Abstract
Infections by oral pathogens are one of the most common health problems worldwide. Due to the intimate connection between exhaled breath and the oral cavity, breath analysis could potentially be used to diagnose these infections. However, little is known about the volatile emissions of important oral pathogens that are connected with gingivitis and periodontitis. In this study, we have performed in vitro headspace measurements on four important oral pathogens (P. gingivalis, T. forsythia, P. intermedia and P. nigrescens) using proton transfer reaction time-of-flight mass spectrometry (PTR-TOF-MS). Some of the most abundant compounds produced by the bacteria include hydrogen sulphide, methanethiol, acetone, dimethylsulphide, isoprene, cyclopentanone and indole as tentatively assigned from the mass spectra. Several other abundant mass signals were recorded but the assignment of these is less certain. Some of the bacterial species can be separated from each other by the emitted volatile fingerprints. The results of this study can be used in potential development of a diagnostic breath test for oral infections. In addition, as several of the measured compounds are known to be toxic, the results point to an intriguing possibility of studying the connection between the bacterial virulence and the emitted volatile compounds.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
The number of bacterial species present in the human mouth is estimated to be somewhere between 500 and 700 [1, 2]. Exhaled breath is directly connected with the oral cavity, where these oral microorganisms can produce significant amounts of volatile compounds. Oral infections are some of the most common diseases worldwide, with over 11% of the whole adult population having severe periodontal disease [3], and about 30% of elderly people having no natural teeth [4]. Nevertheless, this obvious diagnostic application has been mostly neglected by the exhaled breath community, with the exception of sulphur containing compounds and the diagnostics of halitosis [5–10]. Volatiles produced by other human pathogenic bacteria, such as Pseudomonas aeruginosa, Mycobacterium tuberculosis and Helicobacter pylori, have been studied extensively in recent years [11, 12], but comprehensive reports of oral bacteria and the volatiles they produce are scarce. Volatiles originating from the oral cavity have been mainly considered as a hindrance for breath analysis [13–15], and many of the possibilities they provide have been overlooked. However, some recent publications specifically concentrate on the volatile compounds produced by oral pathogens, and the usage of those compounds as biomarkers [16–18]. The analysis of volatile organic compounds (VOCs) for faster and easier screening of infections has also been a subject of growing interest in general [19]. Sensitive and specific breath biomarkers for oral pathogens could represent a major breakthrough in the non-invasive diagnostics of oral infections and decrease the yearly economic burden of over 400 billion USD consumed by oral diseases [3].
Most of the adult population around the world have some inflammation of the gum tissue, known as gingivitis [20, 21]. When the numbers of anaerobic, Gram-negative, rod-shaped bacteria and spirochetes increase compared to the other bacteria in the subgingival plaque, gingivitis is more likely to occur [22]. These bacteria utilize the gingival tissue for nutrients and produce compounds that irritate and destroy it. The bacteria and the degradation of the gingival tissue cause immune response, which can lead to more serious damage as the surrounding tissue detaches from the tooth. Consequently, the plaque on the tooth surface grows further below in the developing periodontal pockets and causes more inflammation. The bacteria in the subgingival plaque together with heightened immune response cause further destruction of the periodontal ligaments, and eventually alveolar bone is permanently destroyed [23, 24]. At this point gingivitis has developed into periodontitis, a destructive disease with irreversible effects.
It has been established that the risk for developing periodontitis partly depends on the kinds of bacteria present in the subgingival plaque [22, 25]. Traditionally, these oral bacteria have been placed in colour-designated complexes based on their likelihood of causing periodontitis and their association with each other [26, 27]. Red complex bacteria impose a high risk for periodontitis and are strongly co-operative with each other. Orange and yellow complex bacteria are connected to moderate and low risk of periodontitis, respectively. However, in the recent years this colour-complex grouping has been criticized to be an over-simplification, and some new bacterial species have been connected to the development of periodontitis. The dominance of gram-negative bacteria as major pathogens for periodontitis over gram-positive species has also been reassessed [28]. Nevertheless, in this study, our focus was on the red and orange complexes, and the bacteria traditionally thought to cause the highest risk for periodontitis. Red complex bacteria include Porphyromonas gingivalis, Tannerella forsythia, and Treponema denticola. Orange complex bacteria include Fusobacterium nucleatum, Prevotella intermedia, Prevotella nigrescens, Parvimonas micra, Eubacterium nodatum, and various Campylobacter species [26]. For this study, we chose two representative species from both complexes: P. gingivalis, T. forsythia, P. intermedia and P. nigrescens. In addition, we chose three different strains of P. gingivalis to investigate the differences in volatile biomarkers between different strains of the same species.
Despite its global prevalence, periodontal disease is often left untreated, because of high costs and poor availability of dental care in some regions [29, 30]. In addition, periodontal disease has been connected to several other highly prevalent conditions, such as diabetes mellitus, cardiovascular diseases, the human immunodeficiency virus (HIV) and Alzheimer's disease [31–35]. Early diagnosis and treatment can decrease the probability of gingivitis developing into periodontitis, and consequently, increase the well-being of the patient as well as reduce the time and cost of the recovery. VOCs produced by the oral bacteria could be used as biomarkers for oral infections, and they could aid in the identification of bacterial species present in the oral cavity. Microbial VOCs could also help us to better understand the virulence factors, metabolic processes, signalling, and biofilm formation of bacteria, as well as the tissue damage they are involved in during periodontitis. In this study, we measured the volatile in vitro fingerprints of some of the major pathogenic bacteria connected to periodontitis. Volatiles produced by the bacteria were measured with proton transfer reaction time-of-fight mass spectrometry (PTR-TOF-MS). We also aimed to tentatively identify the produced VOCs and evaluate their potential as volatile biomarkers for the studied pathogens.
2. Materials and methods
2.1. Bacterial strains and culturing method
Bacterial strains used in this study were: P. gingivalis ATCC 33277, P. gingivalis ATCC 53978 (W50), P. gingivalis OMG 434, P. intermedia ATCC 25611, P. nigrescens ATCC 35563, and T. forsythia ATCC 43037. With the exception of P. gingivalis OMG 434 from the Gothenburg Culture Collection, all other strains were obtained from American Type Culture Collection (ATCC).
All the strains were stored at −80 °C in frozen skim milk. Strains were activated by streaking onto Brucella blood agar (BBLTM, 211086) plates, supplemented with horse blood (5% v/v), hemin (5 mg l−1) and vitamin K1, except T. forsythia ATCC 43037, which was cultured on tryptic soy agar (TSA), with n-acetylmuramic acid and sheep blood. All bacterial strains were incubated in anaerobic gas mixture (5% CO2, 10% H2 and 85% N2) at 37 °C for 72 to 120 h. After the incubation, 3.0 ml of phosphate-buffered saline (PBS) was pipetted onto the agar plate, bacteria were gently scraped from the agar, and transferred into a FalconTM tube. This bacterial suspension was homogenized by gently pipetting. The initial amount of bacteria in the suspensions used ranged between 1.0 and 4.0 × 107 colony forming units (CFUs) per ml. From the 3.0 ml of bacterial suspension, 0.250 ml was pipetted onto a new agar plate, which was placed in an airtight headspace measurement container. Triplicates of each bacterial strain were prepared.
