Abstract
A Polymerase Chain Reaction (PCR) test of a nasal swab is still the 'gold standard' for detecting a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. However, PCR testing could be usefully complemented by non-invasive, fast, reliable, cheap methods for detecting infected individuals in busy areas (e.g. airports and railway stations) or remote areas. Detection of the volatile, semivolatile and non-volatile compound signature of SARS-CoV-2 infection by trained sniffer dogs might meet these requirements. Previous studies have shown that well-trained dogs can detect SARS-CoV-2 in sweat, saliva and urine samples. The objective of the present study was to assess the performance of dogs trained to detect the presence of SARS-CoV-2 in axillary-sweat-stained gauzes and on expired breath trapped in surgical masks. The samples were provided by individuals suffering from mild-to-severe coronavirus disease 2019 (COVID-19), asymptomatic individuals, and individuals vaccinated against COVID-19. Results: Seven trained dogs tested on 886 presentations of sweat samples from 241 subjects and detected SARS-CoV-2 with a diagnostic sensitivity (relative to the PCR test result) of 89.6% (95% confidence interval (CI): 86.4%–92.2%) and a specificity of 83.9% (95% CI: 80.3%–87.0%)—even when people with a low viral load were included in the analysis. When considering the 207 presentations of sweat samples from vaccinated individuals, the sensitivity and specificity were respectively 85.7% (95% CI: 68.5%–94.3%) and 86.0% (95% CI: 80.2%–90.3%). The likelihood of a false-positive result was greater in the two weeks immediately after COVID-19 vaccination. Four of the seven dogs also tested on 262 presentations of mask samples from 98 subjects; the diagnostic sensitivity was 83.1% (95% CI: 73.2%–89.9%) and the specificity was 88.6% (95% CI: 83.3%–92.4%). There was no difference (McNemar's test P = 0.999) in the dogs' abilities to detect the presence of SARS-CoV-2 in paired samples of sweat-stained gauzes vs surgical masks worn for only 10 min. Conclusion: Our findings confirm the promise of SARS-CoV-2 screening by detection dogs and broaden the method's scope to vaccinated individuals and easy-to-obtain face masks, and suggest that a 'dogs + confirmatory rapid antigen detection tests' screening strategy might be worth investigating.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
People infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) may experience various typical or atypical clinical manifestations (ranging from mild to life-threatening in their intensity) or may be asymptomatic. The incidence of asymptomatic SARS-CoV-2 infections and the characteristics of individuals who progress (or not) to clinical coronavirus disease 2019 (COVID-19) have not been ambiguously determined. Furthermore, some asymptomatic individuals may even have objective radiographic findings that are consistent with COVID-19 pneumonia [1–4].
Diagnostic testing for SARS-CoV-2 infections is based on either a nucleic acid amplification test (NAAT, such as a reverse-transcriptase (RT-) PCR test) or a rapid antigen detection test (RADT) of a sample collected from the upper respiratory tract (i.e. a nasopharyngeal, nasal or oropharyngeal specimen). Indeed, an RT-PCR of a nasopharyngeal sample is considered to be the 'gold standard' for detecting an ongoing SARS-CoV-2 infection; a positive test in a patient with recent-onset, COVID-19-compatible symptoms is enough for diagnosis. These methods nevertheless have some limitations. Firstly, viral nucleic acids are not necessarily detectable in the first five days after the infection. Secondly, the likelihood of recovering replication-competent virus more than ten days after symptom onset is very low in individuals with mild COVID-19 [1, 5]. Although antigen tests are generally as specific as most NAATs, they are less sensitive—particularly before symptom onset or late in the course of disease. However, antigen-based tests are relatively cheap and have a rapid turnaround time [1, 5].
Many microorganisms and infectious diseases are associated with the emission of specific odours—typically volatile organic compounds (VOCs) [6, 7]. The VOCs and other semivolatile and non-volatile compounds are variously produced by host cells alone (for viral infections), by host cells and bacteria (for bacterial infections), or by the host's immune cells or cancer cells (for many non-infectious diseases). These compounds are released in the breath, saliva, sweat, urine, faeces, skin emanations, and blood [6–10]. It is well proven that dogs can use their olfactory sense to detect VOCs in the ppm to ppt range [10]. This extraordinary ability has led to the global deployment of trained sniffer dogs for the real-time, mobile detection of drugs of abuse and explosives (by the police, army and customs authorities) and human scents (for search-and-rescue missions) [10, 11]. Although biomedical detection dogs are far less common, they have been assessed for the detection of infectious and (primarily) non-infectious diseases [11–13]. In this context, several proof-of-concept studies have described the use of sniffer dogs to detect the presence of SARS-CoV-2 in a wide variety of human samples. Most studies screened sweat or saliva samples, although urine samples, tracheobronchial secretions, nasopharyngeal swabs, face masks, and socks have also been examined [12 (review), 14–25].
