Abstract
Blue carbon sequestration in seagrass meadows has been proposed as a low-risk, nature-based solution to offset carbon emissions and reduce the effects of climate change. Although the timescale of seagrass carbon burial is too short to offset emissions of ancient fossil fuel carbon, it has a role to play in reaching net zero within the modern carbon cycle. This review documents and discusses recent advances (from 2015 onwards) in the field of seagrass blue carbon. The net burial of carbon is affected by seagrass species, meadow connectivity, sediment bioturbation, grainsize, the energy of the local environment, and calcium carbonate formation. The burial rate of organic carbon can be calculated as the product of the sediment accumulation rate below the mixed layer and the burial concentration of organic carbon attributable to seagrass. A combination of biomarkers can identify seagrass material more precisely than bulk isotopes alone. The main threats related to climate change are sea-level rise, leading to a shoreline squeeze, and temperature rise, particularly during extreme events such as heat domes. In conclusion, some of the disagreement in the literature over methodology and the main controls on organic carbon burial likely results from real, regional differences in seagrasses and their habitat. Inter-regional collaboration could help to resolve the methodological differences and provide a more robust understanding of the global role of blue carbon sequestration in seagrass meadows.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
The effects of global climate change are now unmistakable: droughts, floods, extreme heat, extreme cold, hurricanes, devastating forest fires, and—in the ocean—acidification, deoxygenation, temperature rise and marine heat waves. The Intergovernmental Panel on Climate Change has stated that these effects will worsen as atmospheric CO2 concentrations continue to rise (IPCC 2018). To arrest the global temperature increase at 1.5 °C above pre-industrial values and avoid the most catastrophic effects, fossil fuel emissions must reach net zero by 2050 (IPCC 2018). Even if that target were reached, the world would still see increased climate change effects beyond those experienced to date.
Reducing atmospheric CO2 below current concentrations to reverse some of the effects would require, in addition to an end to fossil fuel emissions, some mechanism to remove CO2 from the atmosphere (negative emissions technology; IPCC 2018). Various negative-emissions technologies have been proposed, all with varying degrees of uncertainty and risk. One option that appears to pose little risk is expanding 'blue carbon' sequestration in the sediment of vegetated coastal ecosystems, including seagrass meadows.
The global extent of seagrass has declined dramatically, largely due to changes in land use (e.g. Harcourt et al 2018, Green et al 2021, Macreadie et al 2021). Consequently, there appears to be the potential to restore seagrass meadows on a large scale, increasing sedimentary blue carbon storage commensurately.
Blue carbon research has expanded rapidly over the last decade. Policy-makers are eager to incorporate blue carbon into climate change mitigation plans, in the hope that coastal carbon sequestration will help meet nationally-defined targets for reducing net greenhouse gas emissions (e.g. Herr et al 2016, Wylie et al 2016, Taillardat et al 2018). For example, the United Nations Decade of Ocean Science for Sustainable Development has endorsed a blue carbon programme (www.oceandecade.org/actions/global-ocean-decade-programme-for-blue-carbon/).
Methods have been developed to put a value on blue carbon sequestration (Emmer et al 2015a, 2015b, Zarate-Barrera and Maldonaldo 2015). Proponents claim that blue carbon burial has an enormous potential relative to the area covered: >50% of organic C burial in the ocean, in approximately 0.5% of the seafloor area (Macreadie et al 2021). Part of the appeal of this solution is that vegetated ecosystems provide enormous ecological and social benefits: they provide critical habitat for numerous species, food security for people, and shoreline protection against storms (Hejnowicz et al 2015, Ruiz-Frau et al 2017, Quevedo et al 2020, Quiros et al 2021, Shilland et al 2021).
A geochemical review demonstrated that the published global and regional estimates for blue carbon sequestration in seagrasses were significantly too high—possibly by more than an order of magnitude—because of misunderstandings about how marine sediments process and bury carbon (Johannessen and Macdonald 2016, 2018). Since those papers were published, the field has advanced and diversified. However, there is still widespread confusion about the role of blue carbon in climate change mitigation, and errors persist in the interpretation of sedimentary processes.
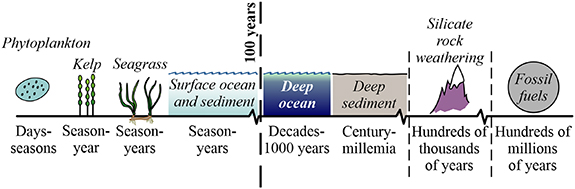
Blue carbon sequestration cannot truly offset fossil fuel emissions because of the many orders of magnitude difference in timescale: 1–100 years for blue carbon vs. hundreds of thousands of years for silicate weathering, or 100 million to 300 million years for fossil fuels (Archer et al 2009) (figure 1). So long as ancient carbon continues to be added to the modern system (air, water, vegetation and surface sediment), the total amount of carbon in the modern system will continue to increase, regardless of how it is distributed.
Figure 1. Timescale for carbon sequestration in different environments. Only burial for >100 years qualifies for carbon credits.
Download figure:
Standard image High-resolution imageIn addition, the total size of the coastal carbon reservoir (10–45 Pg) is very small relative to other reservoirs (e.g. open ocean surface sediment, 1750 Pg C; seawater, 38 700 Pg C) (Friedlingstein et al 2020).
Nevertheless, blue carbon may make an important contribution to reaching net zero CO2 emissions in the modern carbon cycle, so long as its role is clearly understood. To qualify for carbon credits under the Verified Carbon Standard (Emmer et al 2015a, 2015b), a sequestration project must demonstrate additionality (a net increase in C sequestration beyond what would have occurred otherwise) and permanence (on a time scale >100 years).
Consequently, for blue carbon to contribute usefully to the carbon balance, it must: (a) increase the amount of carbon sequestered; (b) comprise carbon that would not otherwise have been preserved; and (c) persist for at least 100 years. Existing stocks, existing burial fluxes, and carbon stored in short-term reservoirs do not qualify for carbon credits.
As identified by Macreadie et al (2019a), there are several areas in need of more research to help quantify the global role of seagrasses. These include methodology for identifying the source of buried carbon, the factors affecting its long-term fate, the role of carbonate, and the effects of climate change on carbon accumulation and loss.
