Abstract
Objective: Complex regional pain syndrome (CRPS) is a complication after surgery or trauma and is characterized by a continuing regional pain in a distal extremity. The pain is disproportionate in severity and duration in relation to the preceding trauma. Currently, the diagnosis is based on the patients' signs and symptoms. There is no objective clinically applicable test available to confirm the diagnosis of CRPS, however this could contribute to a more reliable and valid diagnosis. Since the treatment of CRPS differs from that of other types of pain this could thereby lead to earlier and (more) appropriate treatment and possibly to lower medical costs. The Aeonose™ is a diagnostic test device which detects volatile organic profiles in exhaled air. Exhaled breath analysis using an electronic nose has been successfully applied to differentiate between sick and healthy persons for various indications. This study was a feasibility study in which we investigated whether the Aeonose™ is able to measure a difference in the volatome of CRPS patients compared to the volatome of healthy controls. Design: Prospective observational study. Setting: University Center for Pain Medicine. Subjects: Adult patients diagnosed with CRPS according to the latest IASP criteria (n = 36) and matched healthy controls (n = 36). Methods: Breath profiles were sampled by breathing in and out through the Aeonose™. Data were compressed using a Tucker3-like solution and subsequently used for training an artificial neural network together with the classification 'CRPS: Yes' or 'CRPS: No'. Cross-validation was applied using the leave-10%-out method. Results: Data of the 72 participants were analyzed, resulting in a sensitivity of 83% (95% CI 67%–93%), specificity of 78% (95% CI 60%–89%), and an overall accuracy of 81%. Conclusions: This study suggests that the Aeonose™ can possibly distinguish patients with CRPS from healthy controls based on analysis of their volatome (MEC-2014-149).
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
Introduction
Complex regional pain syndrome (CRPS) is characterized by a continuing regional pain, often combined with vasomotor, sudomotor and motor/trophic disturbances, in a distal extremity. Although in most cases the syndrome is preceded by a fracture, CRPS can also be preceded by sprains, contusions, crush injuries or surgery. The pain is disproportionate in severity and duration in relation to the preceding trauma [1]. In a part of the patients CRPS can have a serious course with development of severe disability. The estimated incidence of CRPS is 20–26.6 per 100.000 person years [2, 3].
As no objective (laboratory) test is available to diagnose CRPS, its diagnosis is based on the patients' signs and symptoms [4]. To this end the Budapest criteria are used [4], which have been updated according to the latest IASP criteria [5]. An objective laboratory test for the diagnosis of CRPS could contribute to a more reliable and valid diagnosis of CRPS. Moreover, since the treatment of CRPS differs from that of other types of pain, misdiagnosis can lead to a delay in more appropriate treatment and (consequently) to higher medical costs [5].
Several studies have indicated that CRPS is an auto-inflammatory disease [6–10]. Especially in patients with acute CRPS, 'classic' signs of inflammation (e.g. pain, redness, swelling, increase in temperature asymmetry) and loss of function can be seen [11]. Earlier research found increased levels of sIL2-R in peripheral blood of patients with CRPS compared to healthy controls [7]. Interleukin-2 is a cytokine involved in regulating activation, proliferation, and survival of T-lymphocytes [12, 13]. In sarcoidosis, also an auto-inflammatory disease, the measurement of sIL2-R is already clinically used to estimate disease activity [14]. The measurement is not disease specific but is presumably helpful in decisions to treat the patient with immune modulating drugs. The specific role of sIL-2R in CRPS is currently under investigation. Another inflammatory marker that is supposed to play a role in the pathogenesis of both CRPS and sarcoidosis is TNF-alpha [6, 9, 10, 15]. In patients with sarcoidosis TNF-alpha was found together with other inflammatory markers, IGF-1, PAI-1, TGF-beta1 and VEGF in exhaled breath condensate using an enzyme immunoassay [16, 17]. This was followed by a study of Dragonieri et al who showed that an electronic nose was able to distinguish between sarcoidosis and healthy controls [18].
