a heated block to prevent cold-spots ( T 4 in table S2 and figure S1) Environmental air samples were collected using the same type of syringe and injected into the GC-IMS in the same way.
Figure 2. Top: Haldane breath-sampler made of cut Eppendorf tube (B) and two one-way mouthpieces (A) with polypropylene syringe (C) in 1:0.55 scale. Bottom left: Breath collection method. Reprinted from [19], Copyright (2020), with permission from Elsevier. This method was used in Centres 1 and 3. Bottom: Schematic of the breath sampling system used in Centre 2, with Loccioni® CO2 sensor directly connected to the GC-IMS transfer line. A—Bacterial mouthpiece, B—CO2 sensor, C—Orifice, D—Connection line between CO2 sensor and Loccioni® breath-sampler, E—Heated inlet line to GC-IMS.
Download figure:
Standard image High-resolution image2.3.2. Method 2 (Study 2 and 3)
Breath-samples were collected with a commercial Loccioni® CO2 sensor-controlled unit coupled to a heated line of the GC-IMS (figure 2 Bottom). The Loccioni® sampler provides control over the sampling parameters, such as exhalation flow, and the phase of breath that is sampled. Exhaled CO2 concentration was monitored whilst the participant maintained a constant exhalation flow. Participants were asked to exhale for a minimum of 20 s at 50 cm3 s−1 before starting the analysis program. Monitoring the CO2 and exhalation flow ensured that only the end tidal portion of the breath was sampled and analysed.
2.3.3. Sampling safety precautions
This study was undertaken before the current pandemic outbreak; nevertheless, infection control safety procedures were used to protect participants and researchers. Nitrile gloves were worn when handling samplers and whenever participants were present. The GC-IMS was cleaned and wiped down with a paper towel wetted with distilled water after each sample was run. The Haldane samplers were single use disposable items (figure 2 Top), disposed accordingly to local procedures for waste management (clinics), or as general laboratory waste for studies undertaken in university laboratories. For the breath collection with in-line sampling (figure 2 Bottom) a new disposable bio-HEPA filter was attached between participant and the Loccioni® sampler for each participant. The filter was disposed through general laboratory waste.
Since the onset of the SARS-CoV-2 pandemic, new guidelines for breath sampling and analysis have been prepared and evaluated, and these have been reported in a separate technical note [21]. Fixed sampling lines are now heated to above 60 °C for more than 20 min between participants [22]. Gas exhausts from GC-IMS systems are now vented through a HEPA bio-filter.
2.4. Instrumentation
The BreathSpec GC-IMS breath analyser was used in all studies (GAS Gesellschaft für analytische Sensorsysteme mbH Dortmund (GAS Dortmund)). The system consists of an IMS coupled to a gas chromatographic column supplied with purified air for the GC and IMS from a circular gas flow unit (CGFU; figure S1) containing a pump ( P ) and gas purification filters ( F1 to F3 ). The IMS was fitted with a tritium (3H) beta ionisation source, (5.68 keV, 370 MBq) and was operated in the positive mode. The drift gas flow for the IMS was maintained using an electronic pressure control unit ( EPC1 ). A 30 m long 0.52 mm internal diameter (ID) capillary column with a 1 µm thick trifluoropropylmethylpolysiloxane stationary phase FS-OV-210 was used for Studies 1, 2, 3, 5, and 6, and a 30 m long, 0.53 mm ID and 0.5 µm thickness MXT-WAX (Study 4) were used for chromatographic separations. The carrier gas flow for the column was maintained with a second electronic pressure control unit ( EPC2 ). Both the IMS and capillary column were heated ( T1 and T2 ). Injected breath-samples (figure 2: Top) were drawn into a 1 cm3 heated stainless steel sample loop fitted to a heated 6-port-valve ( V , T3 ) by an internal pump ( P ). Switching the 6-port valve injected the breath-sample onto the column (figure S1) that eluted into the ionisation region of the IMS through a heated transfer line ( T5 ).
The centres used slightly different methods with some variations in injection-time, injection-volume, chromatographic conditions, gas-flows and temperatures across the GC-IMS systems, and these are summarised in table S2. Chromatographic separation was controlled by carrier-gas pressure programming and the IMS drift gas flow was set to 150 cm3 min−1 in all instances. The six port-valve was set to the loading Position A and switched to the injection Position B at the start of the analysis.