In four recent studies, we showed that trained dogs were able to detect sweat samples from COVID-19 patients with sensitivity and specificity values of at least 80% and often 90% or more [22–25]. Although these results for sweat samples from SARS-CoV-2-infected individuals are promising, it remains to be determined whether dogs can also detect metabolite compounds in the breath (i.e. the expired air) trapped on surgical masks worn only for a short time; the latter are much easier to collect than sweat, urine or even saliva samples. The primary objective of the present study was therefore to determine whether dogs trained on axillary-sweat-stained gauzes from SARS-CoV-2-infected individuals (as in our earlier studies [22–25]) are also able to recognize a disease signature in breath samples trapped on surgical masks. The study's other objectives were to determine whether COVID-19 vaccination can interfere with a dog's ability to detect an infection and to establish the method's diagnostic performance in infected patients with a low viral load and in patients admitted to an emergency service for mild-to-severe COVID-19 (relative to patients admitted for other reasons All precautions were taken to ensure the safety of the handlers and their dogs, as implemented in our previous studies [22, 24].
2. Materials and methods
All the test samples had been obtained from participants (patients or healthy volunteers) in consecutive twin clinical studies ('VOC-COVID-Diag' and 'VOC-SARSCOV-Dep') conducted at a single study centre (Hôpital Foch, Suresnes, France). The only difference between the two studies was that axillary sweat samples only were collected in the first, whereas both axillary sweat and surgical mask samples were collected in the second. Both studies had been approved by an independent ethics committee (IEC): the VOC-COVID-Diag study was approved by the CPP Nord Ouest IV IEC (Lille, France) on 8 October 2020 (EudraCT number: 2020-A02682-37), and the VOC-SARSCOV-Dep study was approved by the CPP Sud-Est II IEC on 22 February 2021 (EudraCT number: 2021-A00167-34). All participants gave their written, informed consent prior to inclusion.
Furthermore, the testing sessions with the dogs were carried out in strict accordance with the French legislation on the care and use of animals. The testing protocols were approved by the animal care and use committee at Alfort School of Veterinary Medicine (Ecole Nationale Vétérinaire d'Alfort, Maisons-Alfort, France).
2.1. Study participants
The study population consisted of adults recruited either during a visit to the hospital's emergency department or among the hospital's medical and non-medical staff. A documented SARS-CoV-2 RT-PCR test result (Abbott Alinity M®, Abbott Park, Illinois, USA) was required for all patients recruited at the hospital emergency department and all symptomatic or asymptomatic individuals having recently been in contact with and/or having cared for COVID-19 patients. A negative NAAT was not mandatory for the vaccinated, healthy volunteers from the hospital staff. Furthermore, all individuals with a suspected history of SARS-CoV-2 underwent serology testing (Biosynex COVID-19 BSS®, Illkirch-Graffenstaden, France) to detect evidence of a past infection.
2.2. Sampling procedure
The sampling materials were sterile gauze (2 × 2 inches) and a three-layer surgical mask (17.5 × 9 cm) provided by the hospital. Two sterile gauzes were rolled up and placed respectively in the left and right armpits for 10 min. An individual's left and right gauzes were stored separately and presented to the dogs separately. The reasons for choosing this sweat sampling method and this site (axillary sweat is a key odour for search-and-rescue dogs) have been described elsewhere [22]. The surgical mask was worn by the participant during the 10 min sweat sampling session.
The samples were collected by trained staff wearing surgical gloves and (for the samples collected from COVID-19 patients) personal protective equipment. The staff also recorded demographic and medical data on a case report form. The medical data focused on the symptoms frequently associated with COVID-19 (fatigue, fever, cough, and headache), the most typical and predictive symptoms of COVID-19 (myalgia, anosmia, ageusia, dyspnoea, and hypoxemia) [26], underlying health conditions (including risk factors for severe COVID-19, such as hypertension, diabetes, and obesity), any history of COVID-19 or recent contact with a SARS-CoV-2-infected individuals, and any medications at the time of the sampling. Each sample (the gauzes from the right and left armpits and the surgical mask) were placed in individual, anonymously coded anti-UV glass containers and stored at +4 °C. All samples were transferred to the testing site once a week in coolers and stored at +4 °C until the testing session by the dogs.