2.2. Bacterial headspace measurements
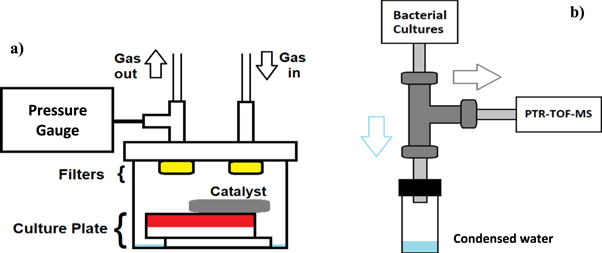
Our custom-built sampling line is fully automated and able to support up to six bacterial cultures simultaneously. The sampling line enables both continuous and one-point measurements of the headspace gas. Figure 1(a) describes our custom-build containers for bacterial headspace measurements. The main body of these containers is made from stainless steel, and the lid from polycarbonate. The fittings between the containers and the sampling line are stainless steel (Swagelok®). The filters used in the containers are syringe filters (Sterile Millex®, Merck Millipore) with 0.22 μm pore size. During the measurements, containers are kept in an incubator at 37 °C. The total volume of one container is 300 ml.
Figure 1. (a) Schematic representation of the bacterial container designed for bacterial headspace measurements. The pressure inside the container is monitored. The containers are suitable for both aerobic and anaerobic culturing. With anaerobic bacteria, excess oxygen is removed with a palladium catalyst placed in the container. Bacterial filters placed on inlet and outlet are used to minimize contamination to and from the sampling line. Distilled, sterilized water on the bottom of the container is used to humidify the headspace and prevent bacterial cultures from drying. (b) Schematic representation of the cold traps used in the sampling line. When warm headspace gas from the bacterial containers flows through the PTFE tubing (in light grey) and the three-way fitting (in dark grey), kept at room temperature, moisture condenses and drips downwards into the water collector. The headspace gas, with excess moisture removed, flows further into the measurement instrument.
Download figure:
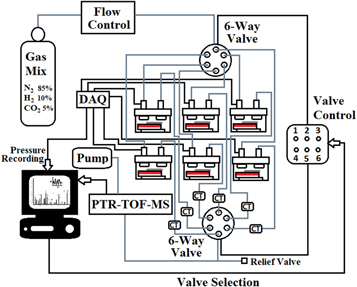
Standard image High-resolution imageThe full sampling system used for the headspace measurements is described in figure 2. Most of the sampling line is composed of inert polytetrafluoroethylene (PTFE) tubing. Cold traps are placed outside the incubator to collect excess moisture produced by the bacterial cultures, and to prevent it from condensing to the sampling line downstream or inside the instrument. The cold traps used consist of a stainless steel three-way fitting (Swagelok®) and a small container for condensed water. Cold traps used are described in figure 1(b). Two six-way valves (080T6 series Flow Selection Valve, Bio-Chem ValveTM) with PTFE bodies, are used to control the flow of gas in and out of containers. These valves are connected to the PTR-TOF-MS and controlled with the measurement program of the instrument (IoniTOF, Ionicon). The valves can be programmed to open and close automatically. A mass flow controller is used to regulate the flow of the carrier gas from the bottle to the sampling line. For the online measurements of bacterial cultures, this flow is kept at 20 ml min−1. Pressure inside the bacterial containers was recorded and controlled to be approximately 1 bar (=105 Pa) during the measurements.
Figure 2. Schematic representation of the automated sampling and PTR-TOF-MS measurement system for volatiles emitted by bacterial cultures. This system supports up to six cultures—aerobic or anaerobic. Grey lines indicate PTFE tubing and black lines signal cables. CT refers to cold traps used for collecting condensed water, and DAQ to a data acquisition card. Two six-way valves are used for the selection of containers, and this valve control is fully automated.
Download figure:
Standard image High-resolution imageIn our measurements, three out of the six possible bacterial containers were used simultaneously. Bacterial headspace from each container was successively sampled for 20 min, with a continuous gas flow of 20 ml min−1 directed through the container. During this 20 min period, the spectrum and concentrations were monitored. The concentrations were allowed to reach a steady state at around 18 min. The last 2 min of the data was considered to represent the true composition of the headspace at the time, and was averaged as an hourly measurement point. During the measurement of the two other containers (40 min), the gas flow to the container was stopped. The 60 min cycle (3 × 20 min) was repeated 160 times.
2.3. PTR-TOF-MS instrument
PTR-TOF-MS is an online mass spectrometry technique, which uses chemical ionization to enable the measurements of individual VOCs ranging from parts-per-million (ppm, 10–6) down to parts-per-trillion (ppt, 10–12) levels. Sample compounds are ionized via proton transfer reactions with H3O+ ions, and therefore the resulting sample ions are detected one atomic mass unit higher than the molecular weight of the neutral equivalents. Successful proton transfer reaction requires the sample molecule to have higher proton affinity than water, which does not allow reliable measurement of some volatile compounds, such as CO2 and CH4. This limitation can be overcome by changing the precursor ion to NO+ or O2+. The measurements in this study were conducted with H3O+. A separate sensor for CO2 (Vaisala, GMP251) was used. A commercially available PTR-TOF-MS instrument (PTR-TOF 1000, Ionicon) was used for all measurements. This instrument uses the TOF method for analysing the ions after ionization. The specified mass resolution of the instrument is 1500 m/Δm (full-width at half-maximum).
PTR-TOF-MS is a direct mass spectrometry method, and it is unable to distinguish isobaric compounds nor structural isomers from each other. Consequently, the identification of VOCs responsible for specific peaks in the mass spectrum is difficult, when several compounds with the same nominal mass, but different exact mass, are present. Identification of compounds can further be complicated by fragmentation of product ions and water clustering. Because PTR-TOF-MS does not provide direct structural identification of signals, additional measurements with pure reference samples were carried out to help identify some of the VOCs. All reference samples were obtained from Sigma-Aldrich®.
Mass scans were performed from mass-to-charge ratio (m/z) 17 to 239. PTR-TOF-MS operating conditions were as follows: field density ratio (E/N) of 127 Td; drift tube pressure of 2.20 mbar, H2O flow of 5.0 standard cubic centimetres per minute (sccm); ion source current of 3.0 mA; inlet flow of 20 sccm and drift tube voltage of 551 V. Drift tube and inlet temperatures were kept at 70 °C. Sampling frequency was 1 Hz, meaning one spectrum was recorded every second.
3. Results
Tentative identification of the compounds produced by the bacteria was aided by measuring the spectra of pure reference substances. Nitrogen gas was directed through a test tube containing the reference substance, and spectrum was measured from the headspace gas with the PTR-TOF-MS instruments. Spectra were recorded for single headspace concentration of each reference sample. Based on fragmentation patterns in the reference spectra and literature references, we offer tentative identities for some of the most interesting signals produced. Correlations between the fragment concentrations and assumed original compounds were also assessed to further verify the connection. However, measurements of the reference samples and the analysis of resulting spectra was rudimentary, and the main motivation was to gain additional aid in the tentative identification of the signals. Information from the reference spectra was always used alongside literature references from earlier research. Absolute identification of the produced compounds requires further analysis, for example, by gas chromatography-mass spectrometry (GC-MS).
