Abstract
Hydrocarbon fuel production and utilization are considered water intensive processes due to the high volumes of water used in source development and fuel processing. At the same time, there is significant water formed during combustion. However, this water is not currently widely harvested at the site of production. Instead, it is added to the hydrologic cycle, often in a different location from the fuel production site. This study quantifies the water formed from combustion of these fuels and analyzes the magnitudes of formation in the context of other hydrologic sources and sinks in order to facilitate future assessments of water harvesting technology and/or atmospheric impacts of combustion. Annual water formation from stoichiometric combustion of hydrocarbon fuels, including natural gas, oil- and natural gas liquid-derived products, and coal, in the United States and worldwide are presented and compared with quantities of water sequestered, evaporated, and stored in the atmosphere. Water production factors in terms of mass and energy of fuel consumed, WPFm and WPFe, respectively, are defined for the comparison of fuels and incorporation into future life cycle analyses (LCAs). Results show that water formation from combustion has increased worldwide from 2005 to 2015, with the largest increase coming from growth in combustion of natural gas. Water formation from combustion of hydrocarbon fuels equals or exceeds water sequestered from the hydrologic cycle through deep well injection in the US annually. Overall, water formation is deemed significant enough to warrant consideration by LCAs of water intensity in fuel production and use, and should be included in future analyses.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence.
Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
Introduction
Hydrocarbon fuel production and utilization are widely considered to be water intensive processes due to the substantial water requirements of production, including oil and gas well drilling, waterflooding, and hydraulic fracturing; processing, including compression and transportation; refining, including steam production for process heat; and utilization, including cooling water in power plants. Increasing awareness of the interdependence of energy and water is leading to extensive and ongoing studies of the energy-water nexus [1, 2]. Life cycle analyses (LCAs) and other research efforts have quantified the water needs of oil, gas and coal production, focusing on water withdrawal and consumption at each stage of a fuel's life cycle. Water consumption at each stage of conventional and shale oil and gas production, including consumption due to well drilling, cementing, fracturing, gas processing and transporting, as well as water savings by flowback water recycling and capture of produced water, have been assessed [3, 4]. The water sources and sinks during hydrocarbon fuel production are generally well characterized for LCAs. However, a critical aspect is often overlooked: water production during combustion. Water is formed in combustion by oxidation of hydrogen in hydrocarbon fuels, and thereby adds water to the hydrologic cycle and atmospheric water stores. Current LCA frameworks for hydrocarbon fuel combustion typically report on greenhouse gas emissions and other environmental impacts, but not on water produced [5–8]. The purpose of this work is to provide greater insight into how much annual water is produced globally as a result of hydrocarbon combustion. The production of this water may be co-located with the fuel production site, but is more likely to be distributed and/or far removed from the site of fuel production, thereby not directly offsetting the water intensity of fuel production. Thus, the magnitude of water production is critical to understand from the perspective of atmospheric stores, which influence climate and weather, as well as for assessment of combustion water harvesting potential which, while not currently widely implemented, has the potential to offset the water intensity of fuel production. While the technoeconomic feasibility of water harvesting is outside the scope of this study, the findings of this study can inform such investigations.
Table 1. Typical composition of natural gas [23].
| Component | Concentration (vol %) |
|---|---|
| Methane | 94.9 |
| Ethane | 2.5 |
| Propane | 0.2 |
| Isobutane | 0.03 |
| n-Butane | 0.03 |
| Isopentane | 0.01 |
| n-Pentane | 0.01 |
| Hexane | 0.01 |
| Nitrogen | 1.6 |
| Carbon dioxide | 0.7 |
| Oxygen | 0.02 |
| HHV | 52 225 kJ·kg−1 |
| LHV | 47 141 kJ·kg−1 |
There are two prominent research areas in which the impact of water production from hydrocarbon combustion might be important to consider. The first area is the energy-water nexus, including LCAs that estimate water intensity of hydrocarbon fuel production [3, 9, 10–12]. Much of the prior work in the energy-water nexus field has focused on withdrawal and consumption of water for extraction and end-use cooling at power plants [13], but has often overlooked the magnitude of water production from hydrocarbon combustion. Water harvesting to provide water for oil- and gas-field operations has been examined, such as recent studies that examined the potential for flared natural gas at oilfield operations to be used for atmospheric water harvesting or wastewater treatment, although the water formed during natural gas combustion has not been targeted for harvest [14–16]. The second group of researchers are climate scientists studying the broader impacts of hydrocarbon fuel consumption and anthropogenic emissions. In general, climate research assumes that water production due to hydrocarbon combustion is negligible in comparison to natural evaporative cycles [17].