2.3. Detection dogs and initial training
Seven operational search-and-rescue dogs were involved in this study (figure 1). They had been trained to detect SARS-CoV-2 for 8 weeks, using line-ups of olfaction cones, positive marking by sitting, barking or scratching in front of the cone, and positive reinforcement (as described previously by our group) [22]. The dogs' welfare was fully considered, with toy rewards and the avoidance of physical or mental fatigue. Each dog's sensitivity and specificity in detecting SARS-CoV-2 have been recently reported [24].
Figure 1. Characteristics and photos of the seven detection dogs and photo of a dog marking an olfaction cone. SAR: search and rescue; ENVA: Ecole Nationale Vétérinaire d'Alfort (Alfort School of Veterinary Medicine); SDIS78: Service Départemental d'Incendie et de Secours 78 (Yvelines County Fire and Rescue Service); SDIS60: Service Départemental d'Incendie et de Secours 60 (Oise County Fire and Rescue Service).
Download figure:
Standard image High-resolution imageStudies performed at Idexx laboratories, Inc. (Westbrook, ME, USA), the Alfort School of Veterinary Medicine (France) and the Hong Kong's Public Health Department have provided evidence of the absence of SARS-CoV-2 infection in dogs [27–29]. The US Centers for Disease Control and Prevention, the World Organisation for Animal Health, and the French Agency for Food, Environmental and Occupational Health and Safety have all stated that there is no evidence to suggest that pet animals (and especially dogs) have a significant role in SARS-CoV-2 transmission or spreading [30, 31]. Furthermore, the olfaction cone's design avoids contact between the dog and the samples [22]. The samples were allowed to sit for at least 72 h before being presented to a dog; this further reduces any virologic safety concerns, as it has been shown that the viability of SARS-CoV-2 declines rapidly (within hours to days) on cotton and on face masks [32–34].
2.4. Testing protocol
The testing sessions took place in a dedicated room at the Alfort School of Veterinary Medicine, where a line-up of five to eight olfaction cones was placed. The handler was blinded to the numbers of positive and negative samples and their positions in the line-up. While the room was empty, the samples were placed randomly in their test position by an investigator wearing disposable gloves (the same brand was used for all the testing sessions) and a mask (in order not to contaminate the olfactive environment). When the dog entered the room with his/her handler, it had to sniff each cone once, one after the other. Samples considered positive by the dog were marked by sitting, barking or scratching in front of the corresponding cone; the dog was then rewarded (regardless of whether the marking was correct or not) by the handler. After marking, the handler then asked the dog to resume the task for the remaining cones in the line-up (i.e. a sequential line-up). Once the dog had sniffed all the cones in the line-up, the dog and the handler left the room. The cones were cleaned rapidly with distilled water by the investigator, in order to remove traces left by the dog. The next dog then entered the room and sniffed the cones in the same line-up. Only when all the dogs had completed the line-up were the dog handlers informed of the COVID-19-positive sample's or samples' location in the line-up. Once all seven dogs had performed the line-up, the cones were cleaned with 3% acetone solution. The new samples were randomly placed in the cones 10 min after cleaning, and a new testing session could be started. None of the dogs sniffed the sweat or the mask samples from an individual more than once, and not all of the dogs sniffed samples from all of the individuals since all the dogs were not available at each of the testing sessions.
2.5. Statistical analysis
Binary and qualitative variables were presented as the frequency (percentage), and quantitative variables were presented as the mean ± standard deviation (SD) and/or the median (range). Fisher's exact test or a chi-square test was used as appropriate to analyse the contingency tables. The 95% confidence intervals (CIs) for the sensitivity, specificity, positive predictive value (PPV), and negative predictive value were calculated using the hybrid Wilson/Brown method [35, 36]. The results for paired sweat and face mask samples were analysed with McNemar's test, computing the P value using the binomial test. Quantitative variables were compared using an unpaired Student's t test. Skewed data were log-transformed. The threshold for statistical significance was set to p < 0.05. All analyses were performed with Prism 7 software (GraphPad Software Inc., La Jolla, CA, USA).