An electronic nose is a device that measures the volatome. Some of these devices focus on detecting specific gas components, while others investigate the integrated breath profile. The volatome consists of volatile organic compounds (VOCs) and can be detected in exhaled breath. These can be either exogenous, endogenous or microbial [19, 20]. Exogenous volatiles consist of components produced after eating food, components derived from smoking cigarettes, or components inhaled from the external environment. Endogenous volatiles originate from internal metabolic production [21]. Microbial VOCs are produced by microorganisms that populate the epithelium of the mouth, intestines and lungs [20]. Endogenous and microbial VOCs can be clinically interesting. Endogenous VOCs reflect the physiological status of an individual while microbial VOCs can be an indication of the pathogen present in an infection [22].
Earlier research showed that electronic noses were not only able to distinguish sarcoidosis from healthy controls. The electronic nose used in this study, the Aeonose™ ( The eNose Company, Zutphen, the Netherlands) was earlier successful in distinguishing between healthy controls and patients with tuberculosis [23] lung cancer [24] gastric cancer [25] and with untreated histologically proven primary prostate carcinoma [26]. The Aeonose™ was also successful in distinguishing COPD patients with and without infection [27] and in distinguishing head and neck cancer from other types of cancer including lung, bladder and colon carcinoma [28, 29].
The above results are sufficiently promising to investigate whether an electronic nose might be useful in diagnosing patients with CRPS. Hence, this study examines the potential of an electronic nose to distinguish between patients with CRPS and healthy controls.
Methods
Participants
Between October 2013 and April 2015 consecutive outpatients, diagnosed with CRPS according to the latest IASP criteria [5], were enrolled from the Center for Pain Medicine (Erasmus University Medical Center Rotterdam). Patients had to be aged ≥18 years.
Healthy controls, also aged ≥18 years, were recruited among hospital employees, medical students, or companions of the patients. If the diagnosis CRPS had ever been considered a possibility in their medical history they were excluded from the study.
The patient group was pairwise matched with the healthy controls based on gender and age (±5 years), as both these can influence the volatome [30]. All participants gave informed consent.
The protocol was approved by the Medical Ethics Committee of the Erasmus MC University Medical Center Rotterdam (MEC-2014-149) and the study was performed in accordance with the Declaration of Helsinki (amended version of 2008).
Data collection
For all participants, information was collected on demographics, general medical history, medication use, and whether or not they smoked or used coffee. This information was collected because there might be multiple confounders which can potentially influence breath analysis, including tobacco use, cardiopulmonary status and interference from other molecules [31]. For each patient, the signs and symptoms of CRPS were recorded by taking a medical history followed by a physical examination. For each patient we also established (i) which extremity was affected, (ii) pain intensity using a numerical rating scale (NRS-11) on the day that the breath sample was taken, and (iii) disease duration.
Participants were asked to breathe gently through an electronic nose (the Aeonose™, The eNose Company, Zutphen, the Netherlands) for 5 min. A nose clip was used to ensure that the participants could only breathe through the device. All participants were asked to refrain from drinking alcohol, coffee and smoking for at least 1 h before the test.
Materials
The Aeonose™ has three semi-conducting metal-oxide sensors that are guided through a temperature profile. These sensors differ in terms of surface characteristics. VOCs in exhaled breath induce redox reactions at the sensor surfaces, changing surface conductivity. These changes in conductivity are dependent on the VOCs, the temperature (dynamics), and the sensor-surface properties. During a measurement cycle, a breath pattern is recorded consisting of over two-thousand conductivity values per sensor [32].
Statistical analysis
Descriptive statistics were used to determine the frequency of the demographic and outcome parameters, and to describe measures of central tendency and variability, depending on the shape of their distribution. The Shapiro–Wilk test was used to analyze whether or not these parameters were normally distributed. Differences between the two groups in proportions of dichotomous variables were analyzed using Fisher's exact test. Differences in continuous variables were evaluated using the Mann-Whitney U test if the parameter was not normally distributed and a T-test if the parameter was normally distributed. Analyses were performed with the IBM SPSS Statistics 21 and α was set at the 0.05 level.
Data from the breath samples were analyzed by The eNose Company using a proprietary software package (AethenaTM). Data were compressed using a Tucker3-like solution [33], resulting in a vector of limited size per patient. These vectors were subsequently used for training an artificial neural network (ANN). An ANN reflects the 'learning' and 'generalization' abilities of the human neural architecture by a mathematical representation. This approach is widely used when the relationship between the variables is unknown or is complex.