In some limited cases the impact of combustion on the water cycle has been investigated. An LCA of greenhouse gas emissions and freshwater consumption in the Marcellus shale field acknowledged, but did not include in the analysis, a substantial amount of water formed from natural gas combustion. It was estimated that the global ecosystem gains a net 5.7 million gallons of condensed water over the life cycle of a well, considering consumption by hydraulic fracturing and wastewater disposal versus production by combustion [18]. Chesapeake Energy has estimated that enough water is produced from combustion of natural gas from their wells to offset the water used in the development of those wells [19]. Gaffen and Ross estimated that global combustion of hydrocarbon fuels contributed on the order of 1012–1013 kg·yr−1 of water vapor to the atmosphere between 1960–1990, although they did not provide a detailed assessment by hydrocarbon fuel source or describe the methodology by which this tally was quantified [20].
The present study provides a detailed estimate of water formation in the US and worldwide from hydrocarbon fuel combustion organized by major fuel types: natural gas, natural gas liquids, oil and coal. The distinction between water formation from different fuel types is particularly important as primary energy sources gain and lose favor. While natural gas has a smaller carbon footprint than coal due to its lower carbon-to-hydrogen ratio, the converse consideration of methane as a higher hydrogen-containing, and therefore higher water-producing, energy source has received less attention. A water production factor (WPF) is defined that gives the water produced per unit of fuel consumed. Modeled after water consumption and water use factors (WCF and WUF, respectively), which have been used in LCAs to represent the water consumed or used per unit of fuel or fuel intermediate output from a given process [21, 22], WPF allows for the comparison of water production under different fuel use scenarios. Furthermore, water formation quantities are contextualized through comparison among different fuels, water contained in the atmosphere, evaporation rates, and volumes of water from oil, gas and coal development that are sequestered by deep well injection.
Table 2. Typical compositions of anthracite [24], bituminous, sub-bituminous, and lignite coals [25] are presented, including ultimate analysis of dry ash free (daf) coal, moisture and ash contents of raw coal on an as-received basis (ar), and higher and lower heating values on dry ash free and as-received bases.
| Composition (wt%, daf) | Anthracite | Bituminous | Sub-bituminous | Lignite |
|---|---|---|---|---|
| Carbon (C) | 92.0 | 80.5 | 73.8 | 71.1 |
| Hydrogen (H) | 3.5 | 5.7 | 5.0 | 5.7 |
| Nitrogen (N) | 1.3 | 1.6 | 1.0 | 1.3 |
| Sulfur (S) | 0.9 | 3.2 | 0.3 | 1.7 |
| Oxygen (O) | 2.3 | 8.7 | 19.9 | 20.2 |
| Moisture, ar (wt%) | 2.5 | 11.1 | 27.4 | 32.0 |
| Ash, ar (wt%) | 4.0 | 9.7 | 4.5 | 15.0 |
| HHV, ar (kJ kg−1) | 32 024 | 27 113 | 20 469 | 15 243 |
| HHV, daf (kJ kg−1) | 34 250 | 34 242 | 28 631 | 28 762 |
| LHV, ar (kJ kg−1) | 31 089 | 26 151 | 19 738 | 14 601 |
| LHV, daf (kJ kg−1) | 33 250 | 33 050 | 27 559 | 27 549 |
Table 3. Representative compositions of oil products [26].