This paper presents a broad review of recent blue carbon research, in the context of the areas identified by Macreadie et al (2019a), to address the question of additionality and permanence of carbon sequestered in seagrass meadows.
2. Methods
The literature review began with a Google Scholar search in July, 2021, using the terms 'blue carbon' AND 'seagrass,' for the years 2015–2021. The top 100 search results were selected, including only peer-reviewed journal articles. Duplicate results were eliminated. A second search of the top 100 results from Web of Science, using the same search criteria, yielded an additional 25 papers.
The papers were then categorized according to themes that emerged from the results. (see section 3). Changes in methodology, new topics, and progress toward answering the central question defined above were identified.
Older papers were added, as needed, to provide context for points in the discussion. These were identified based on the author's expertise, or from references cited in the recent papers. New papers that were published after the initial search and that came to the author's attention were also included.
The results for each theme are presented, with a short discussion in each section. The paper concludes with an overall summary and recommendations for next steps.
3. Results and discussion
The main themes of the retrieved papers were:
- Global estimates and case studies of blue carbon stock or burial rate
- Preservation in sediment: bioturbation, respiration, grain size, etc.
- Source attribution and biomarkers in sediment
- The role of calcium carbonate
- Effects of climate change
- Management and policy, including national emissions targets and recommendations for management actions.
3.1. Global estimates and regional case studies of carbon stock
Global estimates of organic carbon stock in seagrass meadow sediment depend on knowing the extent of seagrass habitat. The extent of the seagrass biome may be modelled, based on occurrence records and environmental variables (especially sea surface temperature and distance from land) (Jayathilake and Costello 2018). Alternatively, the existing extent of seagrass may be estimated using a satellite-based approach, including ocean colour or acoustic measurements (Harcourt et al 2018, Sani et al 2019). These values are sometimes converted to carbon burial using carbon conversion factors (Sani et al 2019). Based on published values, Macreadie et al (2021) estimated that the area covered by seagrass meadows could be increased by 8.3–25.4 million ha globally, with a large associated increase in carbon storage.
Local or regional case studies are carried out by measuring the total organic carbon stored in the uppermost layer of sediment over a specified depth. International protocols (e.g. IPCC 2019) call for a sum over the top 1 m of sediment. Regional studies vary in the depth of sediment assessed, with a range of 5 cm–3 m (table 1). In areas with low sedimentation, there might only be a shallow layer of sediment overlying rock, in which case the shallow layer represents the total stock.
Table 1. Depth of sediment used in some examples of recent carbon stock estimates.
| Location | Depth (cm) | Reference |
|---|---|---|
| China | 5 | Jiang et al (2017) |
| Denmark and Finland | 25 | Röhr et al (2016) |
| India | 20 | Kaladharan et al (2020) |
| United States | 20 | Ward et al (2021) |
| Spain | 30 | Bañolas et al (2020) |
| England | 30 | Lima et al (2020) |
| Australia | 30 | York et al (2018) |
| Australia | 30 | Ewers Lewis et al (2020) |
| Scotland | 50 | Potouroglou et al (2021) |
| Korea | 50 | Kim et al (2022) |
| Kenya | 50 | Githaiga et al (2017) |
| Mexico | 20–100 | Lucero and Herrera-Silveira (2021) |
| United Kingdom | 100 | Green et al (2018) |
| Southeast Asia | 100 | Thorhaug et al (2020) |
| Indonesia | 100 | Alongi et al (2016) |
| United Arab Emirates | 100 | Campbell et al (2015) |
| United States/Mexico | 100 | Thorhaug et al (2019) |
| Mexico | 100 standardized in review article | Herrera-Silveira et al (2020) |
| Colombia | 50–300 | Serrano et al (2021) |
| United States | 100 and 300 | Kauffman et al (2020) |
Sometimes only a shallower depth can be assessed because the deeper sediment has become too compressed to force the corer any deeper, in which case the stock is generally reported to the 'depth of refusal' and extrapolated to 1 m (e.g. Green et al 2018). Extrapolation would usually overestimate the 1 m stock, because organic carbon is remineralized with depth in marine sediment (e.g. Stolpovsky et al 2015), leaving the highest concentration at the surface. Conversely, where the % organic carbon increases with depth due to some change that has occurred in the ecosystem, extrapolation from a shallow surface layer would underestimate the stock.
It should be noted that the top meter does not always represent all of the carbon stock in a particular location. For example, working in a seagrass meadow in the American Pacific Northwest, Kauffman et al (2020) determined that the top 1 m of sediment contained only 48%–53% total ecosystem C stock, while >98% resided in the top 3 m.
A review by Githaiga et al (2016) identified a major, regional gap in carbon stock estimates: although they found some studies of seagrass extent in Africa, particularly in east Africa, they found no reports of sediment carbon stock from that continent. Githaiga et al (2017) subsequently measured carbon stock in a seagrass meadow in Kenya, and Gullström et al (2018) published sedimentary carbon data from southeastern Africa (Tanania to Mozambique). Even so, the lack of data from the whole west coast of Africa represents a significant uncertainty for global estimates.
Blue carbon stock is vulnerable to degradation and re-release to the atmosphere as CO2 (Lovelock et al 2017). These losses may be localized within the seagrass meadow (i.e. greater loss in the center of a patch during a marine heat wave; Aoki et al 2021), or the losses may be widespread. Lafratta et al (2020) reported that 75%–90% of stored organic carbon was remineralized in the top meter in Australian Posidonia meadows following their loss and partial restoration. A shading experiment in the Gulf of Mexico that induced a small-scale die off resulted in a 50%–65% loss of organic matter in the top 8 cm of sediment, including 50% lost from the top 1 cm (Trevathan-Tackett et al 2017). In a disturbed former seagrass bed, Macreadie et al (2015) estimated that 72% of the carbon, which had taken hundreds of thousands of years to accumulate, had been lost. They warned that 'disturbance to seagrass ecosystems can cause release of ancient carbon, with potentially major global warming consequences.'