2.5. Verification and calibration
The GC-IMS response to the peppermint-oil capsule was evaluated by opening the gelatine shell and transferring the peppermint oil to a 20 cm3 headspace vial that was immediately sealed. The vial was maintained at 39 °C for 20 min before 0.5 cm3 of headspace was withdrawn and serially diluted 100-fold before injecting 1 cm3 of the diluted headspace into the GC-IMS. This procedure was undertaken using the Study 1 and 4 methods (table S2).
The GC-IMS sample inlets were modified for injections of test atmosphere standards and peppermint capsule headspace samples by: replacing the breath-sample inlet with a stainless steel injection port fitted with a GC septum enabling the sample loop to be flushed and loaded directly with a gas sample for injection onto the column for the Study 1 set up; directly connecting to a diluted mixture of gas standards (Prepared for this study by the National Physical Laboratory, Teddington, UK.) for the Study 2 and 3 configuration; and, for Study 4, directly injecting diluted headspace vapour standards into the sampling port of the instrument.
In Study 1 (table S2) eucalyptol, α-pinene and β-pinene, menthone and menthol were used to make individual gas-standards by injecting 10 µl of each compound into a sealed 20 cm3 headspace vial maintained at 39 °C in a heated block. After 20 min equilibration, the standard had vapourised and mixed into the vial, and 1 cm3 of the test-atmosphere was extracted with a headspace syringe followed by serial dilutions with environmental air to produce gas-phase concentrations over the range 20–643 µg m−3. Additionally, a mixture of these standards was produced by mixing 1 cm3 of each standard vapour into a 20 cm3 headspace vial.
For Study 3 (table S2, figure S2), a gas cylinder containing a traceable standard mixture of eucalyptol, menthone, α-pinene, and R-(+)- limonene at 500 ppb (v/v) was prepared by the UK National Physical Laboratory and used to provide a traceable calibration. The gas standard mixture, operating within a flow range between 3.3 and 16.7 cm3 min−1, was diluted with a clean air supply (66.7–80 cm3 min−1) to generate a range of concentrations from 129 µg m−3 to a maximum of 643 µg m−3.
In Study 4 (table S2), permeation sources were made from 8 mm crimp-top chromatography vials fitted with an aluminium crimp seal fitted with a polytetrafluoroethylene (PTFE) septum (Thermo scientific C40086A) containing either eucalyptol, α-pinene or β-pinene. These were maintained at 20 °C and gravimetrically calibrated for more than 6 weeks (200 ng min−1) before use. Static headspace test-atmospheres were generated by placing a permeation tube, or tubes, into a 100 cm3 airtight glass mixing vessel for 1 min, to produce vapour concentrations of 2 mg m−3. 1 cm3 of the resultant permeation tube headspace within the mixing vessel was withdrawn with a 5 cm3 polypropylene syringe, and then serially diluted to concentrations across the range 30–550 µg m−3. 1 cm3 of the diluted standard-headspace was injected into the inlet of the instrument and analysed using the Study 4 method.
2.6. Quality control and data evaluation
2.6.1. Study 1
Statistical process control was used to evaluate and verify instrument performance. Before the start of the Peppermint Experiment, nine instrumentation and responses (table S3) were recorded from 20 environmental blank runs collected over 5 d using the Study 1 method (table S2). Table S3 summarises the data collected that were used to evaluate the  -scores used to monitor the GC-IMS performance throughout the Peppermint Experiment study (figure S3). Thirty QC runs were performed, and no response was observed to exceed the
-scores used to monitor the GC-IMS performance throughout the Peppermint Experiment study (figure S3). Thirty QC runs were performed, and no response was observed to exceed the  limit. The drift time of the RIP increased to a value of 7.48 ms from 7.34 ms, indicative of an increase in the water concentration in the drift gas over the course of 59 breath-samples; however, this was not statistically significant, and no other changes in the performance of this instrument were observed throughout Study 1. Additionally, breath samples were evaluated against a minimum intensity level for the acetone signal intensity (2.5 V) to evaluate injection/sampling process. All of the samples in the data set 1 passed this evaluation criteria.
limit. The drift time of the RIP increased to a value of 7.48 ms from 7.34 ms, indicative of an increase in the water concentration in the drift gas over the course of 59 breath-samples; however, this was not statistically significant, and no other changes in the performance of this instrument were observed throughout Study 1. Additionally, breath samples were evaluated against a minimum intensity level for the acetone signal intensity (2.5 V) to evaluate injection/sampling process. All of the samples in the data set 1 passed this evaluation criteria.