To build an algorithm that can predict for disease, the ANN has first to be trained using patient data and classifications (diagnosis) [34]. In the present study, the database consisted of breath profiles of the participants with an added label 'CRPS: Yes' or 'CRPS: No'.
To enhance the ability of the algorithm to discriminate between sick and healthy participants, cross-validation was applied using the 'leave-10%-out' cross-validation. Recently, Kort et al provided a more detailed description of the processing and analysis of breath samples using AethenaTM [35]. After cross-validation, the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were calculated. The results are presented in a scatterplot.
Results
Characteristics of the participants
For this study 72 persons were enrolled: 36 patients and 36 healthy controls. Demographic information and data on smoking and coffee consumption are presented in table 1; no significant differences were found between the two groups. The percentage of participants suffering from a comorbidity are shown per category, subdivided by experimental group (table 2). Significant differences were found between patients and healthy controls in proportions suffering from cardiac, pulmonary, upper gastro-intestinal and lower gastro-intestinal comorbidities. The percentages of participants suffering from these comorbidities were lower in the healthy control group than they were in the CRPS group.
Table 1. Characteristics of patients and healthy controls.
| Patients | Healthy controls | ||
|---|---|---|---|
| n | 36 | 36 | p |
| Gender (female) | 29 (81%) | 29 (81%) | 1.00 |
| Age: years median (IQR) | 36.5 (54.0–23.5) | 35.5 (53.0–23.25) | 0.77 |
| Smoking (yes) | 9 (25%) | 5 (14%) | 0.37 |
| Consumption of coffee (yes) | 26 (72%) | 31 (86%) | 0.25 |
Table 2. Comorbidities of participants per category.
| Category of comorbidities | Number of patients (N = 36) | Number of healthy controls (N = 36) | P-value |
|---|---|---|---|
| Cardiac | 9 (25%) | 2 (5.6%) | 0.046 |
| Vascular | 11 (31%) | 7 (19.4%) | 0.41 |
| Hematopoietic | 4 (11%) | 1 (2.8%) | 0.36 |
| Respiratory | 12 (33%) | 3 (8.3%) | 0.02 |
| Eyes, ears, nose, throat, larynx | 10 (28%) | 4 (11.1%) | 0.14 |
| Upper gastro-intestinal | 12 (33%) | 4 (11.1%) | 0.045 |
| Lower gastro-intestinal | 10 (28%) | 0 (0%) | 0.001 |
| Liver, gall, pancreas | 2 (6%) | 2 (5.6%) | 1.000 |
| Kidneys | 4 (11%) | 1 (2.8%) | 0.36 |
| Urogenital | 8 (22%) | 4 (11.1%) | 0.34 |
| Muscle, bone, skin | 9 (25%) | 7 (19.4%) | 0.78 |
| Neurology | 8 (22%) | 5 (13.9%) | 0.54 |
| Endocrine/metabolic | 5 (14%) | 1 (2.8%) | 0.20 |
| Psychiatry | 1 (3%) | 2 (5.6%) | 1.000 |
Data on the NRS score and the duration of CRPS are listed in table 3. In 30 of the 36 patients, one extremity was affected by CRPS and in six patients two or more extremities were affected. Two patients had had a limb amputation because of CRPS; these two patients still suffer from CRPS in one or more other extremities. The signs and symptoms of the patients are presented in table 4.
Table 3. Patients characteristics.
| Patients | |
|---|---|
| Duration of CRPS in months; median (IQR) | 49.0 (79.0–20.00) |
| NRS-pain (scale 0–10) median (IQR) | 7.0 (8.0–4.0) |
Table 4. Symptoms and signs (N = 36).