Protecting existing stock does not reduce the amount of CO2 in the atmosphere, so protection alone does not qualify for carbon credits under the Verified Carbon Standard (Emmer et al 2015b). Only the expansion of burial counts. However, the stock is important to determine, because it is a measure of the vulnerable carbon that could be re-released due to storms, slope failure, dredging, or climate change.
3.1.1. Macroalgae
Unlike seagrasses, macroalgae, such as kelp, usually grow on rocky or sandy substrate, so there is little potential for carbon burial in situ. However, they are highly productive and might act as donors of storable organic carbon to adjacent seagrass meadows or the deep sea (Hill et al 2015, Duarte and Krause-Jensen 2017, Filbee-Dexter and Wernberg 2020). In contrast, bacteria from macroalgal blooms could enhance breakdown and remineralization of seagrass fronds by co-metabolism, reducing carbon sequestration by seagrasses (Liu et al 2020). The net effect of the interaction between seagrasses and macroalgae on carbon storage is complex and requires further study.
3.2. Source attribution and biomarkers
A variety of biomarkers has been used to identify sources of sequestered C. The most commonly used are the stable isotopes of carbon and nitrogen (δ13C, δ15N) measured in bulk organic matter or sediment. Recent examples include work in Japan (Tanaya et al 2018), on the eastern coast of the Red Sea (Garcias-Bonet et al 2019) and in eastern North America (Greiner et al 2016). Combined with organic carbon concentration or the ratio of organic carbon to total nitrogen, these stable isotopes provide useful information about the relative proportions of allochthonous (mainly terrigenous) and autochthonous (seagrass + macroalgae + phytoplankton) material.
Bulk stable isotopes, while convenient to use, do not separate multiple marine sources very well (Geraldi et al 2019). Other biomarkers for seagrasses include fatty acids (e.g. Dongen et al 2000), lignins (Barry et al 2018), hydrogen isotopes (Duarte et al 2018b), polysaccharides (Kaal et al 2020), and environmental DNA (Reef et al 2017). In some cases, the chemical biomarker results can be confirmed by microscopy (Dongen et al 2000).
In a broad review and critique of biomarker methods, Geraldi et al (2019) recommended the use of a combination of complementary methods, particularly stable isotopic analysis of lipids combined with eDNA analysis, although they noted that the method still required validation. Using multiple biomarkers helps to compensate for the weaknesses of individual methods. For example, lignin ratios can be different in sediment from those in the nearby, fresh seagrass (Barry et al 2018). Similarly, Nakakuni et al (2021) noted that the contribution of long-chain fatty acids in near-surface sediment declined with depth (apparently due to degradation), while lignin phenols were selectively preserved.
Since different methods give different information and can shore up each others areas of weakness, a combination of biomarkers seems the most powerful approach. Endmembers will likely need to be defined locally, or at least regionally, before the combined biomarkers will give reliable, quantitative information about the flux and burial of organic carbon from seagrasses.
3.3. Factors that determine net sequestration in sediment
3.3.1. Meadow connectivity
Both the size of a seagrass meadow and how closely it is connected to other vegetated ecosystems affect the rate of carbon sequestration in the meadow. In a restored seagrass meadow, Oreska et al (2017) found that organic carbon stock related more to proximity to the meadow edge than to the age of the plot. The middle of the plot stored more carbon, probably because of the accumulation of fine-grained sediment in the low-energy environment. They concluded that a larger, more highly connected meadow would store more carbon than several small meadows.
Connected seascapes can also be hotspots for organic carbon storage when material is advected sequentially from one location to another. Asplund et al (2021), for example, found enhanced carbon burial in a seagrass meadow next to deforested mangrove, suggesting that mangrove material had been washed into seagrass meadow.
3.3.2. Grainsize
Grainsize is a determining factor in some situations. Using data from 79 cores collected in seagrass sediment and 21 cores from bare sediment, Serrano et al (2016) found that % mud correlated to % organic carbon when the local contribution of seagrass was low. In Zostera meadows, for example, the % mud accounted for 34%–91% of variability in % organic carbon inside the plot, and 78% outside the plot, mainly as a result of high allochthonous input. However, they found poor or no correlation with grainsize in meadows composed of Posidonia or other large seagrasses, where there was a higher contribution of seagrass carbon. Barry et al (2018) also found that hydrodynamics was important to carbon storage, with a significant negative relationship between bulk density and % organic carbon.
In the United Arab Emirates aridity results in weakly-reduced sediment which, combined with the coarse grainsize and active movement of particles, results in lower carbon storage than reported elsewhere (Schile et al 2017).
3.3.3. Bioturbation
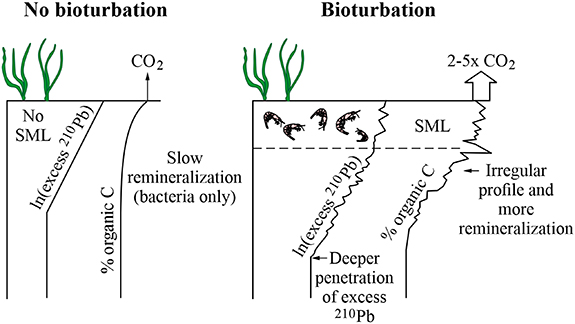
Coastal sediment is nearly always bioturbated. Bioturbation distributes material deeper into the sediment than would have been the case in an undisturbed sediment and increases the rate of remineralization in surface sediment (Johannessen and Macdonald 2016). The global mean depth of the sediment surface mixed layer is 9.8 ± 4.5 cm, probably as a result of the energetic cost of burrowing (Boudreau 1994). A model based on food availability and the intensity of bioturbation (Boudreau 1998) predicted a similar global average (9.7 cm). Neglecting bioturbation tends to exaggerate sediment accumulation rates, whether they are based on 210Pb or on a tracer that represents a known time horizon (Johannessen and Macdonald 2016). This has been a widespread problem in the blue carbon literature.
Remineralization reduces the amount of organic carbon available to be buried (Thomson 2017). During a lab experiment in which seagrass fronds were buried in sediment with or without bioturbating shrimp (Thomson 2017, Thomson et al 2019), the treatment with shrimp released 2–5 times as much CO2 as the control without shrimp (figure 2).