Figures 3(a)–(c) presents an example of the fragment analysis done in order to identify some of the compounds. Additional fragmentation spectra are included in the supplementary information, which is available online at stacks.iop.org/JBR/14/016010/mmedia. It should be noted that the humidity level in the reference measurements was lower than in the bacterial headspace, which can have a significant effect on the fragmentation pattern.
Figure 3. (a)–(c) An example of a reference mass spectrum of a pure compound and a corresponding mass spectrum of a headspace bacterial measurement. In this case, the reference spectrum of isoprene gives a better correspondence to the bacterial headspace spectrum than furan, and therefore, isoprene is the more probable origin for the signal at m/z 69. Fragment ion (C5H9+) from larger compounds, such as certain aldehydes, alcohols and alkenes, can also contribute to this signal. In fact, the bacterial spectra of P. gingivalis W50 shows an ion series (m/z 41, 55, and 69) characteristic for E-2-hexenal, which could indicate contribution from this compound.
Download figure:
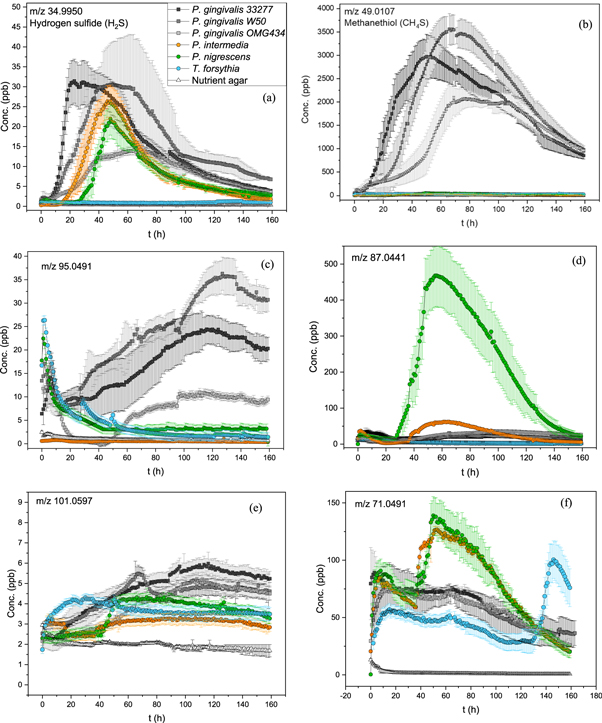
Standard image High-resolution imageIn the headspace measurements, several compounds were found to be emitted by the bacteria, some of which were clearly indicative of certain bacterial species. Table 1 lists the mass-to-charge ratios and concentrations of those compounds we found to have the largest potential as volatile biomarkers for oral bacteria. Concentrations of the compounds produced by the bacteria are further illustrated by a bar graph in figure 4. These compounds are either solely specific for certain bacterial species, or produced by multiple bacteria, but considered in our opinion to be interesting. Concentrations listed are the maximum values of the dynamic production profiles, examples of which are presented in figures 5(a)–(f). The dynamic production profiles describe the compound concentrations produced by the bacteria as a function of time. Note that the place of the maxima can vary between compounds and different bacterial species as well as according to how a specific culture is growing. Example spectra recorded for each bacterial species, as well as, additional dynamic production profiles are included in the supplementary information. All compounds considered to be potential biomarkers were produced by the bacteria in clearly distinct levels compared to the empty agar background. The dynamic production profiles for all of these compounds also showed resemblance to the general bacterial growth curve, with a clear exponential phase and a maximum production.
Table 1. Most prominent volatiles emitted by the oral anaerobes. Values are expressed as in ppb mean ± standard deviation (N = 3) of the maxima of the dynamic production profiles described in figures 5(a)–(f). Note that the place of the maxima can differ between different compounds and bacterial species, as can be observed from the production profiles. Some of the compounds were tentatively identified (in bold) with fragmentation patterns and references from literature.
| m/z | P. gingivalis ATCC 33277 | P. gingivalis W50 | P. gingivalis OMG 434 | P. intermedia ATCC 25611 | P. nigrescens ATCC 35563 | T. forsythia ATCC 43037 | Brucella horse blood agar | Tyrptic soy sheep blood agar |
|---|---|---|---|---|---|---|---|---|
| 35.00 Hydrogen sulphide | 31 ± 7.6 | 31 ± 11 | 13 ± 1.2 | 26 ± 5.0 | 22 ± 1.3 | 1.0 ± 0.3 | 0.4 ± 0.01 | 1.5 ± 0.1 |
| 49.01 Methanethiol | 2969 ± 475 | 3561 ± 332 | 2284 ± 231 | 26 ± 4.3 | 55 ± 6.3 | 30 ± 10 | 6.1 ± 0.9 | 3.8 ± 0.2 |
| 57.03 Butene, Acrolein | 280 ± 6.2 | 68 ± 25 | 62 ± 29 | 22 ± 3.1 | 29 ± 0.4 | 6.6 ± 0.9 | 2.5 ± 0.3 | 1.0 ± 0.01 |
| 59.05 Acetone | 1647 ± 262 | 1648 ± 595 | 1220 ± 646 | 155 ± 43 | 211 ± 76 | 31 ± 1.5 | 26 ± 5.8 | 30 ± 6.0 |
| 63.03 DMS | 11 ± 3.9 | 18 ± 8.0 | 19 ± 0.7 | 6.9 ± 2.3 | 18 ± 1.1 | 3.1 ± 0.1 | 5.3 ± 0.8 | 5.0 ± 0.4 |
| 69.03 Isoprene, fragment ion (C5H9+) | 23 ± 5.3 | 237 ± 19 | 115 ± 75 | 258 ± 20 | 1897 ± 263 | 7.2 ± 0.9 | 5.4 ± 0.1 | 5.8 ± 0.2 |
| 71.05 Pentene/Isopentene, crotonaldehyde | 76 ± 3.8 | 72 ± 7.8 | 161 ± 94 | 127 ± 12 | 139 ± 16 | 100 ± 17 | 1.3 ± 0.3 | 1.3 ± 0.1 |
| 85.06 Cyclopentanone | 5.5 ± 0.7 | 14 ± 5.7 | 11 ± 3.8 | 3.6 ± 0.5 | 5.3 ± 0.4 | 0.8 ± 0.1 | 0.5 ± 0.02 | 0.4 ± 0.03 |
| 87.04 Pentanone/methyl butanal, diacetyl, methyl butenol | 20 ± 1.0 | 26 ± 2.7 | 33 ± 2.8 | 62 ± 5.0 | 468 ± 85 | 2.5 ± 0.2 | 2.6 ± 0.1 | 2.6 ± 0.2 |
| 91.04 Butanethiol, methylthiourea, tropylium ion/other fragment ion (C7H7+) | 5.4 ± 2.1 | 11 ± 5.4 | 11 ± 1.6 | 2.2 ± 0.2 | 4.8 ± 0.5 | 1.3 ± 0.01 | 1.6 ± 0.3 | 1.0 ± 0.1 |
| 95.05 DMDS, phenol, fragment ion (C7H11+) | 24 ± 11 | 36 ± 6.9 | 34 ± 16 | 0.6 ± 0.4 | 3.0 ± 1.0 | 1.8 ± 1.0 | 1.1 ± 0.2 | 0.3 ± 0.03 |
| 101.06 Hexanal, acetyl acetone, MIBK | 6.0 ± 0.6 | 5.5 ± 0.7 | 4.3 ± 0.9 | 3.2 ± 0.4 | 4.3 ± 0.4 | 3.5 ± 0.9 | 2.1 ± 0.1 | 1.2 ± 0.1 |
| 118.07 Indole, benzyl cyanide | 37 ± 6.5 | 26 ± 2.5 | 40 ± 5.4 | 51 ± 3.2 | 55 ± 4.1 | 6.4 ± 0.2 | 7.0 ± 0.9 | 1.8 ± 0.02 |
Figure 4. Description of the 13 signals most potential as biomarkers for specific oral bacteria, or for pathogenic oral infections in general. Signals at m/z 49, 59, 95 and 57 are produced in larger amounts by P. gingivalis than by the other three bacteria. Signals at m/z 69 and 87 are produced in larger amounts by P. nigrescens and P. intermedia. T. forsythia produces significantly less compounds and in lower levels than the other bacteria, however, production of signals at m/z 49, 71 and 101 reach levels comparable to those of the other species. Some signals, such as m/z 35 and m/z 118 are produced in similar levels by most of the bacteria.