3. Results
3.1. Baseline characteristics of the study population
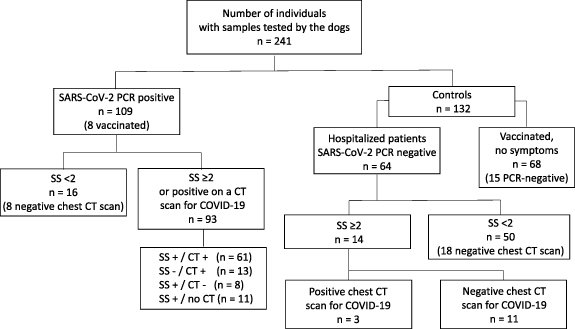
A total of 241 individuals with samples tested by the dogs were recruited in the twin studies (124 in the VOC-COVID-Diag study and 117 in the VOC-SARSCOV-Dep study). The PCR-positive group and control group differed with regard to several demographic and clinical variables (table 1). These differences were mainly due to the 118 members of the control group who lacked any obvious COVID symptoms, including 68 healthy, vaccinated individuals (figure 2). In the present study, a COVID symptom score (SS) of 2 or more on a scale of 0–4 (table 1 and figure 2) was based on the presence of two of the most predictive and typical signs or symptoms (TSs) of COVID-19 (myalgia, anosmia, ageusia, dyspnoea, or hypoxemia) [25] or at least one TS with a very common but less predictive sign or symptom of COVID-19 (fever, cough, sore throat, nausea, vomiting, diarrhoea, headache, or malaise). The 64 PCR-negative patients recruited through the emergency department (mean ± SD age: 57.8 ± 16.6) were similar to the PCR-positive patients, most of whom were also recruited through the emergency department. However, the prevalence of concomitant chronic diseases was higher (78.4%) in the PCR-negative group because an acute recurrence, complication or aggravation of the said diseases was the main reason for their visit to the emergency department.
Figure 2. Distribution of the 241 included individuals within the PCR-positive and control groups. CT: CT scan of the chest; SS+: a symptom score of 2 or more; SS−: a symptom score below 2; CT+: a positive CT scan, with the presence of ground-glass opacities or areas of consolidation; CT−: negative CT scan (absence of ground-glass opacities or areas of consolidation).
Download figure:
Standard image High-resolution imageTable 1. Demographics and baseline characteristics.
| Characteristics | Positive in a SARS-CoV-2 PCR (n = 109) | Controls (n = 132) | P |
|---|---|---|---|
| Sex | |||
| Sex Male, % (n) | 51.4 (56) | 48.5 (64) | 0.6986 |
| Age, years | |||
| mean ± SD | 57.5 ± 15.0 | 47.4 ± 17.8 | <0.0001 |
| COVID symptom score | |||
| Median [range] | 2 [0–4] | 0 [0–4] | <0.0001 |
| Score ⩾2, % (n) | 85.3 (93) | 10.6 (14) | <0.0001 |
| CT scan of the chest, % (n) | 83.5 (91) | 27.3 (36) | |
| Percentage of pulmonary opacity a | |||
| 17.6 (16) | 91.7 (33) |

|
| 22.0 (20) | 0 | |
| 20.9 (19) | 0 | |
| 24.2 (22) | 5.5 (2) | |
| 15.4 (14) | 3.1 (1) | |
| Patients with at least one concomitant disease, % (n) | 67.0 (73) | 34.1 (45) | |
| 31.2 (34) | 12.8 (17) | 0.0008 |
| 15.6 (17) | 4.5 (6) | 0.0042 |
| 12.8 (14) | 7.6 (10) | 0.1987 |
| 10.1 (11) | 3.8 (5) | 0.0680 |
| 52.3 (57) | 25.8 (34) | <0.0001 |
| CRP, % (n) | 93.6 (102) | 35.6 (47) | |
| Geometric mean (95%CI) | 35.9 (26.6–48.5) | 13.5 (7.9–22.8) | 0.0007 |
| Median (range) | 49.5 (1–350) | 7.5 (1–342) | |
| Oxygen, % (n) | 49.5 (54) | 7.8 (10) | <0.0001 |
| Vaccinated (at least one injection), % (n) | 4.6 (5) | 51.5 (68) | <0.0001 |
a The area with pulmonary opacity (ground-glass opacities or areas of consolidation) as a percentage of the entire lung, on CT images. CRP: C-reactive protein.
The distribution of the 241 individuals (figure 2) within the PCR-positive and control groups shows that three patients in the PCR-negative group were diagnosed as having COVID-19 through the presence of TSs (two patients with an SS of 3, and one patient with an SS of 4) and ground-glass opacities on the chest computed tomography (CT) scan (table 1). Furthermore, 16 patients in the PCR-positive group had no COVID-19-consistent symptoms and were classified as asymptomatic, SARS-CoV-2 infected patients [1]. The 96 COVID-19 patients (including the three from the PCR-negative group) could be subdivided into three severity categories (mild (n = 18, 18.7%), moderate (n = 31, 32.3%) and severe (n = 47, 48.9%)), even though the criteria for each category may overlap or may vary from one clinical guideline to another [1].