Figure 2. Effects of bioturbation on profiles of 210Pb and organic carbon, and on CO2 released from sediment. SML = surface mixed layer. Bioturbation smears tracers and moves them deeper into the sediment. Illustration adapted from Johannessen and Macdonald (2016), combined with conclusions of Thomson (2017) and Thomson et al (2019). © IOP Publishing Ltd CC BY 3.0.
Download figure:
Standard image High-resolution image3.3.4. Nutrients, bacteria and microbial priming
Experiments and observations of the effects of nutrients on carbon sequestration by seagrasses have shown mixed results. Even if seagrass biomass increases due to nutrient addition, there might be a null or even negative effect on sedimentary organic carbon storage.
In the South China Sea, for example, nutrient loading (from fish farms) enhances bacterial activity and is associated with lower sediment % org C, possibly by microbial priming, which prepares bacteria to break down more of the organic carbon from the seagrass (Liu et al 2017a, Jiang et al 2018). The resulting effect on carbon sequestration varies among species (Liu et al 2017b), due to different microbial activity associated with different species of seagrass.
Long-term nutrient fertilization can increase the shoot density at a site, without increasing the sequestration of carbon (Howard et al 2016). Howard et al (2016) observed one seagrass meadow that had been fertilized by roosting birds for 30 years, and a nearby site that had not. The two sites had different seagrass composition (dense Halodule at fertilized site, sparse Thalassia at the other) and different shoot densities, but the organic carbon stock in the top 15 cm was the same at the two sites, and comparable to that measured at other sites around the world (Howard et al 2016).
Adding to the complexity, a 17 month phosphorus addition experiment (Armitage and Fourqurean 2016) resulted in higher seagrass biomass stored in sediment under phosphorus enrichment, but lower organic carbon burial in the sediment itself.
The net effect of nutrient addition on carbon storage in seagrass meadows is at yet unclear, varying as it does with the type of nutrient and species of seagrass, as well as other environmental factors.
3.3.5. Herbivores and predators
Herbivores can reduce the rate of carbon storage in seagrass meadows or even release stored carbon. A rapidly-increased sea urchin population in south-eastern Australia caused heavy grazing of seagrass and, ultimately, the erosion of the top 30 ± 20 cm of sediment (Carnell et al 2020). Similarly, an experiment using simulated grazing and shading resulted in a loss of below-ground seagrass biomass and carbon sequestration capacity (Dahl et al 2016). Although the authors did not observe a reduction in sedimentary carbon over the course of the experiment, they suggested that a long-term loss would be likely, due to erosion once the seagrass cover had been reduced.
Marine predators can indirectly enhance carbon storage in sediment (Carnell et al 2020). For example, sharks and other predatory species keep herbivores away or reduce their populations, which favours slow-growing plants that store a high proportion of carbon (Atwood et al 2015).
However, predators can sometimes have the opposite effect. The direction of the effect of predators depends on the environmental context and on the length of the food chain (odd-number vs. even-number of trophic levels; Atwood et al 2015). Some bioturbators and herbivores can be useful for promoting healthy seagrass growth, but high numbers tend to increase the breakdown of organic carbon (Atwood et al 2015).
3.3.6. Emissions of other GHGs
A thorough exploration of non-CO2 greenhouse gas emissions from seagrass meadows is beyond the scope of this review. However, it is worth mentioning that the anoxic decomposition of seagrass carbon can result in significant methanogenesis (Al-Haj and Fulweiler 2020, Chuan et al 2020). The net effect of organic carbon burial in coastal wetlands can be opposed or offset by emissions of methane and nitrous oxide (Rosentreter et al 2021). Such emissions can be patchy within a single wetland and over time, so it is difficult to evaluate their long-term effects.
3.3.7. Role of calcium carbonate
Calcium carbonate can be precipitated, buried, remineralized and/or dissolved in seagrass meadows. This is relevant to the net drawdown of carbon dioxide, because CO2 is released when calcium carbonate forms (calcification) and consumed when it dissolves (figure 3) (Frankignoulle et al 1994).
Figure 3. Effect of calcification on net carbon burial. Precipitation of calcium carbonate releases CO2, reducing the net burial of carbon. Advection of solid calcium carbonate into a seagrass bed from land does not change the net burial in the seagrass bed.
Download figure:
Standard image High-resolution imageCarbonate is often enriched in seagrass sediment (Gullström et al 2018). Some of this comes from calcium carbonate structures in seagrasses themselves, some from associated CaCO3-producing organisms, and some is washed in from terrigenous sources. The molar ratio of CO2 released to CaCO3 formed varies but is generally about 0.6 in the modern ocean (Gullström et al 2018). However, as pH declines globally due to ocean acidification, the CO2:CaCO3 ratio will increase, resulting in a positive feedback that leads to acidification, and reducing the net burial of carbon (Frankignoulle et al 1994). Lower sedimentary carbon storage under low-pH conditions was observed near two CO2 vent systems in the Mediterranean (Vizzini et al 2019), emphasizing that, although acidification might favour the growth of seagrass, it does not necessarily favour carbon sequestration in sediment.
Calcium carbonate formed in situ in a seagrass meadow can offset the CO2-drawdown effect of the burial of organic carbon (Saderne et al 2019, Sanders et al 2019). However, the accumulation of even carbonate-rich sediment can have a beneficial effect of helping seafloor accretion to keep pace with sea-level rise (Saderne et al 2019).
The ratio of inorganic to organic carbon stored in sediment varies around the world. In the western Indian Ocean (Tanzania to Mozambique), % organic C in seagrass sediment is positively related to % CaCO3 (Gullström et al 2018). Sediment in Brazil and Florida has higher CaCO3 than organic C burial, which might negate atmospheric drawdown of CO2 by organic C burial (Howard et al 2018). In coastal sediment of the temperate west coast of Canada, in contrast, the concentration of organic carbon in sediment and sinking particles can be an order of magnitude higher that of inorganic carbon (e.g. Johannessen et al 2019). Overall, CaCO3 burial is higher in the tropics than in temperate waters (Saderne et al 2019).