Download figure:
Standard image High-resolution imageFigure 5. (a), (b) Dynamic production profiles of m/z 35 (hydrogen sulphide) and m/z 49 (methanethiol). Colour coding of the different bacteria is same in both dynamic production profiles. Each data point represents the hourly mean value. Error bars indicate the standard deviation of triplicate measurements. (c), (d) Dynamic production profiles for m/z 95 and m/z 87. Colour coding of the different bacteria is same as in the first production profile (5a). Each data point represents the hourly mean value. Error bars indicate the standard deviation of triplicate measurements. (e), (f) Dynamic production profiles m/z 101 and m/z 71. Colour coding of the different bacteria is same as in the first production profile (5a). Each data point represents the hourly mean value. Error bars indicate the standard deviation of triplicate measurements.
Download figure:
Standard image High-resolution imageThe PTR-TOF-MS instrument was not specifically calibrated for the analysed compounds. Therefore, the concentrations presented in this study are estimates. The aim of this study was predominantly to investigate the kinds of compounds the orange and red complex oral bacteria are able to produce. Quantification was not our primary concern. However, the concentrations were reasonably similar between the triplicate measurements, so we are confident that the concentrations reported are reasonably precise. In the future, accurate concentrations can be achieved by calibrating the PTR-MS system with each compound separately. The culture conditions and the amount of bacteria effect the production levels drastically, and therefore, we aimed to keep them constant during our measurements. Furthermore, nutrient agars produce a large number of compounds, which was acknowledged by considering agar production as a background in the bacterial measurements.
4. Discussion
We considered 13 of the signals produced to be potential biomarkers: m/z 35, 49, 57, 59, 63, 69, 71, 85, 87, 91, 95, 101, and 118. Several of these compounds were produced by all of the bacterial species studied. However, the levels at which they were produced varied significantly between different bacteria. These volatiles could be used in the future to identify the studied oral pathogens. Although, differentiating between the bacteria according to their volatile fingerprints should be further confirmed, with measurement of mixed cultures as well as blinded experiments. Many of the other compounds produced by the bacteria can be considered as common, and more importantly, have been identified to be a part of the normal composition of human exhaled breath. Some of them have also been found from the volatile fingerprint of microbes unrelated to periodontitis, but present in the oral cavity. Consequently, these compounds are not ideal considering breath biomarkers for oral infections, because they can originate from other sources. Such compounds are for example methanol, ethanol, and acetoacetic acid, which were not considered further in this study. The possible identities of the biomarker signals and the production levels according to bacterial species are discussed next.
The signal at m/z 49 is one of the most prominent peaks in the mass spectra, especially for P. gingivalis. This peak was assigned to methanethiol (CH4S) based on reference spectra comparison and because methanethiol production of P. gingivalis has been confirmed in earlier studies [6, 36–38]. Methanethiol, also known as methyl mercaptan, is produced by some human pathogenic bacteria, including P. gingivalis, T. denticola, F. nucleatum, Citrobacter freundii, Staphylococcus aureus, P. aeruginosa, Streptococcus pneumoniae and Haemophilus influenzae, mostly by enzymatic degradation of L-methionine [38–41]. Most of the pathogenic bacteria capable of producing methanethiol are related to oral infections, and its pungent smell is thought to be the main culprit in halitosis [6, 8–10]. It is also produced by several pathogenic species present in small amounts in the microbiota of the respiratory tract [40, 41]. Methanethiol is a toxic compound, and the general health effects of exposure by inhalation are well known. The long-term effects on cells and tissues are still mostly unclear, but it has been suggested that methanethiol may contribute to the pathogenesis of periodontitis [6, 37, 42, 43]. In this study, we report the concentrations produced by three different P. gingivalis strains (33277, W50 and OMG 434) to be several ppm (3.0, 3.5 and 2.0). These amounts are significant, considering that the occupational exposure limit of inhaled methanethiol is 0.5 ppm. The levels of methanethiol produced by P. gingivalis are most likely not as high in the oral cavity, because of the less ideal growing conditions and the smaller number of bacteria. However, considering that the exposure to bacterial volatiles in the oral cavity is continuous and long-term, the possible effects on oral health cannot be ignored. Concentrations of methanethiol measured from the human exhaled breath vary between 0 ppb and 40 ppb for healthy subjects and between 0 ppb and around 700 ppb for people with halitosis [8, 9]. These levels are considerably lower than the maximum production capacity of P. gingivalis. The trend of methanethiol production between the different P. gingivalis strains are consistent with previous studies, with larger production from the more virulent W50 strain than from the others [36–38]. Other bacterial species capable of producing methanethiol could hinder its usage as a biomarker for oral infections. However, the levels of methanethiol produced in our culture model by all P. gingivalis strains are 10 to 100 times larger than previously reported for bacterial species of the respiratory tract [40, 41].