3.2. The detection dogs' performance with sweat samples
Using the olfaction cone line-ups, the seven detection dogs were tested on 886 presentations of sweat samples from the 241 individuals (428 presentations of samples from the PCR-positive group and 458 presentations of samples from the control group). When compared with the PCR results, the dogs detected SARS-CoV-2 with a diagnostic sensitivity of 89.6%, a specificity of 83.9%, and an accuracy of 87%. The sweat samples from the three PCR-negative COVID-19 patients and a PCR-negative contact individual were all marked as positive by all seven dogs. However, we did not obtain additional information on the contact's infection status. Hence, we transferred the three PCR-negative COVID-19 patients to the group of infected patients (PCR-positive or COVID-19 patients, n = 112); this gave slightly higher sensitivity (89.9%) and specificity (86.4%) values and a similar accuracy (88%) (table 2). Overall, the average prevalence of a SARS-CoV-2 infection in our study population was about 46%. No disease, symptoms or clinical abnormalities have been reported for any of the dogs involved in the study.
Table 2. The detection dogs' diagnostic performance. Sensitivity, specificity, positive predictive value and negative predictive value are presented as percentages, with the 95% confidence interval in brackets.
| Type of sample and reference method | Sample presentations (n) | Diagnostic specificity | Diagnostic sensitivity | Positive predictive value | Negative predictive value |
|---|---|---|---|---|---|
| Sweat samples (PCR-based testing) | 886 | 89.65 (86.39–92.20) | 83.95 (80.32–87.02) | 83.74 (80.07–86.84) | 89.79 (86.57–92.31) |
| Sweat samples (PCR-based testing, clinical and CT-imaging) | 886 | 89.95 (86.78–92.43) | 86.38 (82.90–89.25) | 86.59 (83.15–89.42) | 89.79 (86.57–92.31) |
| Sweat samples vaccinated individuals (PCR-based testing) | 207 | 85.71 (68.51–94.30) | 86.03 (80.20–90.36) | 48.98 (35.58–62.53) | 97.47 (93.67–99.01) |
| Mask samples (PCR-based testing) | 262 | 83.12 (73.23–89.86) | 88.65 (83.27–92.45) | 75.29 (65.17–83.24) | 92.66 (87.84–95.66) |
When considering the 207 presentations of sweat samples from 76 vaccinated individuals (including 8 PCR-positive or COVID-19 individuals) having received at least one injection, the sensitivity and specificity were respectively 85.7% and 86.0% (table 2), and the accuracy was 86%. However, the PPV was only 49% since 28 sweat samples from 14 healthy vaccinated individuals (10 with the Pfizer-BioNTech vaccine, and 4 with the Astra-Zeneca vaccine) were marked as positive ('false positives'). Of the ten tested individuals vaccinated with the Pfizer-BioNTech vaccine, seven had been vaccinated in the two weeks preceding the sample collection. The percentage of 'false positives' among the negative samples was significantly higher in this immediately post-vaccination group than in individuals vaccinated longer before the sample collection (17% vs 7.6%, P = 0.007 in Fisher's exact test). One can reasonably hypothesize that an initial, short-lived production of VOCs was induced by transient expression of the full-length transmembrane spike glycoprotein and/or the immune response to vaccination [37] and that this might have confused the detection dogs. However, the transient elevation in the relative risk of 'false positive' detection by the dogs should be confirmed in a study of a larger number of recently vaccinated individuals.
3.3. Viral load and the detection dogs' performance
The PCR cycle threshold (Ct) value is the number of PCR cycles at which the nucleic acid target in the sample becomes detectable. In general, the Ct value is inversely related to the SARS-CoV-2 viral load. A Ct value ⩽30 is considered to be a threshold for infectivity and is an important cut-off for identifying contagious individuals infected with SARS-CoV-2 [38, 39]. In our population of non-vaccinated individuals, the sensitivity and specificity were above 87% when considering samples from both PCR-positive individuals with a Ct-value >30 (n = 79) and those with a Ct-value ⩽30 (n = 363). The intergroup difference in the percentage of 'false negatives' (11.3% for Ct-value >30 vs 12.7% for Ct-value ⩽30) was not significant (P = 0.70). However, the percentage of 'false negatives' among the samples from the PCR-positive individuals with no COVID symptoms or mild atypical COVID symptoms (SS < 2) was higher than that among samples from PCR-positive/COVID individuals with at least one TS (SS ⩾ 2): 23% (n = 113) vs 7.6% (n = 329), respectively, P < 0.0001. The sensitivity for samples from PCR-positive individuals with an SS < 2 was 77% (95% CI: 68–84), although the specificity was 89% (95% CI: 84–92).