Both organic and inorganic carbon come from a mixture of autochthonous and allocthonous sources (York et al 2018), and not all buried organic or inorganic carbon is equally reactive. The variability among sites and seasons depends on local factors (Howard et al 2018), so the CaCO3 offset/buffering effect needs to be studied along with organic C storage.
Macreadie et al (2017b) summarized the main uncertainties around the net effect of carbonate burial in seagrass meadows: the stoichiometry varies; some released CO2 could be taken up by seagrasses and epiphytes; some carbonate in sediment comes from geological not local biological sources; and further acidification might redissolve precipitated CaCO3, buffering the effect.
The magnitude and direction of the net effect of carbonate burial in seagrass meadows worldwide are unclear. The effect will need to be quantified site by site. Certainly it will be important to separate allochthonous from autochthonous carbonate. The reburial of terrigenous CaCO3 could be considered a net zero effect (figure 3), since it is just a matter of moving the solid carbonate from one reservoir to another.
3.4. Different factors predominate in different environments
From the results reported above, it is clear that different factors determine the rate of net organic carbon sequestration in different environments: while biotic factors predominate in the tropics and subtropics, abiotic factors are more important in temperate seagrass meadows. Some recent review papers have also made this point.
In a broad review of studies of tropical, subtropical and some temperate seagrasses, Mazarrasa et al (2017) determined that the habitat characteristics that promoted high org C sequestration were: an extensive, continuous meadow, large persistent species, fine-grained sediment, a low-energy environment, clear water, moderate nutrient concentration, predator protection (to reduce grazing and bioturbation), protection against disturbance, and the ability to expand landward with sea-level rise. They also commented that for smaller, short-lived seagrasses such as Zostera, grainsize could be a useful indicator of carbon storage.
Despite the importance of seagrass species and meadows size in tropical and subtropical regions, Samper-Villarreal et al (2018) observed significant variation in carbon stock over a gradient of water turbidity, from riverine to coastal, in their subtropical study area. Mazarrasa et al (2021) found that different factors predominated, depending on bioregion, plant body size and environment (estuarine vs coastal).
A similar review centred on temperate, northern hemisphere Zostera marina meadows (Röhr et al 2018) determined that high organic carbon stock (measured to 25 cm) was promoted principally by abiotic factors (grainsize, dry density, degree of sorting, salinity, water depth). The authors found that those factors were more important to total organic carbon stock than plant-related parameters (biomass and shoot density). Based on δ13C measurements, less than half of the organic carbon buried in these systems came from seagrass, and this proportion was highly variable.
Environmental factors, particularly the relative energy of the environment, have also been found to determine carbon burial in temperate environments on the west coast of North America (Prentice et al 2019), Korea (Kim et al 2022), England (Lima et al 2020), and the northeastern United States (Novak et al 2020).
3.5. Burial rate methodology
Carbon stored in surface sediments that undergo active re-mineralization or are vulnerable to resuspension cannot be said to be buried on the 100 year timescale required for carbon credits (figure 1). Only burial below the vulnerable surface layer represents sequestration. Quantifying carbon burial rates in seagrass meadows and comparing the calculated rates amongst sites globally has been problematic, due to the widespread lack of understanding of how to make that calculation.
Some authors use accretion above an introduced marker layer, such as a layer of crushed feldspar (e.g. Ewers Lewis et al 2018). Others divide the carbon inventory over the top few cm by the sedimentation rate determined from 210Pb (e.g. Bedulli et al 2020). Some do not specify their burial rate methodology, and some use global average rates from Duarte et al (2013) and do not measure the local rates at all.
Lafratta et al (2020) commented that 210Pb and 14C do not always work in low-depositional seagrass environments and that no additionality is demonstrable using these methods. That likely indicates that there is no significant accumulation of sediment or organic carbon at these sites.
Many authors use a sediment accumulation rate model known as constant rate of supply (CRS), which was designed for unmixed lake sediments (Appleby 2001). Appleby commented that the method was not suitable for use in bioturbated sediments (figure 2). Blue carbon researchers frequently note bioturbation in their cores but then use the CRS model, regardless. Some note bioturbation, but then assign unique dates to specific layers in their cores, or report different sedimentation rates or different carbon accumulation rates for different core segments, which is not possible in a mixed core (Johannessen and Macdonald 2016).
Reviews sometimes compare burial rates from different studies that used a variety of methods, some accounting for surface sediment mixing and others not. Although the lack of standard methodology limits the comparison of burial rates from one study to another, it does seem likely that carbon burial rates in temperate North America are lower than those in tropical and subtropical areas (Postlethwaite et al 2018, Prentice et al 2020).
A universal methodology would assist with inter-regional comparisons and global sums. One method that has been used for decades in coastal marine sediment outside seagrass meadows is illustrated in figure 4. First the sediment accumulation rate is determined from the decay of 210Pb below the surface mixed layer, following Lavelle et al (1986). Next the burial concentration of organic carbon is determined from the depth profile of organic carbon, as the depth at which the organic carbon concentration has declined to a near-constant value (Krause et al 2022). The seagrass proportion of the organic carbon would ideally be determined using a combination of biomarkers, as noted above. Finally, the burial concentration of seagrass organic carbon is multiplied by the sediment accumulation rate (Johannessen and Macdonald 2016).
Figure 4. How to calculate carbon burial (long-term sequestration) in a bioturbated or wave-mixed sediment core. Nearly all coastal sediment has a surface mixed layer. See text for more detail.
Download figure:
Standard image High-resolution imageIf the organic carbon concentration does not decline to a constant value with depth, then either the burial depth has not yet been reached (if the concentration is still decreasing at the bottom of the core), or else something has changed in the environment. For example, a down-core increase indicates that the flux of organic carbon has decreased at that site.
Even the simple situation of down-core decline with depth due to remineralization can, in some situations, mask an increase in carbon flux. These two processes can be separated, but the only method developed to date (Johannessen et al 2020) requires multiple cores from the same area, which might be prohibitively expensive, depending on the situation.
The method described in figure 4 works for either mixed or unmixed sediment, where the sediment accumulation rate and carbon flux have remained approximately constant over the time represented by the core. It is more complicated if either of these rates has changed significantly, which might well have happened in areas where changes in vegetation or in human activities have changed the depositional environment.