Several of the compounds produced by the studied oral bacteria have a distinct smell. The signal at m/z 63 is tentatively assigned to dimethyl sulphide (DMS, C2H6S) and the signal at m/z 35 to hydrogen sulfide (H2S), according to inspection of reference spectra and literature [43–45]. Both of these sulphur-containing compounds have an unpleasant smell, and therefore, are among the main culprits in halitosis [6, 8–10, 42]. In addition to oral bacteria, DMS has also been measured from the headspace of several other human pathogenic bacteria in levels varying from a few ppb to some hundreds [40, 41]. Concentrations in the human exhaled breath of healthy people vary between 0 and 30 ppb for DMS, and between 0 and 70 ppb for hydrogen sulfide. However, in people with halitosis the concentrations can increase up to 50 ppb and 700 ppb, respectively [9]. In our study, P. gingivalis and P. nigrescens produced varying levels of these sulphur containing compounds. P. intermedia and T. forsythia, on the other hand, either produced them in very small amounts or not at all. P. gingivalis and P. nigrescens also produced these sulphur compounds in levels higher than the odour detection limit for humans. It should be noted, however, that the concentrations of both compounds produced by the selected bacteria are lower than the concentrations in human exhaled breath and those measured from some human pathogenic bacteria of the respiratory tract [40, 41]. This indicates that there are other sources for these compounds in the exhaled breath in addition to the oral bacteria studied here. DMS and hydrogen sulfide, together with methanethiol, seem to be some of the most important volatiles for the studied bacteria, and their functions beyond halitosis should be further investigated. Their long-term effects to the oral cavity and gingival tissue should not be overlooked either. It has been shown in earlier studies that the ratio of hydrogen sulphide and methanethiol in exhaled breath could be an indicator of the periodontal stage, with the increased amount of methanethiol compared to hydrogen sulphide suggesting progressed periodontal disease [8, 43]. This trend might suggest that the amount of P. gingivalis, the most important producer of methanethiol, is significantly increased compared to the other microbes in patients with periodontitis. It has also been shown that the concentrations of methanethiol are increased compared to hydrogen sulphide in the microenvironment of the gingival pockets [42]. DMS or hydrogen sulfide originating from the oral cavity can hinder the usage of these compounds as breath biomarkers for other diseases. For example, it has been reported that DMS could be a non-species-specific marker for bacterial presence in patients suffering from ventilation-associated pneumonia (VAP) [46]. Increased concentrations of DMS in the alveolar breath have also been connected to liver disease due to the insufficient metabolism of the sulphur-containing amino acids [47]. As oral bacteria can significantly affect the concentrations measured from the human exhaled breath, the contribution from the oral cavity should be acknowledged, when developing breath analysis for diagnostic purposes.
The signal at m/z 85 is tentatively assigned to cyclopentanone (C5H8O) based on the reference spectra and references from literature [44, 48, 49]. It has a pleasant smell, with no determined exposure limits or hazardous health effects. Its cytotoxicity, which is an important aspect considering tissue damage in oral infections, is also largely unknown. It has been suggested that cyclopentanone is produced from furfural (furan-2-carbaldehyde, C5H4O2) by enzymatic degradation, and at least Escherichia coli and P. aeruginosa are capable of producing it [44, 49]. However, the biological role of cyclopentanone is unclear. Furfural is a genotoxic compound, and therefore, bacterial cells aim to convert it into something less toxic, such as cyclopentanone [49]. Thus, cyclopentanone production by the oral bacteria studied here could also be linked to furfural metabolism. A small signal from furfural is found at m/z 97 in the bacterial spectra, and there is a strong positive correlation (R = 0.92) between the signals at m/z 85 and 97. This further suggests a connection between cyclopentanone production and furfural metabolism. The levels of cyclopentanone produced by the bacteria studied are low. T. forsythia does not producing it above the empty culture plate level. P. gingivalis seems to produce higher levels of cyclopentanone than P. intermedia and P. nigrescens. In addition, two of the P. gingivalis strains, OMG 434 and W50, produce larger amounts than the ATCC 33277 strain. The differences in the volatile fingerprints of different strains of the same species introduce a possibility for even more specific identification of pathogens. It should be noted that other compounds, with the formula C5H8O, as well as some fragment ions, are also possible contributors for the signal at m/z 85. However, previous reports on bacterial emissions of these volatiles are scarce compared to those of cyclopentanone [44, 48, 49]. These literature references, together with the measurements done with reference samples, lead us to our tentative conclusion about the identity of the signal.
The signal at m/z 69 is most likely produced by isoprene (2-methylbuta-1,3-diene, C5H8) according to reference spectra and literature. Most of the biological isoprene is produced by plants and algae, however, several water, soil, and human pathogenic bacteria are also known to produce it [44, 48, 50, 51]. To our knowledge, production of isoprene by oral bacteria has not been reported earlier. It has been suggested that isoprene could be a signalling molecule, in addition to being a by-product of nutrient usage [50, 51]. There is also evidence of isoprene production being a protective stress response to toxins and temperature change, as seen in plants [51]. Isoprene is also found from the human exhaled breath, with concentrations ranging from a few ppb to several hundred [52]. This can make the usage of isoprene as a biomarker for oral infections difficult, because the true origin of the compound in breath may be hard to determine. Isoprene is produced abundantly by P. gingivalis strains OMG 434 and W50, P. intermedia and P. nigrescens. P gingivalis strain ATCC 33277 and T. forsythia produce lower levels of this compound. P. nigrescens produces isoprene in significantly higher levels (>1000 ppb) than the other bacteria, which makes this compound a potential marker especially for P. nigrescens. There are also some differences in the place of the production maxima between different bacterial species. P. nigrescens and P. intermedia produce isoprene during the whole of the active growth phase with production maxima around 60 h, whereas P. gingivalis starts to produce isoprene at a significantly later time-point with maxima at around 100 h. This might be an indication of dissimilar biological functions of isoprene for different bacteria. The production of isoprene by P. gingivalis during the late stationary and death phases might indicate stress response, whereas production during the active growth could be connected to signalling or biofilm formation. This has been suggested briefly in an earlier publications [53], but any empirical proof is lacking. Another possibility for signal at m/z 69 is a fragment ion (C5H9+) originating from larger compounds, such as methylbutanal or methylbutenol (C5H10O), methyl isobutyl ketone (MIBK, C6H12O), some monoterpene, alkene or aldehyde. There is no correlation between the signal at m/z 69 and at m/z 101 (possibly MIBK). However, there is a strong positive correlation (R = 0.99) between m/z 69 and m/z 87 (possibly methylbutanal or methylbutenol). There is no evidence for monoterpene production by the bacteria at m/z 137. However, several bacterial spectra show an ion series (m/z 41, 55, and 69) characteristic for certain aldehydes, such as E-2-hexenal. It is likely that the signal at m/z 69 originates from the combination of isoprene and the C5H9+ fragment ion.