3.4. The detection dogs' performance with mask samples
Four of the seven dogs were also tested on a total of 262 sample presentations of mask samples from 98 individuals (28 PCR-positive patients and 70 controls). The diagnostic sensitivity and specificity were 83.1% and 88.6% respectively (table 2), and the accuracy was 87%. The absence of a difference (McNemar's test = 0.047; P = 0.999) in the dogs' abilities to detect the presence of SARS-CoV-2 in paired samples of sweat-stained gauzes vs surgical masks 0.999) showed that dogs trained on axillary-sweat-stained gauzes of SARS-CoV-2 infected individuals were perfectly capable of transferring this capability to breath samples trapped on surgical masks.
4. Discussion
4.1. Main findings
Our present results showed that (a) trained dogs can even detect SARS-CoV-2 upon presentation of sweat samples from individuals with a low viral load or a negative PCR test, (b) the diagnostic sensitivity and specificity are generally close to or over 85%, although the sensitivity falls to 77% in asymptomatic patients or patients with atypical, mild symptoms, (c) a short time interval after vaccination appears to be associated with a greater risk of false positives, and (d) there is no difference in the dogs' ability to detect the presence of SARS-CoV-2 on sweat-stained gauzes and easy-to-obtain surgical masks worn for only 10 min.
In a recent review [12] and a WHO comprehensive summary [40] of studies of the canine olfactory detection of COVID-19, the sensitivities for various types of human samples ranged from 65% to 100% and the specificities ranged from 76% to 99%. The sensitivities and specificities recorded in the present study (table 2) fall within the range for earlier studies of sweat samples, including our own studies [15, 18, 22–25]. In our proof-of-concept study on axillary sweat samples [22], the detection dogs' success rate ranged from 76% to 100%. However, the scope of our first study was limited by the repeated use of some positive samples during the testing sessions, the use of mocks, and the presence of only one positive sample in each line-up. Our second study [24] had a randomized, double-blind design protocol; the individual sensitivities for the seven trained dogs ranged from 60% to 94% (with values over 85% for six of the seven dogs) and the specificities ranged from 78% to 92% (again with values over 85% for six dogs). The present study involved the same seven trained dogs and was based on the same sampling and testing protocols. In contrast, the samples were retrieved from a single hospital (rather than 13), and the testing was performed in a dedicated facility at Alfort School of Veterinary Medicine (rather than inside a fire station). The risk of severe COVID-19 increases in older adults and as the number of underlying medical conditions (like cardiovascular disease, diabetes, chronic respiratory disease, and cancer) increases in a person. These underlying medical problems could also interfere with the dog's ability to detect the SARS-CoV-2 signature. However, the present study (of patients with well-documented diseases and medical care recruited from an emergency department with frequent concomitant diseases (table 1)), our previous study of samples from 13 French hospitals [24], and a study performed in collaboration with the authorities in the United Arab Emirates [23] suggest that underlying health conditions do not interfere with (or only weakly interfere with) canine olfactory detection of COVID‐19 in sweat. Indeed, another recent study of dogs having been intensively trained for seven weeks with a conventional conditioning method reported similar levels of sensitivity and specificity for the detection of SARS-CoV-2 on face masks or clothes worn for 24 h by 50 PCR-positive and 70 PCR-negative hospitalized patients [18].
4.2. Sweat versus mask samples for sniffer dogs
The present study also provided important new information on detection dog's performance levels and particularly the fact that sweat-stained gauzes and surgical masks yielded similar sensitivities and specificities (table 2). In a recent four-dog study [21] of discarded masks worn for 30–45 min by 24 PCR-positive patients with COVID-19 symptoms vs 10 PCR-negative patients with diabetes or respiratory diseases, the mean accuracy was 85.2% and the mean PPV was 64%. The latter PPV is lower than value calculated in the present study for masks worn for only 10 min. This disparity might be explained by the less intensive training in Mendel et al's study [21], rather than a confounding VOC profile in patients with diabetes and non-COVID-19 respiratory diseases [18, 23, 24], the present study. Another recent study of face masks or clothes worn for 24 h [18] reported a sensitivity of 86.0% and a specificity of 92.9%, although the results for masks vs clothes were not described separately. The present study provides significant new evidence to show that intensively trained detection dogs can screen for SARS-CoV-2 on easy-to-obtain face masks worn by individuals for as little as 10 min.