If the flux of organic carbon has changed with time, but the sediment accumulation rate has remained the same, then the carbon flux can be modelled mathematically as a transient tracer (Guinasso and Schink 1975). If the sediment accumulation rate has also changed, this should be apparent as a break in the slope of the plot of ln(Excess 210Pb) vs depth (in addition to the break at the base of the surface mixed layer; e.g. see figure 3 in Johannessen et al (2008)). However, the studies on transient tracers and changing sedimentation rates were not carried out in seagrass meadows. It would be useful to develop a method specifically for seagrass meadows, to account for changes in the fluxes of sediment and organic carbon.
In summary, the CRS method only applies where there is no bioturbation or other mixing, such as in lakes or in deep anoxic basins. For mixed cores, as in most marine sediments where seagrasses grow, the 210Pb method described by figure 4 is better. For bioturbated cores where there has been a change in the rate of sediment accumulation or of organic carbon flux, the figure 4 method can still be applied, if the change in sedimentation rate was simple. For more complex situations, mathematical modelling is required. Until a method has been developed to deal with complex changes in flux in seagrass meadows, a conservative approach would be to multiply the lowest concentration of organic carbon measured in a core by the lowest sediment accumulation rate that fits the data.
3.5.1. Allochthonous carbon
There has been a great deal of recent discussion over whether to include or exclude allochthonous carbon–carbon that originates from outside the seagrass meadow (i.e. terrigenous carbon, or carbon transferred from another marine environment.) This can include black carbon, which is essentially unreactive (Chew and Gallagher 2018), and microplastics (Huang et al 2021).
Those who propose that allochthonous carbon should be included argue that the seagrass meadow enhances the burial and protection of the allochthonous carbon. For example, in a restored seagrass meadow in Virginia, biomarker analysis (δ13C, δ15N, δ34S) indicated that there was a high, non-seagrass contribution in the middle of the patch that the authors attributed to the burial of benthic microalgae (Oreska et al 2018). The authors argued that this should be included, because its burial was enhanced by the presence of the seagrass.
Similarly, Hidayah et al (2022) found a significant contribution of organic matter from macroalgae and mangroves in seagrass meadow sediment, and argued that this should be included in the total carbon sequestration attributed to the meadow. Krause et al (2022) found seagrass fronds in salt marsh sediment and argued that, although this carbon was allochthonous to the marsh, it had come from a connected, vegetated ecosystem, and that carbon transferred from other, connected vegetated ecosystems should be included. Huxham et al (2018) argued for a similar 'seascape' approach.
In contrast, those who propose that all allochthonous carbon should be excluded argue that the allochthonous carbon would likely have been preserved in nearby, fine-grained basin sediment or shallow, unvegetated sediment, regardless of whether or not the seagrass meadow was there (Chew and Gallagher 2018, Gallagher et al 2022b). They point out that much of the allochthonous carbon is essentially unreactive and would not have been particularly vulnerable to remineralization, even if it had not been captured by the seagrass meadow.
In addition, an expanded seagrass meadow ecosystem necessarily replaces something else, and if the alternative is not bare rock, then the carbon burial potential of the alternative ecosystem should also be subtracted from the credit for the expanded burial of seagrass carbon (Gallagher et al 2022b).
In some environments, nearby unvegetated coastal sediment has been found to contain more carbon than the seagrass meadow sediment, mainly because of the grainsize effect (seagrasses grow in relatively coarse, sandy sediment, not in the adjacent muddy areas) and because the input of allochthonous carbon was greater outside the meadow (Ricart et al 2020). If allochthonous carbon had not been included at either site, then there would, presumably, have been a smaller difference in carbon storage between the two locations.
However, a biomarker study in New Zealand (Bulmer et al 2020) indicated that some of the organic carbon buried in the unvegetated sediment had actually come from the vegetated ecosystem, demonstrating again the importance of biomarkers in determining the fate of carbon exported from seagrass meadows or beds of macroalgae.
There might be cases in which the seagrass provides an environment in which non-seagrass carbon (e.g. from benthic microalgae) is produced and buried. However, excluding allochthonous carbon in general, especially terrigenous organic carbon, which is relatively recalcitrant (e.g. Johannessen et al 2020) helps to avoid the risk of over-inflating the role of seagrass meadows in carbon sequestration. From the point of view of the atmosphere, if the allochthonous carbon would have been preserved in sediment regardless of whether or not the seagrass meadow was there, then the meadow has no net effect on the drawdown of that portion of the carbon.
3.6. Effects of climate change on seagrass meadow carbon storage
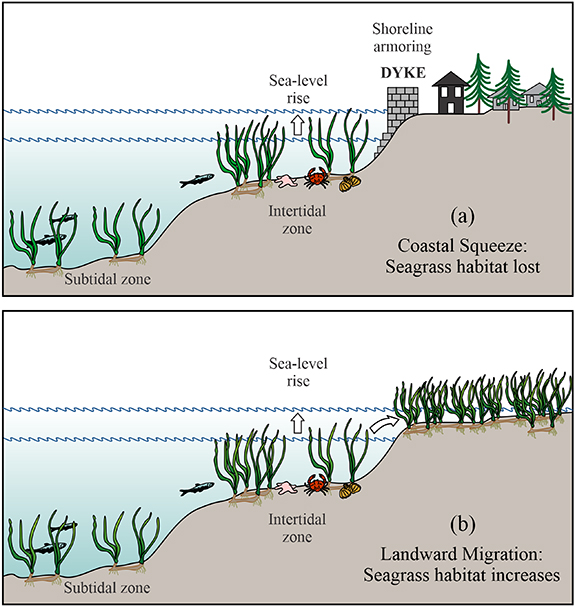
Sea-level rise could result in an expansion of seagrass habitat in places where meadows are able to move landward; however, shoreline armouring, such as building seawalls, would result in a 'coastal squeeze' effect (Lovelock and Reef 2020), which could reduce the area of meadows and the potential for continued storage of carbon (figure 5, table 2). Sea-level rise is likely to reduce seagrass area and meadow carbon stocks, unless the shoreline is managed to permit landward migration of seagrass (Kauffman et al 2020).
