Abstract
Volatile organic compounds (VOCs) in exhaled breath have the potential to be used as biomarkers for screening and diagnosis of diseases. Clinical studies are often complicated by both modifiable and non-modifiable factors influencing the composition of VOCs in exhaled breath. Small laboratory animal studies contribute in obtaining fundamental insight in alterations in VOC composition in exhaled breath and thereby facilitate the design and analysis of clinical research. However, long term animal experiments are often limited by invasive breath collection methods and terminal experiments. To overcome this problem, a novel device was developed for non-invasive breath collection in mice using glass nose-only restrainers thereby omitting the need of anesthetics. C57Bl/6 J mice were used to test reproducibility and different air sampling settings for air-flow (ml min−1) and time (minutes). Exhaled air was collected on desorption tubes and analysed for VOCs by gas chromatography time-of-flight mass spectrometry (GC-tof-MS). In total 27 compounds were putatively identified and used to assess the variability of the VOC measurements in the breath collections. Best reproducibility is obtained when using an air flow of 185 ml min−1 and a collection time of 20 min. Due to the non-invasive nature of breath collections in murine models, this device has the potential to facilitate VOC research in relation to disturbed metabolism and or disease pathways.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
In the past few decades there has been an exponential interest in the diagnostic potential of exhaled breath [1, 2]. Exhaled breath contains volatile organic compounds (VOCs) that are produced during normal, as well as abnormal, metabolic processes in organisms. (Patho-)physiological processes, such as ingestion of food, exercise, infection, and inflammation, result in alterations in the metabolism and subsequently changes in the composition of exhaled VOCs [3]. Collection of breath is a non-invasive procedure and can be performed rapidly. Despite years of research into exhaled breath analysis, only a few breath tests, such as the heart transplant rejection breath test or the 13C-urea breath test for diagnosis of H. pylori infection, are approved for clinical use [4, 5].
In humans, several modifiable and non-modifiable factors influence the VOC composition, thereby complicating analysis of exhaled air [3]. Standardization of breath collection methods is strongly recommended in human breath research [1]. However, options for standardization are limited. Modifiable factors including fasting state, smoking or physical activity, should be taken into consideration when sampling the exhaled breath. Non-modifiable factors on the other hand, including age, gender, comorbidities, and medication used cannot be standardized for breath research. Due to the limited possibilities for standardization, and depending on the incidence of a specific disease, current clinical studies often need large study populations in order to develop diagnostic models [1].
In order to gain fundamental insight in alterations in VOC composition induced by (patho-) physiological processes, in vivo models come into play. Animal studies can provide more standardized models since factors such as comorbidities, diet, housing and other environmental influences can be controlled more easily. In addition, animal studies can be performed in a shorter period of time and require a smaller sample size. Since diseases can be induced, the target number of diseased subjects can be reached earlier. Therefore, animal studies can serve as proof-of-concept before setting up large clinical studies.
Breath collection in small laboratory animals is more difficult compared to human breath or large animals, such as sheep, goat or cattle where a mask or mouthpiece can be used [6]. A potential pitfall of collecting breath from small laboratory animals is the possible contamination of the collected air with VOCs of other origins that should be taken into consideration when collecting exhaled breath from laboratory animals [7].
Most previously published studies use anesthetics and tracheal cannulation or tracheostomy in order to exclusively collect exhaled breath during ventilation. This mainly results in experiments with terminal outcomes and the inability for long term follow up and the loss of animals during breath collection [8–12]. Besides the invasive character of the breath collection, administered anesthetics, such as isoflurane, potentially influence the VOC profile [13]. On the other hand, exhaled breath can also be collected from animals that are awake using either whole body or nose-only techniques [14–16]. However, literature is limited and described sampling techniques are not well standardized.
This proof of concept study describes the proof of concept study of a new custom-made breath sampling device for non-invasive collection of VOCs in exhaled breath from non-anesthesized mice. It gives the possibility to sample up to five animals simultaneously in a flow-controlled, standardized way and minimizing the effects of ambient air. Different settings are tested and a set of 27 targeted compounds were identified to verify the collection of exhaled breath and analyze variation between breath collections.