The signal at m/z 59 is most likely acetone (propan-2-one, C3H6O), based on the reference spectra. Several anaerobic bacteria, referred to as acetogens, are known to produce acetone [48, 54] by the fixing of CO2 and the production of acetyl coenzyme A (CoA) [54]. However, only a few bacteria connected to the human metabolism have been identified as acetogenic. Acetone is also a naturally occurring volatile in the human exhaled breath, originating from the formation of acetyl-CoA from fatty acids in the liver [55]. According to our results, the studied oral bacteria are able to produce high amounts of acetone on culture plates, which to our knowledge is a novel finding. Acetone in human exhaled breath has been one of the major areas of research in the field of breath analysis, and its usage as a breath biomarker especially for diabetes mellitus has been studied extensively [55]. Acetone, originating from the oral microbiota, most likely contributes to the levels of acetone in breath to some extent, and could hinder the usage of breath acetone as a marker for diseases. It could also be one of the reasons for the large variance in breath acetone levels amongst different people. Acetone in the breath of people with diabetes mellitus is considered to be a product of poor glycemic control and to reflect the blood acetone levels [55]. However, poor oral hygiene is also strongly connected to poor glycemic control, with diabetic patients having threefold risk of severe oral diseases [31]. Consequently, acetone in the breath of diabetic patients could also partly originate from oral bacteria, not only from the increased ketone body formation. Acetone is considered hazardous in large amounts (>500 ppm), however, the long-term effect to gingival cells and oral cavity is not well known. In our measurement setup, P. gingivalis is capable of producing acetone at the ppm level, and it is one of the most abundantly produced compounds for all of the studied bacteria. Although it is not a specific biomarker, acetone could be used in combination with other compounds to discriminate between bacteria. For example, all three P. gingivalis strains seem to produce high levels of both methanethiol and acetone, whereas the other species produce both compounds at significantly lower levels. Thus, the combination of acetone and methanthiol could be used as an indicator for the presence of P. gingivalis.
Signals at m/z 57, 71, 87, 91, 95, 101, and 118 are produced by compounds, whose identity is more difficult to determine. Several potential compounds could be responsible for the signals, for example: butene and acrolein for m/z 57; diacetyl, methylbutenol, pentanone and methyl butanal for m/z 87; butanethiol, methylthiourea and tropylium ion for m/z 91; phenol and dimethyl disulphide for m/z 95; hexanal, methyl isobutyl ketone, gamma-valerolactone and acetylacetone for m/z 101; indole and benzyl cyanide for m/z 118. Several of these compounds have been identified in earlier studies as products of certain bacteria, but it is difficult to say without further analysis, which of these compounds are responsible for the signals in our measurements. However, the biological functions of some of these compounds are better known than others, which could indicate that these compounds are more likely something that bacteria could emit.
The signal at m/z 57 could originate from butene (C4H8), acrolein (prop-2-enal, C3H4O) or some fragment ion (for example C4H9+). In a GC-MS study, butene was identified as a product of Pseudomonas fragi [56]. It is most likely a degradation by-product of more complex compounds. Acrolein, on the other hand, has been connected to the microbiota of the gastrointestinal track [57]. Few studies have been published about the bacterial production of these compounds, and consequently, their biological functions in bacteria are unknown. Butene is regarded as a nontoxic substance, but studies investigating the cytotoxicity of long-term exposure to this volatile are sparse. Acrolein is considered harmful and capable of producing direct tissue damage and irritation. It has also been suggested to be carcinogenic and cytotoxic even in small amounts [57]. In this study, P. gingivalis produced the compound responsible for the signal at m/z 57 in levels surpassing the acrolein odour detection limit, and most of the other bacteria also came close. Another possible origin for the signal at m/z 57 is a fragment or a cluster ion. Strong correlation (R > 0.90) between the signal at m/z 57 and several of the other major signals recorded, could indicate the presence of a fragment ion. Overall, the signal at m/z 57 is produced abundantly by all of the studied bacteria, and therefore, it could prove to be a valuable biomarker. Furthermore, if this compound is acrolein, its toxicity to gingival cells and the oral cavity should be studied. It is also possible that a large portion of this signal originates from fragment ions, which should be investigated in detail in the future.
All of the studied bacteria produce the compound responsible for the signal at m/z 71 abundantly. It is also one of the only compounds T. forsythia produces in comparable amounts to the other bacteria. According to reference spectra and literature, this signal is most likely produced by either pentene (C5H10) or methylbutene (C5H10). Methylbutene, also known as isopentene, is a branched isomer of pentene, and therefore, it is difficult to further distinguish between them without additional structural analysis. The biological functions of these compounds are unclear. However, earlier studies suggest 1-pentene and 2-pentene as products of H. pylori and some soil bacteria [58, 59], and 2-methyl-1-butene as a product of Pseudomonas fluorescens SBW25 [60]. As mentioned earlier, simple hydrocarbons are often degradation by-products of more complex molecules, which could also be the case for the signal at m/z 71. Production profiles of this compound differ significantly between the studied bacterial species, with P. nigrescens and P. intermedia being the largest producers, followed by T. forsythia. For these bacterial species, the production of m/z 71 seems to be strongly connected to the active growth, with the production increasing with increasing amount of bacteria. It should be noted that T. forsythia grows slower than the other bacteria, requiring about a week to reach active growth. The production of m/z 71 by P. gingivalis is very different from the other three bacteria, with consistent production during the whole life cycle of the bacteria. Furthermore, the early production levels of all the bacteria are similar, but differ significantly from the empty agar background. P. gingivalis seems to be the only species to stay on the same production level throughout the measurements, whereas for the other three species, the production is significantly increased in the log phase. This might suggest that a portion of the compound responsible for the signal at m/z 71, originates from the nutrient agar, but only in the presence of bacteria. For P. gingivalis, the production might remain constant because the bacteria itself does not produce additional m/z 71, but rather converts the agar nutrient into this compound. P. nigrescens, P. intermedia, and T. forsythia, on the other hand, produce additional levels of this compound. Consequently, the signal at m/z 71 could distinguish P. nigrescens, P. intermedia and T. forsythia from P. gingivalis, and therefore, it could be a promising biomarker for these bacteria. These findings indicate a difference in the metabolic routes of the studied bacteria.
The signal at m/z 87 could originate from several different compounds, such as diacetyl (butane-2,3-dione, C4H6O2), methylbutenol ((2E)-2-penten-2-ol, C5H10O), pentanone (C5H10O), methyl isopropyl ketone (MIPK, 3-methylbutan-2-one, C5H10O) and methylbutanal (C5H10O). It is impossible to identify the compound responsible for this signal without further analysis. However, several previous studies have identified diacetyl as a bacterial volatile [44, 61, 62], although not from oral bacteria. The signal at m/z 87 is not produced in large amounts by P. gingivalis strains, but rather by P. nigrescens and P. intermedia. Out of these two, P. nigrescens produces significantly more of this compound than P. intermedia, which is interesting considering that these bacterial species are very similar in many other aspects and produce other VOCs in comparable amounts. Differences in the volatile fingerprints of these bacteria suggest divergence in their metabolisms and could partly explain their different roles in the pathogenesis of periodontitis. According to our results, all of the studied bacteria produce the compound responsible for the signal at m/z 87 in levels higher than the recommended exposure limit for diacetyl. If this compound proves to be diacetyl, the production of P. nigrescens is especially alarming, since its maximum production capacity is nearly 100 times the recommended long-term limit. The possible role of oral infections in the pathogenesis of respiratory diseases has been debated strongly in recent decades, with contradictory results [63]. However, bacterial volatiles could be one of the keys connecting these diseases. Considering the evidence of diacetyl as a cause for lung disease [64], it is possible that high amounts produced by oral bacteria could contribute to the development of this condition. The biological functions of methylbutanal, MIPK, methylbutenol and pentanone are less clear. Most of these compounds have been reported as products of soil and plant bacteria, as well as, bacteria related to fermentation [44]. The compound responsible for the signal at m/z 87 is produced abundantly by most of the studied bacteria, and therefore, it seems to be an important marker especially for P. nigrescens. Further confirmation of the identity of this signal is essential.