Figure 5. Two scenarios for sea-level rise: (a) rising seawater meets shoreline armouring, causing a coastal squeeze that reduces seagrass habitat; (b) rising seawater is allowed to flood inshore lowlands, expanding seagrass habitat.
Download figure:
Standard image High-resolution imageTable 2. Effects of climate change on seagrass meadows and their ability to store carbon.
| Oceanic effects of climate change | Effect on seagrass and C-storage | Reference |
|---|---|---|
| Sea-level rise |
| Lovelock and Reef (2020)Kauffman et al (2020) |
| Moderate, slow temperature rise (⩽ 4 °C increase) |
| Macreadie and Hardy (2018) |
| Large or rapid (e.g. marine heat wave) temperature rise |
| See temperature rise sectione.g. Duarte et al (2018a) e.g. Aoki et al (2021) |
| Declining coastal salinity |
| Murphy et al (2021) |
| Declining pH (rising pCO2) |
| Murphy et al (2021)Frankignoulle et al (1994) |
| Increasing frequency of extreme events |
| Murphy et al (2021)See temperature section |
| Changing timing of freshwater discharge |
| Bidlack et al (2021) |
Submerged plants, such as seagrasses, are susceptible to temperature increase (Short et al 2016). Global warming is changing the geographic range of seagrasses, with losses in equatorial regions and expansion toward the poles (Duarte et al 2018a). Warming water might favour the expansion of seagrass habitat in temperate regions and the Arctic (Murphy et al 2021).
Short-term marine heat waves, which are becoming more common as a result of climate change (Oliver et al 2019), can cause local or widespread seagrass die-offs (e.g. Smale et al 2019, Strydom et al 2020, Murphy et al 2021). The loss of seagrasses can lead to the loss of associated carbon stocks (Arias-Ortiz et al 2018). These losses may be highly localized in some cases, with a loss of stored carbon at the centre of a patch, followed by a gain in subsequent years around the edges of the patch (Aoki et al 2021). More moderate temperature rise does not appear to threaten seagrass sediment carbon stock. When seagrass cores were transplanted along a thermal gradient generated by hot water released from a coastal power plant (Macreadie and Hardy 2018), there was no secular trend in carbon stock at a temperature elevation of 2 °C or 4 °C. Macreadie and Hardy (2018) speculated that the increased microbial degradation at higher temperatures reported in other studies might require higher temperatures, longer exposure times, or a combination of other stressors.
Overall genetic change is thought to be too slow to keep up with the rate of global change, but changes in microbiome and gene expression (epigenetics) may provide enough plasticity for individual adaptation (Duarte et al 2018a). A range of temperature-tolerance can occur within a single patch (DuBois et al 2022). In an experiment in which a Zostera marina meadow was subjected to five weeks of experimental warming (4.5 °C above ambient), Reynolds et al (2016) observed an initial increase in shoot production, followed by reduced shoot production and a 50% decrease in biomass after the temperature returned to normal. They also observed a change in genotype, favouring some genotypes that had not been dominant at normal temperatures. It seems that some adaptation is possible within genetically-diverse populations.
Deoxygenation is a widespread effect of climate change. Seagrasses experience a large diel range in oxygen concentration (e.g. Berger et al 2020, Altieri et al 2021). They produce oxygen photosynthetically, and are able to counteract the effects of local hypoxia on their associated heterotrophic communities by moving oxygen from blades to sediment and by releasing excess oxygen into the canopy (Altieri et al 2021). In contrast, increased exposure to oxygen—from dredging, for example—can release stored carbon from seagrass meadow sediments (Macreadie et al 2019). Macreadie et al (2019b) observed a marked increase in remineralization (34- to 38-fold) when deeply-buried, thermally-recalcitrant carbon was exposed to oxygen.
The hydrological cycle is also changing in ways that affect nearshore waters and coastal ecosystems. Warming accelerates the melting of glaciers and changes patterns of precipitation. This affects the flux and timing of particles, carbon and nutrients discharged into the coastal ocean (Bidlack et al 2021). Increasing turbidity due to storms or sudden, high-discharge events could reduce the available light and smother seagrass beds (Murphy et al 2021). Reduced light has been shown to reduce the subsurface sedimentary carbon even more than the surface carbon, possibly due to reduced release of dissolved organic carbon by the seagrass roots in low light (Premarathne et al 2021).
Ocean acidification, another effect of increased atmospheric CO2, has a complex effect on carbon storage in seagrass meadow sediment. (See 'Role of calcium carbonate' section above.) Acidification might favour seagrass photosynthesis, but at the same time harm calcareous epiphytes (Murphy et al 2021). It will also increase the ratio of CO2 released per mole of calcium carbonate formed (Frankignoulle et al 1994), reducing the net burial of carbon in sediment.
Declining coastal salinity in some temperate areas could reduce seagrass habitat; a salinity <20 (on the Practical Salinity Scale) has been observed to reduce growth and the establishment of seedlings (Murphy et al 2021), although some seagrass meadows were still healthy with salinity as low as 5 in Arctic waters.
The loss of coral reefs, particularly in the tropics, as a result of climate change, might also reduce seagrass meadow carbon storage; coral reefs can serve to reduce the energy of the environment, permitting greater sediment accumulation in sheltered meadows and reducing erosion (Guerra-Vargas et al 2020).
The effects of climate change on carbon storage in seagrass meadows will be mixed (table 2). The biggest climate-related threats to seagrass meadow carbon storage (and to the meadows themselves) are likely to be the coastal squeeze effect and increased water temperature, particularly as a result of extreme events such as marine heat waves.
3.7. Management strategies to protect seagrass carbon and to qualify for carbon credits
Most papers in this field call for the protection of existing blue carbon stocks in seagrass meadows. Several protective measures have been proposed. For example, the protection of blue carbon stocks could be incorporated into the design of marine protected areas (Howard et al 2017, Sala et al 2021).
Seagrass management studies have been carried out in Australia, which is estimated to hold 5%–11% of the world's blue carbon stock (Kelleway et al 2020). There is interest in including blue carbon in Australia's Emissions Reduction Fund, with the potential to protect, restore and expand blue C ecosystems, using management actions such as reintroducing tidal flow through meadow areas (Kelleway et al 2020).