2. Methods
2.1. Animals and housing conditions
Five male C57Bl/6 J mice, aged 8–10 weeks (Charles River laboratories, The Netherlands) were used for this study. All animals were housed at the Maastricht University animal facilities under standardized conditions. The mice were socially housed with a maximum of five mice per cage and a 12 h day-night cycle. They had ad libitum access to standard rodent chow and water. Food was provided from the same batch before and during the entire experiment. The protocol (AVD1070020185704) was approved by the Animal Ethics Committee (DEC) of the Maastricht University and the Central Authority for Scientific Procedures on Animals (CCD).
To reduce the influence of stress, all mice were trained for four consecutive days to enter and stay in the restrainers. One glass restrainer was placed in the cage of the mice to allow the animals to discover the restrainer and get used to it on the first day. At the end of this day the mice were placed in the restrainer for a brief period of only a few minutes. The days thereafter were used to place the mice in the restrainers multiple times a day with increasing the time up to 25 min. The final training day, the nose cone and airflow along the nose of the animal were added to the training simulating the entire breath collection procedure. During the training procedure and breath collections, the mice were closely observed by the researchers for signs of abnormal behavior or discomfort.
2.2. Collection device
A custom made sampling device was developed in collaboration with CH Technologies (Westwood, New Jersey, USA) for non-invasive collection of exhaled breath from mice (figure 1). The restrainers are made of glass, which is easy to clean, which is known to be inert and which is expected to have little impact on the VOCs secreted by the animals. The tubing used is made of 1/4'' OD Polyurethane or 2.06 mm ID Viton. A schematic overview is provided in figure 2.
Figure 1. Sampling device for non-invasive collection of exhaled breath from mice.
Download figure:
Standard image High-resolution imageFigure 2. Schematic overview of the breath sampling device. With (a) placement of the animal in restrainer, (b) acrylic nose cone with sorbent tube and (c) CO2 monitoring.
Download figure:
Standard image High-resolution imageBefore breath sampling, mice were placed in the glass restrainers (figure 2(a)) after which they inhale filtered air (Clean Air Supply Pump for ReCIVA (CASPER), Owlstone Medical, Cambridge, UK), which is conducted through a transparent acrylic nose cone (figure 2(b)) that is placed over the end of the restrainer. Exhaled breath is immediately collected on stainless steel desorption tubes within the same nose cone. This device offers the possibility to collect exhaled breath of up to five mice simultaneously.
2.3. Restrainers
Glass nose-only restrainers were made to keep the animals with the tip of their nose in a small 6 millimeter opening (figure 2(a)). The restrainers are tailor-made according to the weight range of the animals. Depending on the size or type of animal, restrainers with a different size can be connected to the device. The diameter of the restrainer prevents the animal from turning and ensures a natural breathing pattern. Restrainers are closed with a rubber stopper and mice are held in place with the help of a four arm pusher containing openings for the tail.
2.4. Air supply
An air supply providing a flow up to 40 l min−1, using an activated carbon and HEPA filter (Clean Air Supply Pump for ReCIVA (CASPER), Owlstone Medical, Cambridge, UK) containing Airpel® (Desotec Ltd, Roeselare, Belgium) is used to reduce VOCs and other contamination in inhaled air. According to instructions of the supplier the filter should be replaced after one year of sampling or 900 breath collections. Provided filtered air is divided over six connector pieces via an intake manifold and a total supply air flow meter. Five connector pieces are attached to the acrylic nose only cones that are placed over the opening of the restrainers. This provides an adjustable air flow along the nose of the animals. The sixth connector is placed over an empty restrainer and is used for system blanks and pressure monitoring. At the end of the device, a vacuum system provides a negative pressure to trap the exhaled breath containing the animals VOCs directly onto 1/4'' × 3.5'' stainless steel collection tubes. Tubes with different diameters or sorption material can also be used due to adjustable connector pieces between the collection tube and the air supply.
2.5. Flow control and monitoring
Changeable flow controlling chokes of either 75, 139, 185, 239 or 304 ml min−1, determine the airflow through the connector piece and the collection tubes. A pressure gauge is connected to a sixth empty restrainer to measure the air pressure in the restrainers during breath collection. In order to keep a neutral pressure in the restrainers, a balance between the incoming air and the vacuum pressure at the end has to be obtained. An adjustable air flow meter, which is placed before the connector pieces, is used to control this balance.
Carbon dioxide (CO2) concentrations in the exhaled breath of the animals are monitored by using the Jaeger Metabolic Analysis (JAMA) system, consisting of multiple real-time fast non-dispersive infrared CO2 sensors (CH Technologies, Westwood, New Jersey, USA). Sensors are placed behind each desorption tube for continuous monitoring of the CO2 emission and thus providing an indication of the rodents wellbeing (figure 2(c)).