The signal at m/z 91 could also originate from several different compounds, such as butanethiol/methyl propyl sulfide (C4H10S), butanediol (C4H10O2), methylthioacetate (S-methyl ethanethioate, C3H6OS) or tropylium ion (C7H7+). In addition to tropylium ion, some other structurally different fragment ions with the formula C7H7+ could also be responsible for the signal. These type of fragment ions are known products of many monoterpenes. However, there is no evidence of monoterpene production by the studied oral bacteria, as mentioned earlier. Tropylium ion, on the other hand, is a common fragment product of compounds containing a benzyl group. Compounds such as benzyl cyanide (possible origin of m/z 118) could consequently be the parent compound for the tropylium ion and the signal at m/z 91. However, there is no correlation between these two signals. On the other hand, the tropylium ion could be a product of a benzyl-containing compound that is not volatile, and therefore, is not found from the volatile fingerprints of the bacteria. Isotope patterns in the reference spectra suggest that the signal at m/z 91 originates more likely from a compound with the formula C4H10S, compared to butanediol or methylthioacetate. However, all of these compounds, despite methyl propyl sulfide, have been previously reported as volatile products of bacteria [44]. In a GC/GC-TOF-MS study, Clostridium difficile was proven to produce butanethiol [65]. In another study, C. difficile was reported to produce methylthioacetate [66]. However, this result was obtained with PTR-TOF-MS and identification was not verified. It is possible that the signal assumed to be methylthioacetate was actually butanethiol as in the GC/GC-TOF-MS study. The biological function of butanethiol is unknown. Butanediol, on the other hand, is known to be a product of several plant bacteria, and its production has been linked to the induced growth and systemic resistance of plants. Several rhizobacteria produce 2, 3-butanediol to trigger defence mechanisms in plants to fight against pathogenic species [67]. Methylthioacetate has also been reported to be a product of several plant and soil bacterial species [44, 48], however, its biological functions are unclear. It has been suggested that methylthioacetate could be a growth-stimulating compound used in the interaction between different bacterial species [68]. Methyl propyl sulfide has not been measured from bacteria, although, it is a known component of human exhaled breath [69].
The signal at m/z 95 is most likely caused either by phenol (C6H6O) or dimethyl disulfide (DMDS, C2H6S2). Phenol has been connected in earlier studies to several bacterial species [44, 70], but its biological functions are unclear. Phenol is a toxic substance, and its production could be connected to competition between bacterial species. Phenolic compounds are widespread in nature. Phenol emitted by bacteria could be a degradation by-product of more complex phenolic compounds. It could also contribute to the virulence of the bacteria capable of producing it. DMDS, on the other hand, has been identified as a product of multiple different bacteria, especially those related to soil environments. Several bacteria living in the gastrointestinal system also produce DMDS, and it has also been identified as a product of bacteria present in the oral cavity and the respiratory tract [16, 40, 41, 44]. Similarly to other sulphur containing compounds produced by oral bacteria, DMDS is connected to halitosis and malodour. Concentrations of DMDS in the human exhaled breath range between 0 and 3 ppb [9]. Concentrations of DMDS measured from other pathogenic bacteria, including S. aureus, P. aeruginosa, S. pneumoniae and H. influenza, range from a few ppbs to about 10 ppb [40, 41]. DMDS is considered harmful only in large amounts (>50 ppm), however, the garlic-like odour can be detected by humans at about 10 ppb. In our study, all three P. gingivalis strains produced signal at m/z 95 in levels around 30 ppb, and in similar amounts. The other bacterial species produce significantly lower levels of this compound, and P. intermedia none. Consequently, the compound responsible for the signal at m/z 95 could be one of the most promising biomarkers specifically for P. gingivalis. It can be observed from the dynamic production profiles that the maxima of the m/z 95 production happen at a later time-point in the bacterial life cycle. This might indicate that the compound responsible for the signal at m/z 95 is released mostly in the death phase and is perhaps a compound related to stress response. If phenol is the compound responsible for this signal, the long-term exposure of the oral cavity to this microbial toxin has to be addressed in the future. However, as the production of DMDS by oral bacteria has been reported earlier and the volatile sulphur containing compounds seem to be major metabolites for these bacteria, it is more likely that the signal at m/z 95 originates from DMDS. Inspection of the isotope patterns in the reference spectra supports this as well.
The signal at m/z 101 could be due to several different compounds, such as hexanal (C6H12O), methyl isobutyl ketone (4-methylpentan-2-one, C6H12O) gamma-valerolactone (5-methyloxolan-2-one, C5H8O2), and acetylacetone (pentane-2,4-dione, C5H8O2). Identification of this signal is impossible without further analysis. Gamma-valerolactone has been identified as a product of several soil and marine related bacterial species [44, 68], although, its biological role is unknown. More complex acyl-homoserine lactones have been connected to bacterial quorum sensing [71]. Several other lactones, such as phthalides, have a distinct odour of vegetables and herbs. These volatile, odour-producing compounds could well be something oral bacteria produce, and gamma-valerolactone could be a breakdown product of these more complex compounds. Acetylacetone production, on the other hand, has been connected to several plant and soil bacteria, and it could be connected to both bacterial interaction and growth protomotion [68]. Hexanal has been identified as a product of several bacterial species connected to meat spoilage [56]. It is thought to be a product of hydrolysis of triglycerides or degradation of amino acids. The compound responsible for the signal at m/z 101 is produced in similar, small amounts by all of the studied bacteria. The production of this compound is consistent throughout the life-cycle of the bacteria, which might result from the bacteria converting a nutrient from the agar to a volatile form. However, the production maxima of the signal at m/z 101 are located at the late stationary and death phase of the bacteria, which indicates that it is not a compound related to the active growth. Further identification of this signal should be done in the future, for example by GC-MS methods.