A model study evaluated several management options for blue carbon protection along the coastline of southeastern Australia (Moritsch et al 2021). The study compared the effects of: (a) managed retreat; (b) managed retreat + levee removal; (c) erosion of high-risk areas; and (d) erosion of moderate- to high-risk areas. The authors found that avoiding erosion was more effective than restoration, and that managed retreat + levee removal sequestered the most carbon. Levee removal alone contributed only a small portion to the total carbon sequestered, but the effect began sooner.
Other management actions with the potential to increase carbon sequestration in Australia and beyond include: reducing anthropogenic nutrient loading, restoring water circulation, and reducing the overpopulation of bioturbators (Macreadie et al 2017a). Overfishing of predatory fish can increase the population of grazers and bioturbators, reducing carbon storage in seagrass meadow sediment (Macreadie et al 2017a).
The potential monetary value of blue carbon restoration is as yet unclear. Taillardat et al (2018) commented that, although the global-scale effect of blue carbon was limited because of the small total area of vegetated ecosystems, it could be significant at a national level for countries that have long coastlines and low national emissions, such as Bangladesh. Case studies of carbon-financing in Kenya, India, Vietnam, and Madagascar indicate that small-scale projects under the voluntary carbon standard are the most effective (Wylie et al 2016).
There is still an urgent need to refine and correct blue carbon accounting before it is widely accepted for carbon financing (Johannessen and Macdonald 2016, Gallagher 2017). Recent controversy over land-based carbon crediting schemes in Australia (Macintosh et al 2022, Morton 2022) provide a cautionary tale.
4. Conclusions
In order for blue carbon sequestration in seagrass meadows to qualify for carbon credits, it must represent additional, long-term, net sequestration of carbon. In the context of the Verified Carbon Standard, 'long-term' means >100 years (even though that is many orders of magnitude less than the time required to remove fossil fuel carbon from the modern carbon cycle).
It is easy to get bogged down in a meadow- or plot-scale view, which leads to arguments over how to account for allochthonous organic carbon or carbonate, or what depth to include when calculating carbon stock.
Considering the effect of a blue carbon project from the point of view of the atmosphere provides some clarity. Increased draw-down of atmospheric carbon dioxide requires increased removal (i.e. burial) of organic carbon. The considerations required to increase long-term, net carbon burial are listed in table 3.
Table 3. Additionality/permanence best practices checklist.
| New or restored area only—no existing stock or burial counts |
| Long-term burial—below depth vulnerable to remineralization or resuspension (calculated correctly) |
| Exclude allochthonous carbon; identify and quantify stored seagrass carbon, using biomarkers |
| Subtract pre-existing C-burial of the area (e.g. phytoplankton or other plant production displaced by new seagrass) |
| Account for calcium carbonate (subtract carbonate formed in the meadow; allochthonous carbonate has net zero effect—moved from one location to another—unless reduces local pH) |
| Account for non-CO2 greenhouse gases (CH4, N2O) |
| Consider future effects of climate change (exclude burial likely to be lost due to e.g. sea-level rise) |
| Protect against future disturbance: development, eutrophication, trawling |
4.1. The importance of regional differences
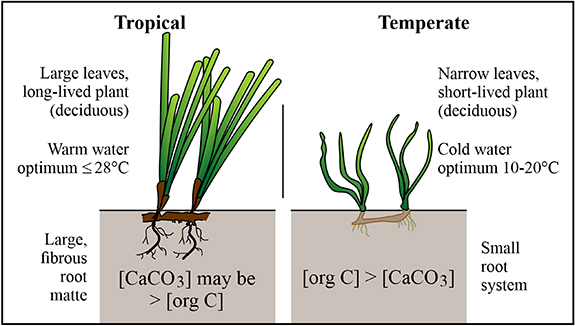
Some of the controversy over blue carbon methodology likely results from real regional differences in ecosystems (figure 6). Seagrass meadows in tropical and subtropical waters tend to be dominated by large, long-lived species that produce extensive root mattes. The underlying sediment often contains high concentrations of carbonate, and the water is warm. In temperate waters, the seagrasses tend to be smaller, short-lived, with short, fine roots, and grow in principally siliciclastic sediment. The replacement ecosystems in the absence of seagrasses may also be different in different regions.
Figure 6. Regional differences in seagrass species, water temperature and sediment composition.
Download figure:
Standard image High-resolution imageConsequently, the primary controls on net carbon burial are different in different kinds of meadows. In areas with large seagrass species, the burial rate depends mainly on the type of seagrass and the meadow extent, while in areas with smaller seagrasses, abiotic factors are more important.
In addition, there are regional or even local differences in the importance of excess nutrients, tidal flow constriction, invasive species, bioturbation, and the likelihood that seagrasses will be able to migrate landward in response to sea-level rise. Even familiar indicators, like bulk stable isotopes, might have different meanings in different environments. For example, in the Red Sea lower δ15N in seagrass and macroalgae indicates oligotrophic conditions and more nitrogen fixation (Duarte et al 2018b), while off the west coast of Canada, lower δ15N in sinking particles indicates bloom conditions, with ample nutrients, and a short food chain (Johannessen et al 2005).
4.2. Recommendation
Given the extensive regional differences, it is not surprising that there has not been a single, consistent interpretation of the role of seagrass meadows in offsetting carbon emissions globally. Inter-regional cooperation could help to resolve this. Rather than making measurements in one type of environment and extrapolating to the world, it might be more effective to assess the potential to expand net, long-term carbon burial meadow by meadow, region by region, with techniques appropriate to each environment, and then sum the parts to estimate the whole. Collaboration among researchers from different parts of the world would facilitate this approach. It will be particularly important to include researchers from Africa, where there have been so few measurements to date.
Acknowledgments
The author thanks Dr Andrew Ross and two anonymous reviewers, who provided very helpful comments on an earlier draft, and Patricia Kimber, who prepared the figures.
This paper is dedicated, in grateful memory, to Dr Robie W Macdonald.
Data availability statement
No new data were created or analysed in this study.