2.6. Breath collection
Prior to the breath collection, mice are placed in the nose-only restrainers with the air supply connected via the connector pieces for approximately 5 min to flush their lungs with clean air. For this study, 1/4'' × 3.5'' stainless steel tubes with Tenax TA 35/60 carbograph 5TD 40/60 sorbent (Camsco©) were used. Based on a tidal volume of 0.2–0.3 ml and a breath rate of 250–290 min−1 [17], an estimation was made for the time needed to collect at least 1.0–1.5 l of exhaled breath from the animal. The settings for the air flow were selected based on the characteristics of the collection tubes. Therefore, an air flow of 185 ml min−1 and 239 ml min−1 were tested with a collection time of 20 or 25 min. Immediately after breath collection, the capped tubes were stored at 4 °C until analysis.
Breath collection was performed on two consecutive days at the same time of day in order to avoid any possible influences on the circadian rhythm of the animals. The first day consisted of five breath collections of three animals (table 1(a)). Each of the animals was placed in one of the restrainers for breath collection and, each time, moved to the next position for the following collection. This way possible influences of the different restrainers on the breath profiles was examined. Differences between air flow and collection time was tested the second day (table 1(b)). Breath sampling consisted of four breath collections per animal (n = 5). Every sample was collected from the same animal at the same position in the device. A flow of 185 ml min−1 and 239 ml min−1 respectively were used with a collection time of 20 and 25 min. Additionally, a total of seven breath collection system blanks were collected from the 6th restrainer.
Table 1. Overview of the breath sampling schedule. (a) Position effect; First day of sampling using three different mice (M) in all different positions (P) over time, the air flow was set on 185 ml min−1 with a collection time of 20 min. (b) Settings effect; Second day of sampling using five different mice in the same position using a flow of 185 ml min−1 and 239 ml min−1 with a collection time of 20 and 25 min.
| (a) | |||||
|---|---|---|---|---|---|
| Time (hh:mm) | Position | ||||
| P1 | P2 | P3 | P4 | P5 | |
| 10:00 | M1 | M2 | M3 | X | X |
| 11:30 | X | M1 | M2 | M3 | X |
| 13:00 | X | X | M1 | M2 | M3 |
| 14:30 | M3 | X | X | M1 | M2 |
| 16:00 | M2 | M3 | X | X | M1 |
| (b) | |||||
| Time (hh:mm) | Flow (ml min−1) | Time (min) | |||
| 11:30 | 185 | 20 | |||
| 13:00 | 185 | 25 | |||
| 14:30 | 239 | 20 | |||
| 16:00 | 239 | 25 | |||
2.7. Exhaled breath
The collected breath samples were stored until analysis and measured in a randomized order. Samples were measured by thermal desorption (TD)-gas chromatography combined with time-of-flight mass spectrometry (GC-tof-MS) which was able to detect compounds with molecular masses ranging from 35 to 350 [18]. 5-D-bromobenzene was used as internal standard to account for instrument variation over time. In short, the VOCs trapped on the desorption tubes were released at 250 °C using TD under a flow of helium. Subsequently, the vaporous sample was divided into two parts of which 25% was used for analysis and 75% was recollected into the same desorption tube. The sample for analysis was transmitted to an electrically-cooled sorbent trap programmed at 5 °C for sample concentration. Subsequently, the sample was injected into the GC (Thermo Fischer Scientific, Austin, USA, column: Restek RTX-5 ms, 30 m × 0.25 mm ID, coated with 1.0 μm HP-5 phase). Compounds were separated by GC during a gradually increasing temperature: first 40 °C for 5 min, then raised by 10 °C every minute until 300 °C was reached. This final temperature was kept for 5 min.
The separated compounds were detected and identified by tof-MS (Bench TOF-dx, Alsmco International, Llantrisant, Wales, UK) using electron ionization at 70 eV with 5 Hz scanning rate and a mass range of m/z 36–350.