2.6.2. Study 3
Note that the data from Centre 2's first study (Study 2; ten washouts) were discarded during the retrospective evaluation phase when it became clear that the peppermint oil features were suppressed. The sampling system was reinstalled with an updated sampling method and a further six washouts were collected as Study 3; this time, the expected signals were observed; demonstrating how the Peppermint Experiment concept rapidly identified that the analytical system was not operating optimally.
The instrument performance responses in table S3 were evaluated retrospectively using the data from the daily environmental blank runs collected at the beginning, and the end, of each day of analysis over the course of the Peppermint Experiment. The results are summarised in table S3 and figure S3, and indicate that no significant change in the instrument was observed over the course of the studies. The level of acetone in the breath samples was also evaluated, and all the samples passed the minimum intensity level criteria.
2.6.3. Study 4
Statistical process control and acetone evaluation was used to evaluate the instrument performance and breath sampling over the course of the study; similar to Study 1. Two QC environmental samples were run each day during the campaign and were scored for nine spectral and operational responses. The data are summarised in table S3. All responses were within  limit, and no significant change in the instrument was observed over the course of Study 4. All of the samples passed minimum intensity level for acetone presence in breath, figure S3.
limit, and no significant change in the instrument was observed over the course of Study 4. All of the samples passed minimum intensity level for acetone presence in breath, figure S3.
2.7. Data processing
Data were visualised using Excel, Matlab and Origin, while commercial and proprietary software (LAV software version 2.2.1; GAS Dortmund) was used to identify, extract and integrate the GC-IMS peak volumes for features of interest. The reactant ion peak (RIP) with known mobility was used as an internal ion mobility standard to compensate for run-to-run instrument variability in drift time measurements. This was accomplished within the LAV software using a normalisation function defined as the relative drift time,  [23, 24] equation (1):
[23, 24] equation (1):

Eucalyptol washout curves were processed and benchmark washout values were estimated using the method described previously [10].
3. Results and discussion
3.1. Identification of peppermint compounds
The test atmosphere standards verified the assignment of GC-IMS features to peppermint-oil compounds in figure 3 and table 1 that compared responses obtained from gas standards, peppermint oil headspace analysis, and breath-samples. (Menthone and menthol responses were not observed because the chromatographic run time was not long enough to record the elution of these compounds.) This combination of traceable gas standards, prepared by a National Measurement Laboratory, and fieldable methods that may be applied by most laboratories enabled identification and semi-quantification of the VOC of interest. It is helpful to note that the nominal vapour-phase concentrations would have been subject to some wall and adsorption artefacts, and thus the true values may be lower than the values reported. Twelve compounds attributed to peppermint were detected in the headspace analysis of the capsule, and four were identified as: eucalyptol, β-pinene, α-pinene, and limonene. β-pinene, α-pinene, and limonene concentrations in the capsule and standards were high enough to produce both monomer and dimer ions. However, in breath, only monomer signals were observed, apart from limonene. Hence, in this work monomer responses are highlighted, with the exception of Limonene where dimer responses are also noted. Note also that monomer and dimer ion assignments are tentative and require mass selected ion mobility characterisation to verify their identity.
Figure 3. Contour plot of the peppermint standards, capsule and breath (collected at t + 60 min) showed in full (Left) or zoom (Right) scale, collected for Study 1, with main features being highlighted. Three features 1, 2, 3 with level 1 identification, were assigned as: eucalyptol (1), β-pinene (2) and α-pinene (3). As a limonene (4) standard was not run for Study 1, its level 2 identification was based on relative and reduced drift time obtained from the capsule headspace data. tr —retention time in seconds, tDr , relative drift time.