4.3. Viral load and the detection dogs' performance
Furthermore, we also showed that PCR-positive patients with a Ct-value >30 and a Ct-value ⩽30 can be detected with similar, high (>86%) levels of sensitivity and specificity. This finding is in line with a very recent study published online by researchers at the London School of Hygiene & Tropical Medicine; by sniffing socks worn for 3 h, the six detection dogs displayed individual sensitivities of 82% to 94% and individual specificities of 76% to 92% [19]. There was no evidence of an association between sensitivity and the sample's viral load (as estimated through the Ct value), and mathematical modelling suggested that the dogs' level of performance was similar to that of an RT-PCR test [19]. Furthermore, a Bayesian latent class analysis (not requiring the use of an external reference standard) suggested that the detection dog's olfactory sensitivity (89%) for sweat samples was greater than that of an RT-PCR test (73%) performed on nasal swabs in a 99.5%-PCR-negative population attending a COVID-19 screening centre [20].
4.4. Detection dog positioning at the point of care
The RADTs for SARS-CoV-2 infection on nasopharyngeal swabs have very varied sensitivities, ranging from 12% to 98%; their sensitivity in asymptomatic patients or patients with a normal or low viral load is much lower (<50%) than in symptomatic patients or patients with a high viral load [41–45]. The RADTs' relatively low sensitivity can lead to false-negative results—particularly in asymptomatic infected patients and in patients in the early or late phases of the infection typically associated with a low viral load [46]. The risk of false-negative results may limit the usefulness of RADTs for mass screening, since up to 40%–50% of cases may be attributable to transmission from asymptomatic or presymptomatic people [2, 45]. Conversely, the RADTs' high specificity (close to 100%) means that the false‐positive rate is low. RADTs may therefore constitute a quick, easy-to-perform alternative for differentiating between highly contagious and less contagious of individuals infected by the SARS-CoV-2. The European Centre for Disease Prevention and Control (ECDC) has adopted the World Health Organization's minimum performance criteria (sensitivity ⩾80% and specificity ⩾97%) for RADTs [47]; dog detection meets the WHO and ECDC criterion for sensitivity but not the criterion for specificity.
Dog detection and RADTs have several operational advantages over NAATs for the detection of SARS-CoV-2, including their possible use as 'point-of-care'/near-patient screens. The advantage of dog detection include high sensitivity, a very short turn-around time, low cost, and ease of application (particularly for face masks). It has been estimated that a single dog is able to screen hundreds of people a day, at a rate of ∼200 h−1 [40, 48]. RADTs are inexpensive, easy-to-use and more specific than dog detection. The turn-around time ranges from 10 to 30 min. However, RADTs have drawbacks: the invasive nature of nasopharyngeal swabbing, and a lower diagnostic sensitivity than dog detection—particularly in asymptomatic, infected patients and in patients with a low viral load. Highly sensitive tests are best used for screening, while highly specific tests are best for confirming a diagnosis. When considering all these aspects and the results of the present study, a 'dogs + confirmatory RADTs' strategy might be appropriate for the rapid screening of individuals in busy areas (e.g. airports and railway stations) or remote areas.
4.5. The olfactive signature of a SARS-CoV-2 infection
It is likely that the dogs are detecting a signature resulting from SARS-CoV-2 infection, rather than replication-competent virus per se. This hypothesis is supported by the fact that individuals with a Ct value <30 or >30 yield similar sensitivity and specificity values with dog detection. Furthermore, the relative increase in the proportion of false positives in healthy, recently vaccinated individuals and the sweat samples (marked by all the dogs) from the three PCR-negative COVID-19 patients in the present study suggest that the scent is related (at least in part) to the host's response to the viral infection or the vaccine. Furthermore, sweat samples from infected individuals are rarely PCR-positive [15] and urine samples have never yielded virus [49]—suggesting that dogs are able to detect metabolic products of the host response to the infection released into sweat and urine [15, 16].