2.8. Data preprocessing
Preprocessing of the raw data was performed in order to moderate effects of various artifacts during chemical analysis such as noise, baseline shift or column aging. Reducing this variance results in a more reliable data matrix for statistical analysis [19]. Before the initial data preprocessing masses corresponding to carbon dioxide and argon were removed as initial background subtractions. The first consequent step in preprocessing is excluding parts of the chromatograms containing noisy areas at the beginning, i.e. retention time < 1.4, and at the end of each chromatogram, i.e. retention time > 25), followed by background noise removal using wavelets transformation and baseline corrections. The signal to noise ratio was set on 100. Column aging, changes in temperature or other unknown instrumental variations during chemical analysis can result in variations in retention times between samples. Therefore, the next preprocessing step consisted of accurate alignment of all samples, by correlation optimized warping [20], or correction for variation of retention times between samples. This technique aligns each chromatogram into a reference one, here the one having the highest similarity to all chromatograms in the data. The alignment is based on compressing, shifting and stretching of different segments in each chromatogram with the same segment length in a reference chromatogram and consequent alignment of them. The position with highest correlation is then selected as the new position in the aligned chromatograms. The final steps were combining the calculated area under the peak and corresponding mass spectra and using probabilistic quotient normalization.
2.9. Data analysis
All statistical analysis were performed using Matlab R2019a (MathWorks, Natick, Massachusetts, USA). The preprocessed data was first analysed by means of unsupervised random forest (URF) to find natural trends and groupings in the data. URF uses the main principle of random forest ensemble technique and uses sets of predefined number weak decision trees (e.g. 1000), but it makes an assumption that if the data embraces any possible trends (e.g. relevant class groupings), it should be differentiated from a randomly generated version of itself [21]. The possible trend in the data is subsequently visualized using a proximity matrix, which represents a similarity measure among samples. Finally, principal coordinates analysis (PCoA) was performed on the proximity matrix and the trends and groupings in the data were visualized in score plots.
Breath collection from the same animals were used for testing the effects of the collection time and different air flow used in the experiment, resulting in possible intra-individual variation obscuring the data of interest. Variation centering was performed to diminish the influence of intra-individual variation, allowing the relative compound concentration to fluctuations around zero for each individual instead of around the population mean.
Regularized multivariate analysis of variance (rMANOVA) was performed to analyze VOCs that are statistically different between the different factors, i.e. collection time and flow, as well the interaction between them [22].
3. Results
Five male C57Bl/6 mice of 8 weeks old were used. Training of mice consisted of four consecutive days of practice of entering and leaving the restrainers with gradually increasing the time spent in the restrainers for up to 25 min. At the end of the training period, mice were able to enter the restrainers without resistance and leave the restrainers themselves. After sufficient training, mouse were calm in the restrainer and no abnormal behavior was observed when returned to their cage. There was no drop-out of animals during the training period or breath collections.
A representative exhaled murine breath VOCs profile is shown in figure 3. In the chromatogram the compounds ethanol, acetone, pentane, heptane, toluene, xylene, decane, 3-carene, nonanal and decanal were arbitrarily indicated. These VOCs were previously found to be present in exhaled breath and related to either normal or abnormal metabolic processes [23–26].
Figure 3. The representative VOCs profile of a mouse exhaled breath with selected and identified compounds; 1 = ethanol, 2 = acetone, 3 = pentane, 4 = heptane, 5 = toluene, 6 = xylene, 7 = decane, 8 = 3-carene, 9 = nonanal, 10 = decanal.
Download figure:
Standard image High-resolution imageIn order to investigate the reproducibility and overall variations of the presented breath collection device a set of 27 compounds (table 2), both of exogenous and endogenous origin, were putatively identified and extracted from samples collected for mouse breath, breath collection system blanks and TD tubes blanks. For quality control purposes the following compound standards were used; ethanol, acetone, isoprene, butanal, benzene, pentanal, toluene, octane, hexanal, nonane, styrene, heptanal, decane, octanal, limonene, nonanal, dodecane and decanal. The other compounds; carbon disulphide, 2-butanone, xylene, 2-nonene, alpha pinene, 3-carene, undecane, tridecane, undecanal, were tentatively identified based on a match score of >850 in the NIST library (NIST17). For each specific compound the peak area's in the chromatograms were determined and used for calculation of the relative standard deviation (RSD) to examine the variation for the different settings that were tested and are tabulated in table 2. In this case five mice were sampled at the indicated flow and time. The RSD of the internal standard, 5-D-Bromobenzene, is 17.2% which can be interpreted as variations of the entire analytical system. For the majority of the compounds, the lowest RSD is observed for a flow of 185 ml min−1 with a collection time of 20 min.