Download figure:
Standard image High-resolution imageTable 1. Summary of retention times ( ) and relative drift times (
) and relative drift times ( relative to reactant ion peak drift time) for compounds identified in the peppermint oil capsule using GC-IMS. Note: NA*—either not run (Study 1) or not present in the standard mixture (Study 2); M—monomer. D—Dimer; ND—compound not detected; NA*—not available (standard not run).
relative to reactant ion peak drift time) for compounds identified in the peppermint oil capsule using GC-IMS. Note: NA*—either not run (Study 1) or not present in the standard mixture (Study 2); M—monomer. D—Dimer; ND—compound not detected; NA*—not available (standard not run).
| Compound |
 (s) (s) |

| ||||
|---|---|---|---|---|---|---|
| Study 1 | Std | Cap | Br | Std | Cap | Br |
| Eucalyptol M | 352 | 355 | 355 | 1.348 | 1.355 | 1.342 |
| β-pinene M | 293 | 294 | 290 | 1.264 | 1.266 | 1.262 |
| α-pinene M | 258 | 261 | 254 | 1.262 | 1.264 | 1.262 |
| Limonene M | NA* | 323 | 308 | NA* | 1.266 | 1.265 |
| Limonene D | NA* | 323 | 308 | NA* | 1.354 | 1.337 |
| Catabolite unassigned | 317 | 1.078 | ||||
| Study 3 | Std | Cap | Br | Std | Cap | Br |
| Eucalyptol M | 441 | 439 | 440 | 1.328 | 1.345 | 1.342 |
| β-pinene M | NA* | 356 | 355 | NA* | 1.221 | 1.217 |
| α-pinene M | 318 | 316 | ND | 1.250 | 1.222 | ND |
| Limonene M | 403 | 401 | ND | 1.251 | 1.221 | ND |
| Limonene D | 403 | 401 | ND | 1.329 | 1.343 | ND |
| Catabolite unassigned | 389 | 1.049 | ||||
| Study 4 | Std | Cap | Br | Std | Cap | Br |
| Eucalyptol M | 398 | 397 | 396 | 1.338 | 1.338 | 1.340 |
| β-pinene M | 298 | 297 | 295 | 1.258 | 1.257 | 1.259 |
| α-pinene M | 243 | 244 | 242 | 1.258 | 1.257 | 1.259 |
| Limonene M | NA* | 310 | 311 | NA* | 1.259 | 1.261 |
| Limonene D | NA* | 310 | ND | NA* | 1.339 | ND |
Note. Analysis of the standards (std), peppermint-oil headspace (cap) and breath (Br) were not performed at the same time for Studies 1 and 3. Consequently, batch effects with shifts in drift times and retention times, and normalisation methods may be discerned, although statistical process controls indicated the instruments' parameters were all within limits.
Participants exhibited varying peppermint washout profiles. Eucalyptol, β-pinene, α-pinene, and an additional unassigned feature were observed to increase in exhaled concentration and then washout over the following 6 h. (The unassigned feature was not found in current IMS data bases, and was not observed in analysis of peppermint-oil headspace. It was attributed to a catabolite from the peppermint-oil [25]). At t + 60 min eucalyptol was the highest intensity feature observed with all participants in all studies. β-pinene was observed in eight out of ten participants in Study 1, four out of six participants in Study 3 and in six out of ten participants in Study 4. α-pinene was detected in seven out ten, not observed, and in four out of ten participants in Studies 1, 3 and 4 respectively. Limonene monomer and dimer ions were observed sporadically, and definitive washout profiles were not observed in all the studies.
3.2. Calibration
Combining eucalyptol monomer and dimer product ion signal volumes provided calibration data, see figure S4 and table 2 for a summary. The limit of detection (LOD) was defined as the y-axis intercept + 3 ×  of y-axis intercept [26], and at concentrations above 500 µg m−3 the calibration responses become non-linear. The IMS used in Study 1 was also calibrated using a peak extraction method [27] and the IMS detector LOD was estimated to be 33 fg s−1; in line with previous studies see figure S5.
of y-axis intercept [26], and at concentrations above 500 µg m−3 the calibration responses become non-linear. The IMS used in Study 1 was also calibrated using a peak extraction method [27] and the IMS detector LOD was estimated to be 33 fg s−1; in line with previous studies see figure S5.