| Oil products | Composition | HHV [kJ·kg−1] | LHV [kJ·kg−1] |
|---|---|---|---|
| LPG | C3.5H9 | 49 700 | 45 800 |
| Gasoline | C8H15 | 47 300 | 43 000 |
| Diesel | C12.3H22.2 | 44 800 | 42 500 |
| Heavy Diesel | C14.6H24.8 | 43 800 | 41 400 |
Methods
The hydrocarbon fuels considered in this study include natural gas, natural gas liquid products, oil products and coal. Natural gas is comprised of several components including methane, ethane, propane, and butane. While natural gas can vary in composition with production location and time, a representative composition is given in table 1 and used in this study [23]. The US specification for maximum moisture in pipeline quality natural gas is 1.1 × 10−4 kg·m−3; thus, moisture in pipeline natural gas is neglected in this analysis. Coal is generally characterized by carbon content as anthracite, bituminous, sub-bituminous or lignite. Representative compositions, including atomic composition of dry ash free coal by ultimate analysis, moisture and ash contents of as-received coal, and higher and lower heating values for dry ash free and as-received coal, are given in table 2. Average properties of anthracite [24], Illinois No. 6 coal to represent bituminous coal, Wyodak/Anderson Powder River Basin (PRB) coal to represent sub-bituminous coal, and Texas lignite to represent lignite [25] are summarized. Oil products include natural gas liquids (NGL) and petroleum derivatives, and combusted products are categorized as liquified petroleum gas (LPG), light distillates, middle distillates, and fuel oil. Other petroleum derivatives, including solvents, lubricants and petroleum coke, are neglected due to their non-combustion fate or low yield and low hydrogen content. While the compositions of the combusted NGL and oil products vary, they are approximated as a 50/50 vol% blend of propane and butane for LPG due to variability in composition worldwide, gasoline for light distillates, diesel for middle distillates, and heavy diesel for fuel oil in this study, and representative formulas are given in table 3 [26]. Equation (1) represents stoichiometric and complete combustion of a hydrocarbon fuel, which is expected to consist largely of carbon (C) and hydrogen (H) and may contain oxygen (O), nitrogen (N) and sulfur (S), with air
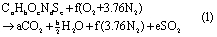
where air is represented by a mixture of diatomic oxygen (O2) and nitrogen (N2) in a 1:3.76 molar ratio, and major products are assumed to be full oxidation of C and H to carbon dioxide (CO2) and water (H2O), respectively. Other components in the fuel, such as carbon dioxide (CO2), N2, or bound H2O are assumed to carry through to the products and can be added to equation (1). Thus, combustion is assumed to proceed by equation (1) for the purpose of quantifying water production. Then, b/2 moles of water are produced per mole of fuel combusted, which can be converted to a water production factor in terms of mass of fuel combusted (WPFm) as defined by equation (2)
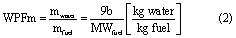
where MWfuel is the molecular weight of the fuel of interest in kg·kmol−1. In the case of coal, for which a molecular weight is ill-defined, the WPMm is most readily calculated from the ultimate analysis of the coal, which gives hydrogen content on a mass fraction basis (XH). Water production factors are defined for dry ash free (WPFmdaf) and as-received (WPFmar) coal, where the latter includes the water contained within the raw coal and the former does not. Given an ultimate analysis on a dry ash free basis, the WPFm for dry ash free (daf) coal is calculated by equation (3)
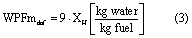
and the WPFm for as-received coal, including moisture bound in the coal, is calculated by equation (4)
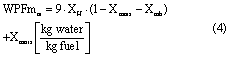
where Xmois and Xash are the mass fractions of water and ash in the as-received coal, respectively, given by proximate analysis as outlined in an ASTM method by which moisture, volatile, fixed carbon and ash fractions of coal are quantified [27].