In figure 4 the PCoA score plot obtained for data containing the set of 27 targeted compounds is shown. As can be seen the breath samples are clearly separated from system blanks and TD blanks. The creation of three separated clusters along PCo1 and PCo2 indicates a clear difference in profile of the selected 27 compounds.
Figure 4. Principal Coordinate Analysis score plot for data containing a set of 27 targeted compounds (table 2) for exhaled breath of mice, system blanks and thermal desorption (TD) tube blanks.
Download figure:
Standard image High-resolution imageTable 2. Compound list with 27 compounds tentatively identified in exhaled breath of mice (n = 5 per group) and the relative standard deviation (%), based on the peak area's, of each compounds for all different settings. The lowest relative standard deviation per compound is highlighted in green.
| Sampling settings | ||||
|---|---|---|---|---|
| Compounds | F185 T20 | F185 T25 | F239 T20 | F239 T25 |
| Ethanol | 18,8 | 15,5 | 31,3 | 13,2 |
| Acetone | 16,4 | 4,6 | 4,5 | 8,2 |
| Isoprene | 43,8 | 90,4 | 122,3 | 104,6 |
| Carbon Disulphide | 55,5 | 48,1 | 17,2 | 52,7 |
| Butanal | 18,1 | 21,1 | 17,9 | 20,7 |
| 2-Butanone | 19,2 | 13,7 | 14 | 7,9 |
| Benzene | 15,5 | 22,1 | 22,1 | 24,1 |
| Pentanal | 31,5 | 13,2 | 16,2 | 11,3 |
| Toluene | 3,9 | 4,1 | 8,5 | 12,2 |
| Octane | 29,5 | 22,8 | 8 | 27,1 |
| Hexanal | 15,4 | 13,4 | 13,4 | 12,2 |
| Xylene | 5,5 | 5,8 | 7 | 7,4 |
| 2-Nonene | 16,1 | 19,5 | 31,4 | 19,1 |
| Nonane | 9,4 | 10,3 | 23,9 | 14,7 |
| Styrene | 4,7 | 6,7 | 7,6 | 10,7 |
| Heptanal | 42,5 | 36,8 | 23,1 | 13,1 |
| Alpha Pinene | 4,9 | 7,2 | 8,6 | 15,6 |
| Decane | 6 | 5,6 | 8 | 10,9 |
| Octanal | 15,5 | 19,6 | 10,6 | 11,1 |
| 3-carene | 6,9 | 9,9 | 13,2 | 17,9 |
| Limonene | 3,3 | 3,4 | 4,2 | 5,5 |
| Undecane | 5,1 | 3,6 | 4,7 | 5,8 |
| Nonanal | 26,1 | 15 | 32,1 | 23 |
| Dodecane | 5,1 | 3,9 | 4,5 | 6,2 |
| Decanal | 29,6 | 17,1 | 36,3 | 33,2 |
| Tridecane | 5,3 | 5,4 | 5,6 | 7,6 |
| Undecanal | 14,2 | 13,9 | 14,2 | 9,4 |
In order to investigate the effect of restrainer position and the time and flow, the complete breath profile was examined by means of PCoA. Different conditions, as indicated in table 1, resulted in a total of 35 breath samples. The PCoA analysis was performed on the VOCs that were present in at least 20% of the data, which accounted for 290 VOCs.