Table 2. Summary of GC-IMS eucalyptol air concentration (![$\left[ i \right]$](https://content.cld.iop.org/journals/1752-7163/16/3/036004/revision2/jbrac6ca0ieqn10.gif) ) calibration data from the three centres for the calibration equation.
) calibration data from the three centres for the calibration equation. ![$I\left( {{\text{m}}{{\text{V}}^{\text{2}}}} \right) = {B_0}\left( {{\text{m}}{{\text{V}}^{\text{2}}}} \right) + {B_1}\left( {{\text{m}}{{\text{V}}^{\text{2}}}{ }{{\text{m}}^{\text{3}}}{{ \mu }}{{\text{g}}^{{ -\text{1}}}}} \right) \times \left[ i \right]{B_1}\left( {{\mu \text{g }}{{\text{m}}^{{-\text{3}}}}} \right)$](https://content.cld.iop.org/journals/1752-7163/16/3/036004/revision2/jbrac6ca0ieqn11.gif)
| Study |
 (mV2) (mV2) |
 (mV2 m3
µg−1) (mV2 m3
µg−1) |

| LOD (µg m−3); ppb (v/v) | Comment |
|---|---|---|---|---|---|
| 1 | 66 (±90) | 5.0 (±0.40) | 0.99 | 23, 3.7 | Static gas standard |
| 3 | −32 (±280) | 6.9 (±0.90) | 0.99 | 61, 9.7 | Dynamic test atmosphere |
| 4 | −12 (±122) | 2.7 (±0.48) | 0.99 | 90, 14.3 | Permeation tube headspace |
Note. Values in brackets denote 95% confidence intervals, and the limit of detection (LOD) value indicates the upper 95% level for the extrapolated estimate.
3.3. Washout profiles
Eucalyptol, β-pinene, α-pinene, as well as the monomer and dimer ions of limonene were all resolved within the GC-IMS data, figure S6. Figure 4 shows how Eucalyptol washout behaviour varied amongst the participants with the data grouped according to the time at which maximum eucalyptol abundance was observed; that is at either  ,
,  or
or  min. The elimination profile followed the power relationship described previously [10], see equations (2) and (3)
min. The elimination profile followed the power relationship described previously [10], see equations (2) and (3)

Figure 4. Box-whisker plots of the consolidated eucalyptol breath washout, based on the peak volume change (V/V0) vs time (t in min) in Studies 1, 3 and 4. The graphs show the median, 25% and 75% inter-quartile range (IQR) with 1.5 × IQR indicated by the whiskers. The mean is indicated by the black square and the averaged observed profiles are highlighted by the dashed lines. Additionally, individual data points, from all data sets are also shown by open symbols. Note that the maximum exhaled levels of eucalyptol were not necessarily coincident with the sampling points and may have been higher than the observed levels indicated here. Plot (a) Consolidated data from all participants. Plots (b)–(d), show the group data from the participants with maximum observed eucalyptol concentrations at  min,
min,  min and
min and  min respectively.
min respectively.
Download figure:
Standard image High-resolution imagewhere, I: intensity [mV2]; t: time [h]; and,  and
and  are coefficients transformed to a linear form, as follows:
are coefficients transformed to a linear form, as follows:
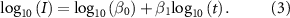
In studies 1, 3 and 4, the highest observed exhaled eucalyptol concentration was at  min for 11 out of 25 participants (44%) with a mean concentration increase of 6.7-fold, varying over the range 1.7-fold to 13.2-fold. Nine out of 25 (36%) participants had a maximum observed exhaled eucalyptol concentration at
min for 11 out of 25 participants (44%) with a mean concentration increase of 6.7-fold, varying over the range 1.7-fold to 13.2-fold. Nine out of 25 (36%) participants had a maximum observed exhaled eucalyptol concentration at  min with an average fold-change of 3.8-fold, ranging from 1.6-fold to 6.0-fold. Five of the 25 participants (20%) had a maximum observed concentration at
min with an average fold-change of 3.8-fold, ranging from 1.6-fold to 6.0-fold. Five of the 25 participants (20%) had a maximum observed concentration at  min, with an average fold-change of 2.0-fold. The results agree with other studies, reporting elimination of the eucalyptol from breath measurements [28–30]. Not all participants had a well-defined washout pattern, with three cases indicating a more complex metabolic pattern such that the maximum observed concentration was not followed by a washout of the form indicated in equation (2).