| Symptoms | Signs | |
|---|---|---|
| Sensory | ||
| Allodynia | 32 (89%) | 30 (83%) |
| Hyper-/hypoesthesia | 36 (100%) | 34 (94%) |
| Vasomotor | ||
| Asymmetry in temperature | 32 (89%) | 29 (81%) |
| Asymmetry in color | 33 (92%) | 31 (86%) |
| Sudomotor | ||
| Edema | 30 (83%) | 22 (61%) |
| Asymmetry in sweating | 30 (83%) | 20 (56%) |
| Motor/trophic | ||
| Weakness | 32 (89%) | 27 (82%) (N = 33) |
| Decreased range of motion | 28 (78%) | 26 (74%) (N = 35) |
| Dystonia or tremor | 29 (81%) | 8 (22%) |
| Change in hair growth | 19 (53%) | 15 (42%) |
| Change in nail growth | 21 (58%) | 17 (47%) |
| Skin atrophy | 16 (46%) (N = 35) | 15 (42%) |
In four patients, one or two signs and symptoms were not assessable, resulting in missing data. Therefore, the percentages of the signs and symptoms listed in table 5 were calculated based on the total number of patients in whom these signs and symptoms were assessable. The use of medication prescribed for CRPS was also registered (table 5).
Table 5. CRPS-related medication use at the time of examining the breath sample.
| No. of patients | |
|---|---|
| NSAID | 6 (17%) |
| Opiates | 16 (44%) |
| Co-analgesics (group of antidepressants) | 7 (19%) |
| Co-analgesics (group op anti-epileptic drugs) | 9 (25%) |
| Other pain medication | 4 (11%) |
| Anti-oxidants | 6 (17%) |
| Vasodilators | 1 (3%) |
| Benzodiazepines | 3 (8%) |
| Ketanserin | 4 (11%) |
| Acetyl-L-carnitine | 4 (11%) |
Aeonose™ classification results
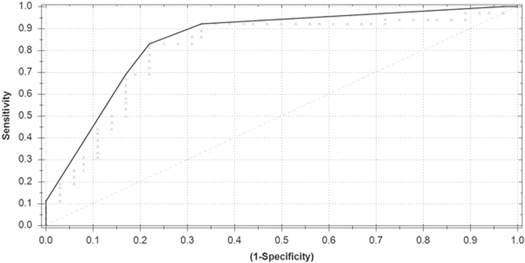
The threshold above which participants were designated as CRPS patient group was set to 0.00 . This is the threshold where the sum of specificity and sensitivity was maximal. This led to eight healthy controls and six patients being classified into the wrong group, with a sensitivity of 83% (95% CI 67%–93%), a specificity of 78% (95% CI 60%–89%), a PPV of 79% (95% CI 62%–90%), and a NPV of 82% (95% CI 65%–93%). The overall accuracy appeared to be 81%. Figure 1 presents the ROC-curve based on the above mentioned sensitivity and specificity.
Figure 1. Receiver operating characteristics curve of the Aeonose™ as a predictor of group (CRPS patients versus healthy controls).
Download figure:
Standard image High-resolution imageDiscussion
The results of this study give a first indication that the Aeonose™ is able to distinguish between patients with CRPS and healthy controls.
The pathophysiology of CRPS is complex and based on multiple mechanisms; any or a combination of these mechanisms, including inflammation, may influence the volatome. Studies on sarcoidosis have shown that it is possible to identify inflammatory markers (e.g. TNF-alpha) in exhaled breath condensate using an enzyme immunoassay [16, 17]. So the ability of the Aeonose™ to distinguish patients with sarcoidosis from healthy controls could (also) be due to the influence of inflammation [18]. In this study the volatome of CRPS patients was only compared with the volatome of healthy controls. Therefore it is unclear whether the Aeonose™ made a discrimination between CRPS patients and healthy controls based on something that is specific for CRPS and not on something that CRPS has in common with other (pain) conditions. For example, with pain patients in general, or for patients with an inflammatory disease or even for patients in distress. The Aeonose™ misclassified eight healthy controls and six patients. The misclassification of patients could be due to the different clinical stages of CRPS (acute, subacute, chronic). Another explanation for misclassification of participants is that it is still unclear whether the Aeonose™ is capable to measure disease specific.
As mentioned above, CRPS is based on consensus-derived criteria. Obviously, because a golden standard validation criterion is missing, it is actually impossible to determine whether the IASP diagnostic criteria set or the—based on empirically obtained data—trained algorithm yields a better predictive validity. So, in this light analyzing the (mis) classifications using the trained algorithm could lead to a better nosological and etiological understanding of CRPS and subsequently to less over- or underdiagnosis. This would then result in improved clinical communication and better generalizability across research samples. In addition, it would enable physicians less familiar with the IASP criteria to diagnose CRPS. Hereby it could lead to (more) appropriate treatment in an earlier stage, better research (more comparative research samples) and possibly to lower medical costs.