3.1. Position in the device
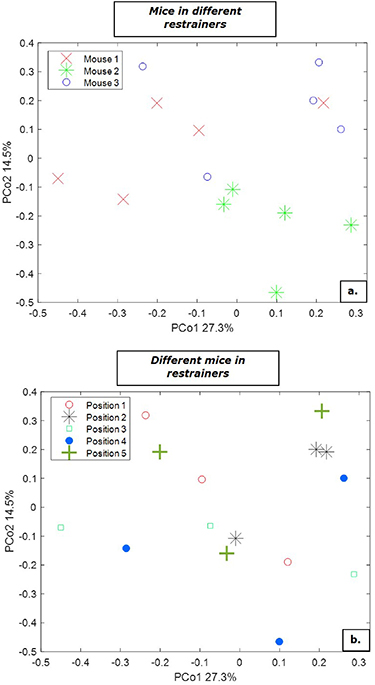
Three different mice were used of which breath samples were collected in all five different restrainers resulting in five repeated measurements per animal. In total 15 breath samples were collected from three different mice. Results are shown in figure 5. Every point in the PCoA represents one exhaled breath profile of a mouse. PCoA score plot shows similar breath profiles for each individual mouse, independent of the position of the restrainer (figure 5(a)). Each of the examined mice create a distinct cluster in the PCoA score plot. Breath samples of mouse 2 show the most similarities, since all points gather together and do not overlap with measurement of other mice. For mouse 1 and 3, there are few points that are separated from its own cluster. Two measurements of mouse 3 overlap with measurements of mouse 1. Similarly, one measurement of mouse 1 shows overlap with measurements of mouse 3. The results shown in figure 5(a) suggest that between-mice-variations are dominating over within-mice-variations. The analysis of different positions in the restrainers is shown in figure 5(b). The score plot shows no clustering with respect to the different restrainers used in the sampling device. These results show that the exhaled breath collections of each mouse are similar and independent of the position in the device. Moreover, the technical aspect of the sampling device does not generate a source of variance that might potentially obscure the information of interest.
Figure 5. Principal coordinate analysis (PCoA) score plot for repeated breath samples of three different mice. The samples are color-coded with respect to (a) mouse number (b) position of a mouse in the different restrainers.
Download figure:
Standard image High-resolution image3.2. Flow and time
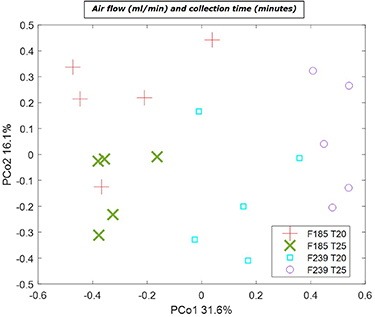
Four different settings for air flow and collection time were tested using five different mice resulting in 20 breath samples. Results are shown in figure 6. Separation based on the air flow is seen along PCo1 with the largest variation observed between samples collected with a flow of 239 ml min−1. In contrast to the collection time of 25 min the samples collected for 20 min show lesser variation for both the air flow of 185 ml min−1 and 239 ml min−1. However, the collection time has the highest effect on variation in the samples collected with a flow of 239 ml min−1. This can be particularly observed in figure 6 where each collection time, 20 and 25 min, for flow 239 ml min−1 create distinct clusters. These distinct clusters are not clearly visible for flow 185 ml min−1.
Figure 6. Principal component analysis (PCA) score plot for repeated breath samples of five different mice using different settings for air flow of 185 or 239 in ml min−1 (F185 and F239, respectively) and collection time in minutes for 20 or 25 min (T20 and T25, respectively).
Download figure:
Standard image High-resolution imageTo further investigate the effect of collection time and flow on the VOCs profile, rMANOVA was performed. The analysis yielded significant differences in VOC profile for different collection time and flow as individual factors, as well the interaction between them with p-value < 0.0001. This significant interaction effect signals the variation is dependent on both factors simultaneously, and thus the commonly encountered One-Variable at a Time approach for optimization would not necessarily result in the best overall optimal condition.
4. Discussion
This study describes a new custom-made breath sampling device for non-invasive collection of exhaled breath in small laboratory animals. In our proof of concept study, we show the ability to collect exhaled breath from mice without the need of anesthetics or invasive procedures. Moreover, the variability of the identified compounds between breath samples of mice in the different settings shows the best results when using an air-flow of 185 ml min−1 and a collection time of 20 min.
The main advantage of this specific device is the ability to collect exhaled breath in animals that are awake, allowing multiple breath collections from the same animal without the need of anesthetics or invasive procedures. In addition, the design is developed to minimize possible contamination with VOCs from different origins, such as environmental air, materials of the set-up, fur, feces or urine.
The device can be used for research with small laboratory animals and, depending on the research model, can easily be adjusted to different sizes of animals or collection tubes. Multiple breath collections over time enables longitudinal follow-up which can contribute to a more fundamental understanding of (patho-)physiological processes. This method could be of specific interest in prospective experiments to identify biomarkers for early detection of diseases, disease progression, or monitoring therapy responses.