min, with an average fold-change of 2.0-fold. The results agree with other studies, reporting elimination of the eucalyptol from breath measurements [28–30]. Not all participants had a well-defined washout pattern, with three cases indicating a more complex metabolic pattern such that the maximum observed concentration was not followed by a washout of the form indicated in equation (2).
Three out of six participants from the ancillary studies (Studies 5 and 6) had a maximum exhaled eucalyptol concentration at  min with an average 2.0-fold increase in exhaled eucalyptol abundance. One of the ancillary participants had a maximum exhaled concentration level at
min with an average 2.0-fold increase in exhaled eucalyptol abundance. One of the ancillary participants had a maximum exhaled concentration level at  min; however, it should be noted that only two out of the six participants had their data collected at
min; however, it should be noted that only two out of the six participants had their data collected at  min.
min.
β-pinene, α-pinene and limonene were present at lower levels with elimination profiles observed for α-pinene and limonene indicative of more complicated processes than release and elimination [31]. β-pinene along with the unassigned catabolite had the same build up and elimination behaviour as that observed for eucalyptol, see figure S7.
Background or baseline concentrations in breath were close to, or below the extrapolated calibration detection limits, with concentrations estimated to be on average 9, 12 and 26 µg m−3 for Study 1, 3 and 4 respectively. Average maximum concentrations at  to
to  min were 64, 79 and 60 µg m−3 (10.1, 12.5, and 9.5 ppb(v/v)) for Studies 1, 3 and 4 respectively, and correspondingly lower maximum exhaled levels were observed with participants as their time to maximum eucalyptol concentration increased, see figure 4.
min were 64, 79 and 60 µg m−3 (10.1, 12.5, and 9.5 ppb(v/v)) for Studies 1, 3 and 4 respectively, and correspondingly lower maximum exhaled levels were observed with participants as their time to maximum eucalyptol concentration increased, see figure 4.
3.3.1. Eucalyptol washout time to reach baseline ( ) values—benchmark
) values—benchmark
Eucalyptol was selected as the peppermint marker for benchmarking analysis for GC-IMS. The lower 95% confidence interval of the x-axis intercept of the washout function (equation (3)) was proposed as the benchmarking metric by the Peppermint Initiative (figure 5). This enabled analytical-sensitivity, as well as sampling and analytical-reproducibility, to be assessed. Data from participants with a maximum exhaled eucalyptol concentration at either 60 min or 90 min were used to produce the model.
Figure 5. Logarithmic plots (equation (3)) of the eucalyptol washout curves of the fold-change change in peak volume vs time for washout data collected over 6 h following ingestion at t = 0 min of a peppermint capsule. These data provide information on sampling and analytical reliability as well as detection-sensitivity of the methods used for: (a) Study 1, (b) Study 3, (c) Study 4, and (d) the consolidated data from all three studies. The different colours indicate individual participants' data. Averaged values are denoted by the solid symbols with 95% confidence intervals indicated by dashed lines. The x-axis intercept of the lower 95% confidence interval for the consolidated data provided a benchmark value for the minimum  time to the loss of peppermint signal.
time to the loss of peppermint signal.
Download figure:
Standard image High-resolution imageThe consolidated benchmark time from Studies 1, 3 and 4 was estimated at t + 367 min, with an average x-axis intercept of t + 422 min, see table 3.