Face masks collect VOCs from exhaled breath and exhaled droplets containing entrapped semivolatile and non-volatile compounds [50]. It has been suggested that trained cancer-sniffing dogs rely on the less volatile compounds from breath aerosol [51]. The VOCs from the exhaled breath of SARS-CoV-2 infected patients have variously been analysed (a) using electronic noses [52, 53], (b) using a real-time, online, proton transfer reaction time-of-flight mass spectrometer [54], (c) by collecting a breath sample in a bag prior to analysis with an electronic nose [55, 56], and (d) by collecting a breath VOC sample in a bag and loading sorbent tubes prior to gas chromatography-mass spectrometry analysis [57]. Taken as a whole, the results of these studies suggest that variations in the breath VOC profile can distinguish between COVID-19 patients and non-COVID-19 (but SARS-CoV-2-infected). The exhaled droplets can be collected as a breath condensate prior to analysis using RADTs or NAATs [51, 58]. In the present study, the dogs trained on axillary-sweat-stained gauzes of SARS-CoV-2 infected individuals were perfectly capable of transferring this capability to breath samples trapped on surgical masks worn for only 10 min. This new finding strongly suggests that there is a degree of overlaps between the compounds contained in and then released respectively by sweat samples and breath samples from SARS-CoV-2 infected individuals. In a recent study [15], the sniffer dogs' ability to transfer performance from saliva samples to urine and sweat samples also suggested that SARS-CoV-2-associated compounds are released in several body secretions.
4.6. Study limitations and strengths
Our study had a number of limitations. Firstly, we presented the detection dogs with samples, rather with people for screening. However, face-mask-based screening could be organised easily in public areas, such as platform access points or airport arrival gates. Secondly, the recruitment of patients and volunteers from a single hospital might have reduced or mitigated the risk of errors induced by background odours from different sampling sites. Thirdly, we studied a small number of PCR-negative patients with cold-like symptoms; hence, we could not demonstrate that the dogs detected a SARS-CoV-2-specific odour and not an odour associated with other viral respiratory infections (e.g. influenza). However, the results of in vitro studies have suggested that different viral infections result in distinct odour profiles [19].
This study also had several strengths. The samples' positions in the line-up were randomized, and the handler and the dog were blinded to the positions of the positive samples. Each dog sniffed a given sample only once and the paired face mask and sweat gauzes were sniffed in a randomized order and in different line-ups. Furthermore, we recruited a large number of individuals, whose true infection status was determined not solely by RT-PCR (which is not a perfect reference test) but also by a patient interview, a clinical examination, and (in 83.5% and 27.3% of the PCR-positive and control patients, respectively) a CT scan of the chest (table 1 and figure 2).
Overall, our present results confirm the dog's extraordinarily sensitive and specific sense of smell. However, dogs are influenced by boredom, a limited attention span, hunger, fatigue, and external distractions; hence, they can typically only work effectively for relatively short periods of time. Furthermore, dog-handler interactions have a major impact on detection work. In particular, the handler must pay special attention to any signs of fatigue in the dog. Furthermore, each dog needs to be individually trained and then continually evaluated for performance using fresh challenge samples, which constitute time-consuming processes [59, 60].
5. Conclusion
The present study's results extended our knowledge of the detection of SARS-CoV-2 infection by trained dogs and strongly supports the concept of dog detection as a front-line, non-invasive, efficient, cost-effective method for screening retrieved face masks for SARS-CoV-2 infection. A 'dogs + confirmatory RADTs' strategy might be appropriate for the rapid screening of individuals in busy areas. Further research is needed to assess (a) the feasibility, performance and acceptability of dog detection as a public health intervention tool in busy areas (e.g. airports and railway stations) or remote areas, (b) means of ensuring the safety of the handlers and their dogs, and (c) ways of overcoming the difficulties of training dogs in low-prevalence settings. In an attempt to resolve this later point, we are now looking at whether gauzes impregnated with recombinant SARS-CoV-2 proteins can be used to train detection dogs.
Data availability statement
The data that support the findings of this study are available upon reasonable request from the authors.
Acknowledgments
The authors thank David Fraser D Phil (Biotech Communication SARL. Ploudalmezeau, France) for medical writing support.
Collaborative group of authors with emails
Sébastien Charreaudeau:sebastien.charreaudeau@sdis78.fr
Louis-Jean Couderc:louisjean.couderc@gmail.com
Chantal Coudert:chantal.coudert@sdis78.fr
Loïc Desquilbet:loic.desquilbet@vet-alfort.fr
Nicolas Dirn:nicolas.dirn@sdis60.fr
Jean-Michel Duquesne:jean-michel.duquesne@sdis78.fr
Alexandre Forget:alexandre.forget@sdis78.fr
Katia Hamon:katia.hamon@sdis78.fr
Fabien Gasmi:fabien.gasmi@sdis78.fr
Antoine Magnan:a.magnan@hopital-foch.com
Quentin Muzzin:quentin.muzzin@vet-alfort.fr
Sébastien Petitjean:sebastien.petitjean@sdis78.fr
Footnotes
** The study was funded by the World Health Organization (WHO BluePrint Program; 2020/1062 930-0) and the Hôpital Foch's endowment for research.