Before the Aeonose™ can be used in clinical practice, research is needed to find out whether or not the Aeonose™ is able to distinguish between patients with CRPS from patients with other diseases showing similar symptoms and signs, and/or similar underlying pathophysiologic mechanisms, especially inflammation. Thereafter a validation study is required in which the trained algorithm classifies blinded samples. If the results of such a study show that the Aeonose™ is able to differentiate CRPS patients from the above mentioned patient populations then we will have convincing scientific evidence of using the Aeonose™ in clinical practice.
Study limitations
Shirasu and Touhara demonstrated that the human volatome varies by age, lifestyle, gender, physiological status, and (possibly) by genetic background [30]. Therefore those parameters should be comparable in the experimental and the control group. In the present study, the two groups were pairwise matched only by age and gender and no significant differences in smoking habits and/or coffee consumption were found between the groups. Nevertheless, a limitation of this study could be the fact that the two groups were matched only on the parameters mentioned above and not on other parameters such as physiological status and genetic background. According to the study of Shirasu and Touhara these aspects also affect the volatome [30]. It would have been better to correct for these aspects as well, but it is unclear what they exactly mean by physiological status and genetic background. Participants were asked to refrain from drinking alcohol, coffee and smoking one hour before the breath sample was taken. Two patients and two healthy controls have not fulfilled this requirement. There was no limit imposed on eating or drinking in general. So far it has never been investigated whether the absence of a period of refrainment affects the classification of the participants by the AeonoseTM, nor has been investigated how long the period of refrainment should be to prevent this. Hypothetically the absence of a period of refrainment on eating and drinking in general could possibly affects the classification. Besides that it is questionable if the hour of refrainment of drinking alcohol, coffee and smoking that was held in this study was long enough to prevent bias of drinking alcohol, coffee or smoking on the classification. Moreover, significant differences were found between the patients and healthy controls in cardiac, pulmonary, upper gastro-intestinal and lower gastro-intestinal comorbidities, which may have influenced the results. Furthermore, some significant differences may be due to multiple comparison. We have not corrected for this, which means that there is an increased change of a type I error. Another explanation for the significant differences we found could be that a medical history of asthma, migraine and osteoporosis is associated with CRPS. The higher percentage of pulmonary comorbidities that were found in the CRPS group confirms the finding of de Mos et al [36]. The higher percentage of gastro-intestinal comorbidities could be caused by use of analgesics and co-analgesics in the CRPS group.
In this study we found a sensitivity of 83% (95% CI 67%–93%), a specificity of 78% (95% CI 60%–89%), a PPV of 79% (95% CI 62%–90%), a NPV of 82% (95% CI 65%–93%). The appreciation of these measures of the predictive validity is dependent on the prevalence of a disease in the studied population—in our study 50%. Therefore, it would be useful to investigate whether a high validity would also be found in a study in the general population
Conclusion
The volatome of 72 participants was analyzed with the objective to investigate whether the Aeonose™ is able to distinguish patients with CRPS from healthy controls, resulting in a sensitivity of 83% (95% CI 67%–93%), specificity of 78% (95% CI 60%–89%), and an overall accuracy of 81%. The patients were diagnosed with CRPS using the IASP diagnostic criteria set, which consists of consensus-derived criteria. The two groups were pairwise matched based on age and gender. There was no significant difference in smoking habits or coffee consumption. Limitations of this study are that we did not correct for lifestyle, physiological status and genetic background, that the percentage of cardiac, pulmonary and gastro-intestinal comorbidities were significant different between the patients and healthy controls and that although patients were asked to refrain from drinking alcohol, coffee and smoking one hour before the breath sample was taken, there was no period of refrainment on eating and drinking in general. The results of this study suggest that the Aeonose™ can possibly be used to distinguish between CRPS patients and healthy controls based on analysis of their volatome. Further validation steps are necessary to establish the value of the AeonoseTM in the differential diagnostic process of CRPS.
Acknowledgments
The authors thank J W Gerritsen, The eNose Company, Zutphen, for comments that greatly improved the manuscript.
Conflict of interest
All authors declare that they have no conflict of interest.