Use of small laboratory animals in exhaled breath research is limited by difficulties in collecting exhaled air without contamination of other possible origins. In contrast to larger animals or non-human primates, where a mask can be placed over the muzzle [6], pure exhaled breath is more difficult to collect in small laboratory animals. Most studies use anesthetized and ventilated animals in order to collect pure exhaled breath from the ventilation system. Although the risk of contamination of exhaled breath by exogenous sources is low, the method of collection is invasive and long-term follow-up is practically impossible. Anesthetizing animals for a longer period of time results in a number of factors that can potentially interfere with the VOCs measurements. The inability to move around, fasting state and alterations in the circadian rhythm might result in changes in metabolism [27].
Respiratory conditions or VOCs derived from the respirator itself may also interfere with the measurements [28]. Inhalation anesthetics, such as isoflurane, can still be detected in exhaled breath even several days after surgery [13, 28].
Identified compounds in the current study were previously described in relation to, amongst others, oxidative stress, dietary response or as product of gut bacteria. Others can be found as an air pollutant in environmental air and can be absorbed by an organism and subsequently detected in exhaled in breath which cannot be removed completely during the wash-out period with filtered air [23, 24, 26]. Before and after each breath collection the animal was placed under normal housing conditions with unlimited access to water and food. Environmental VOCs can be taken up by the animal and will be slowly excreted thereafter. The animals in this experiment did not receive any specific treatment. Therefore, the endogenous compounds that were detected are considered as derived from normal metabolic processes in the body. Compounds related to exercise, such as isoprene, show a rapid increase after movement explaining variation in the breath samples since the animals were still able to move their legs and snout in the restrainers [25].
Until now there are only a few studies describing a breath collection method in animals that are awake [14, 16, 29–31]. These studies use either a whole body respiratory chamber or a conical tube connected to a collection or direct measuring system. As the physiological response to stress results in metabolic changes interfering with the VOCs measurements, the experienced stress should be kept to a minimum. The advantage of a whole body respiratory chamber is the ability to sample VOCs from an unrestrained animal and thereby limiting the amount of stress experienced by the animal. A respiratory chamber is easy to use and the air in the chamber, the headspace, can easily be collected and subsequently analysed [31]. However, VOCs coming from other origins as fur, feces and urine, will obscure the results and obtaining a clean breath sample is not possible. Previous experimental set ups already showed nose-only sampling to prevent the se contaminations by either placing the animal inside the tube with their muzzle in a small opening [16] or by placing the tube on the muzzle when fixating the animal [14].
A possible limitation of the present study with regard to the effect of the position of the restrainer is the non-random assignment of the animals in the restrainers. This may have resulted in a systematic error, even though no clustering is observed in the current data. Additionally, during the development of the device, certain options were considered for reducing animal discomfort and ensuring the quality of collected breath samples. For instance, monitoring the temperature of the animals could be a valuable add on. However, due to the used materials of an acrylic nose cone and a glass restrainer it was not possible to add a temperature sensor, such as an infrared thermometer, to the system as it cannot penetrate the materials and thus cannot provide a reliable reading. Another option that was explored is to add a rectal measurement via the pusher. However, insertion of a rectal probe will inevitably lead to increased animal discomfort and will also limit the ability to maintain an air-closed system. Further, another limitation of the current device may be limited choices of the flow settings due to the supplied flow chokes and that a continuously adjustable system is preferred for more accurate optimization of flow rates for different animal models and/or desorption materials. A compromise has to be made between the relatively high air flow for the mice, causing stress to the animals, and a sufficient flow to maintain quality of the breath samples.
Animal studies provide insights how pathological processes in a host, such as infections or malignancies, lead to alterations in metabolism and subsequently to changes in the composition of VOCs in exhaled breath. Due to the good possibilities for standardization and the wide availability of different disease models, animal studies can be hypothesis generating that will facilitate the design and analysis of clinical research. It will contribute to the discovery of biomarkers and to the initial selection of VOCs in the detection of specific (patho-)physiological processes. Obviously, the translation from animal to human has yet to be made, but results using the current device could steer the more complex clinical studies that are often difficult to standardize and requiring large numbers of participants and time.
5. Conclusion
This custom-made breath sampling device enables breath collection in murine studies in a natural and non-invasive way, without the need of anesthetics and minimizing contamination from exogenous sources. Future animal studies can be improved by the non-invase nature of breath collections, enabling long-term follow up and a reduction in the number animals needed.
Data availability statement
The data that support the findings of this study are available upon reasonable request from the authors.
Footnotes
* Supported by Dutch Digestive Foundation (MLDS career development Grant CDG16-12 to T Lubbers).