Table 3. Summary of regression parameters (equation (3)) obtained from plotting average Eucalyptol logarithmic washout curves of eucalyptol peak volume fold-change vs  time for each benchmarking data sets with
time for each benchmarking data sets with  participants, as well as the consolidated fit of three studies.
participants, as well as the consolidated fit of three studies.
| Study |

|

| R2 | N | t + time benchmark (range)(min) |
|---|---|---|---|---|---|
| 1 | 2.38 ± 0.04 | −0.897 ± 0.02 | 1.00 | 7 | 446 (412–482) |
| 3 | 2.83 ± 0.12 | −1.073 ± 0.09 | 0.98 | 4 | 432 (314–596) |
| 4 | 1.95 ± 0.10 | −0.745 ± 0.05 | 0.99 | 9 | 410 (324–518) |
| Consolidated | 2.26 ± 0.12 | −0.863 ± 0.05 | 0.76 | 20 | 422 (367–484) |
Note. Data from delayed elimination profiles (time to observed maximum >  min was excluded for there were insufficient data points for reliable regression analysis.
min was excluded for there were insufficient data points for reliable regression analysis.
The ancillary studies (Studies 5 and 6) undertaken at clinical point of care settings enabled the application of this benchmark to be evaluated in operational settings. The average washout time for Study 5 was 250 min, and 390 min for study 6. Figure S8 shows the Peppermint Experiment test data obtained during the setup and training phase of a study being undertaken with GC-IMS by a clinical team using GC-IMS for the first time. With the loss of the eucalyptol signal at  min, and comparison of their data against the benchmark data from this work in accordance with the Peppermint Methodology [10], enabled the clinical team to identify enhancements in the test-setup and method; assuring their research before admitting clinical participants into their study.
min, and comparison of their data against the benchmark data from this work in accordance with the Peppermint Methodology [10], enabled the clinical team to identify enhancements in the test-setup and method; assuring their research before admitting clinical participants into their study.
3.3.2. Data fidelity
Slight changes in sampling and instrument operation have the potential to significantly affect the analytical fidelity of the VOC profile obtained from GC-IMS, and the Peppermint Experiment may be augmented through comparison of the cumulative frequency and signal intensity distribution of the exhaled VOC features acquired from breath samples. Figure S9 shows average cumulative frequencies from the three studies with the cumulative numbers of VOC isolated and characterised plotted against retention time. On average 69, 34 and 57 exhaled VOC were isolated in Studies 1, 3 and 4, respectively. It should be noted that Laboratory 2 (Study 3) used an inline biofilter (figure 2 Bottom). The peppermint oil components noted previously are indicated by solid features. Five were found within the Study 1 data, two (eucalyptol and β-pinene) were isolated by Study 3, and 4 were isolated with Study 4 (table 1). The distributions of the signal intensity of the VOC features indicates a log normal distribution across three orders of magnitude with the peppermint oil features highlighted by solid symbols, figure S9.
3.3.3. Biological variability and confounding factors
GC-IMS appears to be well suited for point-of-care applications within a clinical environment. It is ideal for hypothesis testing and biomarker verification, and with the right markers will have significant diagnostic, prognostic and theragnostic capabilities. The low resource burden and speed of analysis means that high frequency sampling and analysis may take place, and the inherent analytical-sensitivity of the technique allows small sample volumes, in this case 0.5 cm3 to 1 cm3, to be used for analysis.
It is helpful to note that the atmospheric pressure chemical ionisation processes within the reaction region of the ion mobility spectrometer mean that only compounds with a proton affinity higher than that of the RIP will be detected, which in this study was water (PA = 697 kJ mol−1). Further, the charge exchange mechanism for ionisation means that a sample overloaded with contaminants, such as, alcoholic hand sanitizer, will encounter charge sequestration causing the suppression of some signals with similar retention times to the contaminant; referred to as a matrix effect in atmospheric pressure chemical ionisation mass spectrometry. Therefore, care is required when setting up studies and operations with GC-IMS, ideally with clean allocated room for sampling and additional cleaning protocols, minimising presence of solvents/contaminant chemicals in the environment, surfaces, and operator.
It is most important that temperature, pressure, and water levels within the ion mobility spectrometer are monitored and maintained at constant levels for reproducible operation. Statistical process control is essential for longer term studies, and interlaboratory collaborations will require standardisation of quality control and instrument calibration procedures as parts of their standard operating procedures.