Table 4. Summary of water production factors for stoichiometric combustion of fuels.
| Fuel | WPFm | WPFeLHV | WPFeHHV |
|---|---|---|---|
![$\rm \left[ {\frac{{kg\;H_2 O}}{{kg\;fuel}}} \right]$](https://content.cld.iop.org/journals/1748-9326/12/9/094019/revision3/erlaa8390ieqn1.gif)
|
![$\rm \left[ {\frac{{g\;H_2 O}}{{MJ}}} \right] $](https://content.cld.iop.org/journals/1748-9326/12/9/094019/revision3/erlaa8390ieqn2.gif)
|
![$\rm \left[ {\frac{{g\;H_2 O}}{{MJ}}} \right] $](https://content.cld.iop.org/journals/1748-9326/12/9/094019/revision3/erlaa8390ieqn3.gif)
| |
| Natural Gas | 2.12 | 44.96 | 40.58 |
| Coal | |||
| Anthracite | 0.32 (ar) | 10.99 (ar) | 10.67 (ar) |
| 0.32 (daf) | 9.47 (daf) | 9.20 (daf) | |
| Bituminous | 0.52 (ar) | 24.92 (ar) | 24.03 (ar) |
| 0.51 (daf) | 15.47 (daf) | 14.93 (daf) | |
| Sub-bituminous | 0.58 (ar) | 43.25 (ar) | 41.71 (ar) |
| 0.45 (daf) | 16.36 (daf) | 15.75 (daf) | |
| Lignite | 0.59 (ar) | 76.23 (ar) | 73.02 (ar) |
| 0.51 (daf) | 18.48 (daf) | 17.71 (daf) | |
| NGL and Oil | |||
| LPG | 1.59 | 34.64 | 31.90 |
| Gasoline | 1.22 | 28.37 | 25.79 |
| Diesel | 1.18 | 27.76 | 26.34 |
| Heavy Diesel | 1.12 | 27.05 | 25.57 |
The water production factor can also be defined per unit of energy available by combustion of the fuel of interest (WPFe) and calculated from WPFm by equation (5)
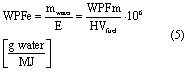
where E is the energy content in MJ that is released during complete combustion of the fuel and HVfuel is the heating value of the fuel in kJ·kg−1. WPFe are defined in terms of the lower heating value (WPFeLHV), which represents the heat of combustion for combustors that emit water to the atmosphere as vapor, and the higher heating value (WPFeHHV), which represents the heat of combustion for combustors that condense the produced water to liquid and utilize the additional latent heat of vaporization of the water. Thus, the power or heat rate obtained from combustion is dictated by the LHV or HHV, the form of the combustion-produced water as it leaves the generation system, and the mass consumption rate of the fuel.
Figure 1. Annual water formation from combustion of fuels worldwide and in the US between 2005 and 2015. Values are shown for water production from oil- and NGL- products (Oil/NGL), natural gas (NG), coal, and the total combined production from these three sources.
Download figure:
Standard image High-resolution imageTable 5. Comparison of anthropogenic and natural sources and sinks of water to and from the atmospheric reservoir.