Based on its vast biological functions, indole (C8H7N) is the most likely origin of the signal at m/z 118. Another possibility is benzyl cyanide (phenylacetonitrile, C8H7N). Further identification between these structural isomers is impossible with the PTR-MS method. Indole is a known metabolite of amino acid tryptophan and has many known functions in bacterial metabolism [44, 60, 62]. It has been connected to bacterial virulence, cell cycle regulation, acid resistance, signalling, quorum sensing, and biofilm formation. It also promotes bacterial excretion of toxins, improves bacterial drug resistance, and regulates the genetic stability of bacteria, for example by managing the maintenance of plasmids copies. There is also evidence of indole regulating the stress response of bacteria and stimulating growth. Indole is widespread in the natural environment and several human pathogenic bacteria have been shown to produce it in large amounts [58, 61, 62]. In addition, indole has a strong odour and it has been connected to halitosis. It exists in the human exhaled breath in levels less than 1 ppb [9]. On the contrary, biological functions of benzyl cyanide are mostly unknown, and only a few bacteria are known to produce it [72]. It is not considered harmful itself, however, its major breakdown product hydrogen cyanide (HCN) is highly toxic. It has been reported earlier that several of the oral bacteria studied in this article can produce HCN [18]. However, because of its biological role and many earlier reports of production by bacteria, it is more likely that the signal at m/z 118 originates from indole. The signal at m/z 118 is produced abundantly by all the bacterial species studied, except for T. forsythia, which produces it only slightly above the background level. All the other bacteria produce this compound in similar amounts, which indicates its importance to these species. As indole has been connected to biofilm formation, the lower indole levels produced by T. forsythia could indicate distinct biofilm formation mechanism from the other bacteria. P. gingivalis, P. intermedia, and P. nigrescens readily form biofilms and adhere to surfaces and other bacteria. However, the virulence factors and biofilm formation mechanisms of T. forsythia are less known. The monospecies biofilm formation of T. forsythia is poor, but it has been shown that it produces mixed synergistic biofilms with F. nucleatum [73]. The virulence factors of T. forsythia are also known to be enhanced, when coexisting with other oral bacteria. N-acetyl muramic acid (NAM) is an important amino sugar essential for the formation of the peptidoglycan cell wall of bacteria. Unlike the other oral bacteria, T. forsythia lacks a metabolic pathway to synthesize its own NAM, and therefore, has to harvest it from the degradation products of the oral biofilm. This further suggests the importance of co-colonization to T. forsythia. It has also been shown that T. forsythia readily co-aggregates with other bacteria, facilitating oral biofilm formation [73]. Often bacteria habiting the same microenvironment take on different functions from each other to adapt better to their shared niche. Although, there are only a few studies explaining the precise relationship between the red complex species, differences in the volatile profiles of these bacteria could be an indicator of different function in their niche. In future studies, we aim to investigate the volatile profiles of bacteria in mixed culture, which is especially important considering T. forsythia. It would be interesting to see, whether the volatile fingerprints change in the presence of other bacteria and how those changes could be connected for example to the biofilm formation or bacterial signalling.
5. Limitations
There are some remaining issues we wish to address. We acknowledge that one of the main limitations of our current study, is the lack of information about the growth rate of the bacterial cultures. In our agar-based model, with combined real time headspace analysis, it is challenging to determine the growth rate of the cultures. This would require us to scrape the bacteria off the plate, and hence, lose our VOC analysis sample. It is possible to have a different set of cultures growing in the same conditions as those used for the VOC analysis, and those could then be scraped at different time points. This would allow us to determine the growth rate of the reference culture, and thus the growth rate of our culture model could be approximated. This method would not, however, allow us to measure the exact growth rate of the culture under VOC analysis. We plan to utilize the preliminary results gained from this study in the next stages of our research, where we aim to improve our in vitro model.
The properties of the biofilms produced in our agar-based closed culture system are not ideal or comparable with in vivo biofilms. In the future, we plan to reproduce the VOC measurements described in this article with broth cultures, for comparison. Many earlier studies have measured VOC productions from broth-based closed cultures [17, 19, 40, 48, 49, 61, 65]. The determination of growth rate from broth cultures is easy, and they provide simpler means to study mixed cultures and the effect of nutrient compositions. Models that more accurately represent the in vivo conditions of the oral cavity, e.g. substrate availability, salivary flow and different colonization surfaces, are based on continuous culture systems. In these, fresh medium is continuously added to the bacterial cultures and waste products are continuously removed. In the future, we will also consider using a filter or flow cell based continuous biofilm model [16, 74].
6. Conclusions
Abundant levels of methane thiol (m/z 49) and acetone (m/z 59) seem to be the major markers for P. gingivalis, regardless of the strain. Other important markers for P. gingvalis are m/z 95 (DMDS or phenol) and m/z 57 (butene or acrolein). Largest differences between P. gingivalis strains were measured for m/z 57 and isoprene (m/z 69). For the most part, P. gingivalis strains OMG 434 and W50 seem to have more similar volatile production profiles compared to ATCC 33277. Analysis of the methanethiol levels in human exhaled breath could be a possible future diagnostic tool for identifying P. gingivalis in the oral cavity, if contribution from the lower respiratory tract and other sources can be assessed or eliminated.
P. nigrescens seems to produce volatiles more abundantly than P. intermedia, with m/z 49, 69, 87, 63 and 91 being the most distinct signals between them. The signals at m/z 87 (pentanone, methyl butanal, diacetyl, methyl butenol) and isoprene (m/z 69) seem to be the distinctive compounds separating P. intermedia and P. nigrescens from P. gingivalis. Lower levels of methanethiol and acetone than those produced by P. gingivalis, and elevated levels of isoprene and m/z 87, could be indicators for P. intermedia and P. nigrescens.
T. forsythia produces fewer volatile compounds and at significantly lower levels than all of the other species studied. T. forsythia produced only signals at m/z 49, 57, 71, 101 and 118 in levels higher than the empty agar plate. Only signals at m/z 71 and 101 were produced in similar levels to some of the other bacteria. All other emissions were significantly lower. Measurements with mixed cultures could prove to be especially important for T. forsythia, because of its co-operative requirements, and its volatile fingerprint might be significantly different in those conditions.
Identification of the unknown signals, as well as, confirmation of the tentatively identified compounds, should be performed in the future by GC-MS or another suitable method. Since tissue destruction is a major complication of some oral infections, it is also essential to know more about the effects of toxic compounds, such as methanethiol, to oral tissues. Continuous, low level exposure to these toxic volatiles produced by oral bacteria could be one of the major causes of both minor and serious issues in oral health. In the next stage of our research, we aim to continue the identification of the compounds found in this study, and also to extend our measurements to other oral bacterial species. It is especially important to move from the highly idealized conditions of the monocultures towards conditions more representative of the actual oral cavity. In addition to the in vitro measurements, we aim to connect the bacterial findings to the volatile fingerprints of human exhaled breath in vivo.
Acknowledgments
KR gratefully acknowledges funding from The Paulo Foundation, Wilhelm and Else Stockmann Foundation and the CHEMS doctoral program of the University of Helsinki.
PHG has received funding from Academy of Finland (275614 and 316664), Novo Nordisk Foundation (#NNF OC0013659), Folkhälsan Research Foundation, Helsinki University Central Hospital Research Funds, and Wilhelm and Else Stockmann Foundation.
Conflict of interest
PHG has received research grants from Eli Lilly and Roche, lecture fees from Astellas, Astra Zeneca, Boehringer-Ingelheim, Eli Lilly, Elo Water, Genzyme, MSD, Mundipharma, Novartis, Novo Nordisk, and Sanofi. He is an advisory board member for AbbVie, Boehringer-Ingelheim, Eli Lilly, Janssen, Medscape, Mundipharma, Novartis, Novo Nordisk, and Sanofi.
All other authors declare that there is no conflict of interest.