Different sampling and operating conditions were used in this benchmarking study and the potential effect on the data from the use of a bio-filter in a sampling line may be discerned in Study 3. This study highlights how changes in configuration of sampling and operation for GC-IMS may be objectively evaluated through a Peppermint Experiment. Although superficially simple, polypropylene Haldane tubes are compatible with IMS operations as the potential impurities outgassed from polypropylene have low proton affinities and tend not to be ionised, and thus are not observed with many GC-IMS ionisation sources. Further, the hydrophobic nature of polypropylene suppresses adsorption losses of IMS-active compounds (those with labile protons, and a proton affinity higher than that of water). It is also helpful to note that raising the temperature of the Haldane tube to 40 °C prior to taking a sample suppresses analyte condensation and condensate losses thus increasing the number of compounds recovered; note, however, that this procedure was not adopted in this study.
Finally, physiological and environmental factors such as ethnicity, BMI, age or diet may also influence the metabolic processes of peppermint oil components and their washout behaviour [31]. Although the Peppermint Experiment has not been designed to answer such questions, the data suggests that these factors contribute to the observed variability in peppermint washout profiles; for example, the wider spread of maximum eucalyptol concentration observed in Study 4 was taken from a cohort with the oldest and most ethnically variable participants (table S4).
4. Conclusion
One hundred and forty-eight peppermint washout samples were analysed in this work with 35 ancillary tests used to evaluate the results. Combined with the results from peppermint-oil headspace analysis, the results indicated that eucalyptol was the most reliable marker for peppermint-oil washout breath analysis using GC-IMS with the collection method. The responses obtained by all centres provided traceable exponential washout profiles within detectable concentration levels and within a given timescale. Following ingestion, the exhaled concentrations of eucalyptol increased over the range 24–153 µg m−3 with 80% of participants reaching maximum exhaled concentration within t + 90 min. The remaining 20% of participants showed either delayed or complex elimination profiles with lower maximum concentrations suggestive of confounding and unknown factors such food intake and/or the effect of age, sex, race/ethnicity, and body mass. The implication of such phenotypic variability within the Peppermint Experiment will be the subject of a future study.
Despite the differences between sampling techniques and fidelity of the data, eucalyptol exhibited stable responses in all studies making it a good candidate for future benchmarking activities. All studies returned similar times to the loss of detection for eucalyptol at elevated levels: 446, 432 and 410 min for Studies 1, 3 and 4 respectively. The minimum benchmark value based on 95% confidence interval obtained from data from all three studies was established to be 367 min. The intra-participant R2 values for the eucalyptol washout model (equation (3)) were typically greater than 0.95 indicating reproducible sampling and analytical activity. The aggregated data for each study reflected phenotypic variability with R2 values of 0.9; the incorporation of phenotype variability into the design is helpful for it enables the methodology to be tested against a range of participant types and therefore, not unduly affected by the results from any single individual.
This benchmarking study has shown how a Peppermint Experiment may be used with GC-IMS in different operational settings, including clinical deployments, notably with the simplest of breath sampling techniques delivering 0.5 cm3 of end-tidal breath to the analyser. Further studies would be helpful in verifying this preliminary benchmark, and additional work is needed to fully describe the effect of bio-filters in sampling lines, as well as the biological variation observed, with specific reference to food intake, diet and other factors such as age, sex, race/ethnicity.
Acknowledgments
Figure 2 Top was reprinted from EclinicalMedicine, 29–30, Ruszkiewicz D, Sanders D, O'Brien R, Hempel F, Reed M, Riepe A, Baillie K, Brodrick E, Darnley K, Ellerkmann R, Mueller O, Skarysz A, Truss M, Wortelmann T, Yordanov S, Thomas C L P, Schaaf B, Eddleston M, Diagnosis of COVID-19 by analysis of breath with gas chromatography-ion mobility spectrometry—a feasibility study, 100609, 2020 with permission from Elsevier.
The authors acknowledge gratefully: Daniel Sanders from GAS Dortmund and Sukhinder Khattra from British Columbia Cancer Research Centre for their support and participation in this study; Peppermint Initiative [10] for the conception of the peppermint protocol and the volunteers without whose participation this study would not have been possible.
We acknowledge gratefully European Union Horizon 2020 funding to Edinburgh and Oslo (TOXI-triage project H2020 funded project 653409).
Data availability statement
The data that support the findings of this study are available upon reasonable request from the authors.