| Water sources and sink | Location | Units (×1015) | Amount of water | Ref |
|---|---|---|---|---|
| Combustion (Source) | US | kg·yr−1 | 0.0024 | This study |
| Deep well injection (Sink) | US | kg·yr−1 | 0.0013 | [32] |
| Evap—Irrigation (Source) | US | kg·yr−1 | 0.1400 | [20] |
| Evap—Natural (Source) | US | kg·yr−1 | 3.9000 | [20] |
| Combustion (Source) | Global | kg·yr−1 | 0.0120 | This study |
| Evap—Irrigation (Source) | Global | kg·yr−1 | 1.0000 | [17] |
| Evap—Irrigation (Source) | Global | kg·yr−1 | 2.6000 | [36] |
| Evap—Natural (Source) | Global | kg·yr−1 | 490.0000 | [33] |
| Atmosphere (Reservoir) | Global | kg | 13.0000 | [33, 34] |
Results and discussion
Table 4 summarizes the WPFm and WPFe calculated by equations (2)–(5) for the fuels considered in this study. Natural gas has the highest WPFm among the fuels considered as a result of its high H-to-C ratio as compared to the other fuels. More specifically, the WPFm of natural gas is greater than those of coal and NGL- and oil-derived products by average factors of 4.5 and 1.7, respectively. Natural gas also has higher WPFeLHV than dry ash free coals and NGL- and oil-derived products by average factors of 3.0 and 1.5, respectively, and higher WPFeHHV by factors of 2.8 and 1.5, respectively. When as-received coals are considered, lignite has the highest WPFeLHV and WPFeHHV at 76.2 and 73.0, respectively, which is attributed to its high moisture and hydrogen contents and low as-received heating values as compared to other coals. In contrast, the WPFm of coal are significantly lower than those of the other fuels due to the relatively low H-to-C ratio of coals. There are potentially significant local and global implications of these relative WPFm and WPFe factors as fuels gain or lose favor for power and heat production; for example, a widespread transition of utility electricity generation from coal or other fuels to natural gas implies substantially more combustion-derived water production, which will contribute to atmospheric stores or might present water harvesting opportunities.
Total annual water formation depends on WPFm (or WPFe) and the quantity of fuel consumed. Fuel consumption data [28, 29] and WPFm are used to generate estimated annual water production quantities for the years 2005–2015. LPG data are extrapolated for years 2014 and 2015 from data provided by [29], and 25% of LPG production is not included in water production calculations in this study due to non-energy uses. The breakdown of total coal consumption by rank in the US is 0.2% anthracite, 45.7% bituminous, 46.7% sub-bituminous, and 7.4% lignite, while the worldwide breakdown is estimated using an average of production by the US and China [30, 31], which are the two largest coal producers and together produce nearly 60% of the world's coal [28], to be 9.6% anthracite, 61.1% bituminous, 23.3% sub-bituminous, and 6.0% lignite. Figure 1 shows the results of annual water formation analyses, including water formed from NGL and oil products, natural gas, and coal on an as-received basis, and total water production, in the US and worldwide. Total global water production increased monotonically between 2005 and 2015, except for 2009 which showed a 1.4% decrease from production in 2008. This decrease in water production is attributed to decreased fossil fuel consumption during the 2008–2009 global recession. Overall, total water production worldwide increased by 22.7% from 2005–2015 with the largest growth seen in water production from natural gas, with a 25% increase, followed by water production from coal with a 22.7% increase and oil-derived products with a 10.8% increase. Total water production in the US varied more substantially, with multiple increases and decreases in production between 2005 and 2015. As observed in total global water production, US production also reached a minimum in 2009. Total water production in the US decreased overall between 2005 and 2015 by 0.7%, which was due to water production from natural gas increasing by 24.8% while water production from coal and oil- and NGL-derived products decreased by 31.0% and 8.2%, respectively.
Table 5 summarizes the water formation results from this study and compares them with water sequestration rates by underground injection, natural and irrigation-induced evaporation rates, and the mass of water held in the atmosphere. Water formation quantities due to combustion in the US averaged 2.3 × 1012 kg·yr−1 over the years 2005–2015. By comparison, 1.3 × 1012 kg of water from oil and gas operations were sequestered by underground injection into US disposal wells in 2012 [32]. Therefore, the combustion of hydrocarbon fuels in the US produces water at a higher rate than the volumes of water sequestered by injection during oil and gas operations. Despite this net water formation, however, pressure on local water resources incurred by water demands of oil and gas production are not necessarily relieved by formed water, which may be added to the hydrologic cycle in a different location or in a widespread manner [18, 20], suggesting the value of water harvesting for fuel production operations. Additionally, irrigation-induced and natural evaporation rates in the US are two and three orders of magnitude larger than water production from combustion, respectively.
The annual global formation of water from combustion of hydrocarbon fuels from 2005–2015 amounted to an average of 1.2 × 1013 kg·yr−1, as shown in figure 1. By comparison, the atmosphere is estimated to hold on average approximately 1.3 × 1016 kg of water, while the global rates of irrigation-induced and natural evaporation are on the orders of 1015 and 1017 kg·yr−1, respectively [20, 33, 34]. Due to the relatively small volumes of water produced from hydrocarbon fuel combustion, it has often not been an area of focus for climate science models as noted by Gaffen and Ross [17]. Climate science has focused mostly on natural evaporative sources of water vapor, with some attention paid to irrigation as it is the dominant anthropogenic source of water vapor contributed to the atmosphere—estimated to provide between 1.0 × 1015 to 2.6 × 1015 kg·yr−1 [17, 35, 36]. While the rate of water production from combustion is small compared to water evaporation rates due to irrigation, it is noteworthy that there is a substantial range in estimates of water contribution to the atmosphere from irrigation, which highlights the challenge of measurement uncertainties for LCAs at a global scale [37] and supports the consideration of water production from combustion. The inclusion of water production from combustion can be readily included in future LCAs through the use of WPFm and WPFe to evaluate the net water intensity of oil and gas production, electricity generation from combustion, and other processes for which water production and consumption are key metrics. While the system boundary for an LCA will be study-specific, it is generally recommended that water production from combustion be treated similarly to CO2 emissions in that the net production of water is more likely to contribute to atmospheric stores, not local supplies.
The WPF derived in this study can also be applied to the assessment of water harvesting, such as at the point of water production in stationary power generation, which might be beneficial for local water supplies. For example, flared natural gas was neglected in the US and global assessments of water production from hydrocarbon fuel combustion in this study due to its small quantity as compared to total natural gas production and consumption in the US and worldwide, at approximately 1% and 4% respectively. There is, however, significant potential benefit to harvesting water for oil and gas production operations, particularly in water deficient regions [14]. Using WPFm factors calculated in this study, and given annual flared gas volumes of approximately 8.9 × 109 and 1.5 × 1011 m3·yr−1 in the US and worldwide [38], respectively, an estimated 1.5 × 1010 and 2.5 × 1011 kg·yr−1 of water are produced in the US and worldwide, respectively, by combustion of flared gas alone. The quantities of water produced from flare gas combustion are comparable to estimated atmospheric water that can be harvested using the energy from flared gas [14]. While on a much smaller scale than total water produced by US and global fossil fuel consumption, water formed from flared gas combustion is produced at oil and gas production sites where the flares are located and the water need can be high. Therefore, the water produced from combustion may be a valuable resource in meeting local water needs.
Conclusions
This work estimates water production in the US and worldwide from the combustion of hydrocarbon fuels over the years from 2005 to 2015 using approximations for fuel composition and stoichiometric assessments. Water production factors are defined on fuel mass and energy bases per kilogram or megajoule of fuel as WPFm and WPFe, respectively, for ease of comparison of fuels and incorporation into future LCAs of water addition to atmospheric stores or assessment of water harvesting potential. Of the fuels analyzed, natural gas has been increasingly utilized for power and heat production over the years from 2005–2015 as compared to coal and oil-derived products. Natural gas produces more water per unit of fuel than oil- and NGL-derived products and dry ash free coal, followed by oil products then coal, as indicated by relative WPFm and WPFe. Thus, the increased utilization of natural gas will increase water production compared to that which would be produced from other fuels. This work finds that the total annual quantity of water formed from combustion of hydrocarbon fuels exceeds the water sequestered from the hydrologic cycle through deep well injection in the US, but is substantially less than water volumes generated through evaporation and irrigation each year. Thus, water formation is deemed significant enough to be considered for inclusion in future life cycle analyses of the water intensity of fuel production and use.
Acknowledgments
This work was funded in part by a National Science Foundation Graduate Research Fellowship (NSF 12–52375), the US Department of Energy (DE-EP0000011), the Alfred P. Sloan Foundation, and the Cynthia and George Mitchell Foundation.



